Abstract
Background
The vasopressin V2-receptor antagonist tolvaptan is used to treat cirrhotic patients with ascites. We investigated the outcome of long-term treatment.
Methods
This was a single-center retrospective study. Overall, 170 cirrhotic patients (95 males, median age 63 years) were enrolled and received tolvaptan orally after hospitalization for ascites, which included treatment with conventional diuretics. We compared patients who withdrew tolvaptan treatment after < 1 year (n = 90) with patients who continued treatment for ≥1 year (n = 37). In continuously treated patients, the pretreatment and post-treatment (1 year) blood biochemistry values were assessed.
Results
Overall, 37 patients received treatment for ≥1 year and showed a higher response after tolvaptan therapy. The reduction in body weight was 2.0 (− 3.4–17.2) kg compared to discontinued cases, which had a body weight reduction of 1.1 (− 6.2–7.5) kg after 1 week. The group that received treatment for ≥1 year had a significantly lower rate of the complication gastroesophageal varices and also showed better liver function. In patients with continued treatment, serum levels of albumin was significantly higher without renal disturbance after 1 year of treatment. The prothrombin time/international normalized ratio and ammonia level were also significantly improved. Multivariate analyses showed that a change in body weight reduction and serum levels of albumin were predictive factors of continued administration.
Conclusions
Long-term tolvaptan treatment increased serum levels of albumin, decreased ammonia levels, and preserved renal function after 1 year of treatment. A reduction in body weight after 1 week was associated with a favorable outcome of tolvaptan therapy.
Similar content being viewed by others
Background
Liver cirrhosis occurs in end-stage liver disease, and ascites accumulation is a sign of decompensated liver cirrhosis. Following the development of ascites, the 5-year survival rate is only 50% [1, 2]. After the development of dilutional hyponatremia, refractory ascites, and type 2 hepatorenal syndrome, the 1-year probability of survival is 25.6, 31.6, and 38.5%, respectively [2]. Ascites treatment for liver cirrhosis patients is important, and the vasopressin V2-receptor antagonist tolvaptan has dramatically improved this treatment [3, 4]. The Japanese Society of Gastroenterology recommended the use of tolvaptan prior to albumin administration or ascites drainage in 2015 [5]. Arginine vasopressin (AVP) triggers vasoconstriction by binding to V1a receptors, and promotes water re-absorption in the kidney by binding to V2 receptors, which are primarily responsible for the antidiuretic effects of AVP [3]. Tolvaptan selectively blocks the binding of vasopressin to V2 receptors.
In previous studies, we have shown that treatment with tolvaptan for 6 months is efficacious and safe and may improve the prognosis of cirrhotic patients [6, 7]. Median survival is significantly longer in patients with less ascites accompanying diuretic treatment [8]. According to a meta-analysis of randomized controlled trials, a tolvaptan-associated reduction in diuretic use is associated with lower serum sodium levels and maintenance of renal function, but no survival benefits were apparent in either the short- or long-term [9].
Tolvaptan response does not depend on albumin or sodium levels [3, 10]. These characteristics are beneficial for cirrhotic patients because cirrhosis results in extremely low levels of albumin and sodium in end-stage liver disease [11]. Conventional diuretics, particularly furosemide, bind reversibly to carrier protein; therefore, their mechanism of action requires albumin as the carrier protein. This leads to a decrease in the action of diuretics in cirrhosis. In addition, cirrhosis is often complicated by hyponatremia. It is sometimes difficult to treat using conventional diuretics because reducing or abolishing sodium chloride reabsorption further reduces serum levels of sodium, and hyponatremia is also associated with poor prognosis [12, 13]. Tolvaptan increases sodium levels and improves survival [13].
However, no studies have investigated long-term tolvaptan administration. Here, we investigated whether long-term tolvaptan treatment may improve liver and renal function, the predictive factors of long-term treatment, and the characteristics of patients who could stop treatment after improvement of ascites.
Methods
Patients and study design
This was a single-center, retrospective, observational study conducted between September 2013 and July 2018. A total of 170 cirrhotic patients (95 males, 56%, 75 females, 44%), complicated with ascites, who received tolvaptan (Samsca™; Otsuka Pharmaceutical Co. Ltd., Tokyo, Japan) after hospitalization with a salt/water-restricted diet (including four retreatment patients) were enrolled. Tolvaptan was started from a half dose (3.75 mg once per day) and it was increased to 7.5 mg/day if the response or body weight reduction was insufficient. Tolvaptan was added on other diuretics including 0–160 mg/day furosemide and/or 0–400 mg/day spironolactone. Excluded criteria was patients with severe renal dysfunction (estimated glomerular filtration rate [eGFR] < 15 mL/min/1.73 m2 or serum levels of creatinine > 3.5 mg/dL) and patients with a hepatic coma scale score > II. Overall, of the patients who were excluded in the discontinued cases (n = 90), eight patients could stop treatment and seven continued treatment for < 1 year. Patients who received tolvaptan treatment for < 1 year and ≥ 1 year (n = 37) were compared (Fig. 1). Denver shunts were placed following the manual of the procedure [14].
Flow chart of the study design. Overall, 170 cases with cirrhosis were treated with tolvaptan. Eight patients finished treatment due to improved ascites, and treatment was stopped in 90 cases due to poor condition, death, and liver transplantation. By contrast, 37 cases were continuously administered tolvaptan for ≥1 year
This study was conducted according to the principles of the Declaration of Helsinki and the ethics rules of Tokyo Women’s Medical University Hospital (TWMU, Tokyo, Japan). The TWMU Institutional Review Board approved the study protocol (approval no. 3258-R).
Clinical parameters
The following baseline characteristics of patients were assessed: age, sex, clinical history, body weight, urine volume, underlying hepatic diseases, rates of complications of cirrhosis (i.e., varices, hepatocellular carcinoma, and hepatic encephalopathy), treatments including the administration of diuretics and branched-chain amino acids (BCAA), and procedures including ascites drainage and implantation of Denver shunts. Blood samples for biochemistry and hematological data were collected at the time of administration of tolvaptan and after 1 year of treatment. Urine volume was determined the next day and the reduction in body weight was evaluated after 1 week. Laboratory tests included serum concentrations of albumin, total bilirubin, aspartate aminotransferase, alanine transaminase, γ-glutamyltransferase, ammonia, alpha-fetoprotein and des-gamma-carboxy prothrombin, platelet counts, hematocrit, prothrombin time (PT), and PT/international normalized ratio (INR). The modified Child-–Pugh (CP) score [15] and the model for end-stage liver disease (MELD) score [16] were used to evaluate liver function.
Follow-up and outcomes
The patients were hospitalized and administered tolvaptan for 1–2 weeks. After discharge, patients were followed every 1–2 months at an outpatient clinic to check biochemical parameters. One year after tolvaptan administration, outcomes were monitored and evaluated the efficacy and the condition.
Statistical analyses
Data are presented as medians with minimum and maximum values. Significant differences between the two groups were assessed using the Mann–Whitney U test and χ2 test with the SPSS statistical software package (SPSS Inc., Chicago, IL, USA). Pretreatment and post-treatment values were compared using Wilcoxon signed-rank tests. Differences were considered statistically significant at p < 0.05. Multivariate logistic regression analyses were performed with the likelihood ratio test to assess their fit after 1 year of tolvaptan treatment.
Results
Baseline characteristics of patients pretreated with tolvaptan
A flow chart of our study is shown in Fig. 1. A total of 170 cirrhotic patients were treated with tolvaptan and 64% of patients had their dose increased to 7.5 mg/day. The median age was 63 (range, 21–90) years old, and 56% were male (Table 1). Underlying liver diseases included hepatitis C virus (HCV, 28%), hepatitis B virus (HBV, 7%), alcoholic liver disease (ALD, 28%), non-alcoholic fatty liver disease (NAFLD, 11%), and primary biliary cholangitis (PBC; 10%). Regarding complications of liver cirrhosis, gastroesophageal varices (68%), hepatocellular carcinoma (HCC; 32%), and hepatic encephalopathy (27%) were observed.
The median urine volume on the next day of treatment was 1595 (120–6630) mL and the body weight change was − 1.5 (− 17.2 to + 6.2) kg after 1 week of treatment.
The distribution of diuretic use is shown in Fig. 2. The majority of cirrhotic patients with ascites who started tolvaptan treatment were taking 0–50 mg/day spironolactone and 20–40 mg/day furosemide.
Patients who could stop treatment due to improved ascites
Of the patients who withdrew from tolvaptan treatment after < 1 year, the reasons included death (n = 59), liver transplantation (n = 22), hemodialysis or non-response to treatment (n = 9), transfer to another hospital (n = 28), and improvement (n = 8). Overall, nine patients stopped tolvaptan treatment (Table 2). Six cases had ALD, and patients who stopped alcohol consumption were able to successfully improve their ascites without tolvaptan treatment. However, two patients required retreatment, which was accompanied by a return to alcohol consumption. In a patient with methotrexate-related liver disease, ascites improved after discontinuing methotrexate. A patient with HBV temporarily recovered by undergoing nucleic acid analog treatment, but required tolvaptan treatment again.
Comparison of laboratory data between patients who withdrew and continued tolvaptan treatment
Tolvaptan was introduced to cirrhotic patients with ascites who were also taking conventional diuretics. However, almost all of the patients could not continue long-term treatment (Fig. 3). Overall, 62 patients discontinued tolvaptan within 3 months, 30 in 3–6 months, and 31 in 6 months to 1 year. Only 37 cases were treated for ≥1 year. The median treatment period was 132 days.
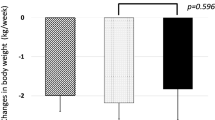
Patients who were treated with tolvaptan for < 1 year (n = 90, without improved and continued treating cases) were compared to those treated for ≥1 year (n = 37, Fig. 1, Table 1). Changes in body weight after 1 week were significantly greater in patients who continued treatment (− 2.0 kg [− 17.2 to + 3.4]; p = 0.03) than in those who discontinued treatment (− 1.1 kg [− 7.5 to + 6.2]). Consistent with the etiology of liver disease, the rate of ALD increased in such patients, who also had less gastroesophageal varices. A Denver shunt was placed in three patients who continued treatment and in only one patient who discontinued treatment. The rates of other ascites therapies including diuretic use, administration of BCAA, and CART or drainage of ascites were not significantly different between the groups.
The serum levels of albumin (p = 0.03) and sodium (p = 0.01) were significantly higher, whereas the level of total bilirubin (p < 0.01) was significantly lower in patients who continued treatment. Although the modified CP score tended to be low (mean value 11 [range: 8–13], p = 0.05) in patients who continued treatment, the eGFR was slightly reduced (49.5 [16.8–107.2] mL/min/1.73 m2 [p = 0.07]) in that group.
Changes in chemical parameters from baseline, and analyses of factors predictive of continued long-term tolvaptan treatment
To investigate whether long-term tolvaptan treatment may improve liver and renal function, patients who were treated for ≥1 year were assessed (n = 37, Table 3). Serum levels of albumin were significantly increased (p < 0.01, Fig. 4a), whereas levels of total bilirubin (p = 0.06, Fig. 4b) and ammonia (p < 0.01, Fig. 4f), and the PT/INR (p = 0.02, Fig. 4d) were significantly reduced after 1 year of tolvaptan treatment. Renal function was not affected (Fig. 4e). The modified CP score improved significantly (p < 0.01, Fig. 4g) and the MELD score was also reduced, but not significantly (p = 0.13, Fig. 4h).
Changes in biochemical parameters in blood after 1 year of tolvaptan. a) Serum levels of albumin, b) total bilirubin, c) PT%, d) PT/INR, e) creatinine, f) ammonia, g) the modified CP score, and h) MELD score. Albumin levels were significantly increased (a, p < 0.01), whereas total bilirubin (b, p = 0.06), PT/INR (d, p = 0.02), and ammonia (f, p < 0.01) were reduced after 1 year of tolvaptan treatment. The modified CP score was significantly improved (g, p < 0.01). The MELD score was also reduced, however, it not significantly (h, p = 0.13). PT, prothrombin time; INR, international normalized ratio; CP, Child- Pugh; MELD, model for end-stage liver disease
To identify predictive factors associated with continued administration of tolvaptan, multivariate logistic regression analyses were used to assess changes in body weight, gastroesophageal varices, HCC, hepatic encephalopathy, eGFR, and levels of albumin, total bilirubin, aspartate aminotransferase, and sodium (Table 4). Changes in body weight and albumin levels were identified as predictive factors (odds ratio [OR]: 1.320, 95% confidence interval [CI]: 1.083–1.608, p = 0.006 and OR: 4.280, 95% CI: 1.136–16.127, p = 0.032, respectively). In addition, eGFR was weakly associated with continued administration (OR: 0.978, 95% CI: 0.958–0.999, p = 0.045).
Discussion
We demonstrated that tolvaptan treatment for ≥1 year significantly increased albumin levels and decreased the PT/INR and ammonia levels. Factors associated with continued administration included body weight reduction after 1 week and better liver function.
In the current study, patients who required liver transplantation were included. Therefore, it was difficult to analyze long-term tolvaptan treatment, because the modified CP score had already attained 11 (range: 6–14). In this condition, 37 cases (22%) with a modified CP score of 11 (8–13) were observed for ≥1 year. Serum levels of albumin were significantly higher in continuously treated cases. While hematocrit values were slightly increased by improving excess water, total bilirubin and ammonia levels were decreased. Therefore, the increase in albumin level was thought to be due to improved condition. Although the MELD score did not differ significantly between the groups, the modified CP score improved significantly in the group that continued treatment (p < 0.01). We speculated that patients with improved ascites had increased appetite and nutritional condition, resulting in increased protein production ability.
Long-term administration of tolvaptan resulted in no significant decrease in renal function. We previously reported that liver transplant recipients maintained their renal function after 1 year of transplantation [17]. Even in chronic kidney disease, tolvaptan reduces the risk for medium-term worsening renal function [18], because tolvaptan is an aquaretic agent that removes excess water without electrolyte excretion. Therefore, renal functional loss was not exacerbated [19]. Furosemide greatly increases urination, independent of the intravascular volume, reducing renal function.
Regarding predictors associated with long-term treatment, changes in body weight were significantly greater in continued cases. Lower complication rates of esophageal varices, total bilirubin levels, and higher serum levels of albumin and sodium were predictive factors for long-term treatment. Portal vein pressure is associated with tolvaptan response [20]. A hepatic venous pressure gradient (HVPG) of 190 mmH2O or less results in a body weight reduction of 2 kg in a week. It has been suggested that ascites accumulation is caused by not only by low albumin levels but also portal hypertension.
Multiple logistic regression analyses showed that changes in body weight reduction (OR: 1.320, 95% CI: 1.083–1.608, p = 0.006) and albumin levels (OR: 4.280, 95% CI: 1.136–16.127, p = 0.032) were predictive factors for continued treatment. In addition to the baseline ability of the liver, tolvaptan response was important for continued administration. Recently, response to tolvaptan was defined as a 1.5 kg/week reduction and this value was expected to be an indicator of symptomatic improvement [21]. Body weight reduction may also be used as a predictor of long-term outcome.
By contrast, few reports have discussed the stopping of tolvaptan treatment because liver cirrhosis is irreversible and almost all patients fail to discontinue treatment. We attempted to stop treatment by improving ascites in nine patients (one patient stopped after ≥1 year treatment); however, three patients relapsed and required retreatment. These patients stopped consuming alcohol, ceased methotrexate use, and underwent nucleic acid analog treatment for HBV. These factors may have been associated with improved ascites, leading to functional improvement of the liver, even if the liver was in a cirrhotic state. In particular, patients with ALD might have a chance to recover if they stop drinking. The number of patients who successfully completed tolvaptan treatment was limited; therefore, the predictive factors were not determined. However, cases with temporarily compromised liver function and those initially engaging in treatment may wish to discontinue the drug.
A limitation of this study was that it was a single-center observational retrospective study. We could not compare patients with and without tolvaptan therapy; therefore, the direct effects of tolvaptan on albumin levels were not determined. Further analyses to determine the outcomes of long-term tolvaptan treatment are required.
Conclusions
In conclusion, long-term treatment with tolvaptan increased serum levels of albumin and decreased ammonia levels after 1 year of treatment. Body weight reduction after 1 week was associated with a favorable outcome of tolvaptan therapy. Among patients who require radical treatment for cirrhosis and still require ascites treatment, tolvaptan administration should be maintained.
Abbreviations
- ALD:
-
Alcoholic liver disease
- BW:
-
Body weight
- CART:
-
Cell-free and concentrated ascites reinfusion therapy
- CP:
-
Child-Pugh
- eGFR:
-
Estimated glomerular filtration rate
- HBV:
-
Hepatitis B virus
- HCV:
-
Hepatitis C virus
- INR:
-
International normalized ratio
- MELD:
-
Model for end-stage liver disease
- NAFLD:
-
Non-alcoholic fatty liver disease
- PBC:
-
Primary biliary cholangitis
References
Ginés P, Quintero E, Arroyo V, Terés J, Bruguera M, Rimola A, et al. Compensated cirrhosis: natural history and prognostic factors. Hepatology. 1987;7:122–8.
Planas R, Ballesté B, Alvarez MA, Rivera M, Montoliu S, Galeras JA, et al. Natural history of decompensated hepatitis C virus-related cirrhosis. A study of 200 patients. J Hepatol. 2004;40:823–30.
Okita K, Sakaida I, Okada M, Kaneko A, Chayama K, Kato M, et al. A multicenter, open-label, dose-ranging study to exploratively evaluate the efficacy, safety, and dose-response of tolvaptan in patients with decompensated liver cirrhosis. J Gastroenterol. 2010;45:979–87.
Kawaratani H, Fukui H, Yoshiji H. Treatment for cirrhotic ascites. Hepatol Res. 2017;47:166–77.
Fukui H, Saito H, Ueno Y, Uto H, Obara K, Sakaida I, et al. Evidence-based clinical practice guidelines for liver cirrhosis. J Gastroenterol. 2015;51:629–50.
Kogiso T, Tokushige K, Hashimoto E, Ikarashi Y, Kodama K, Taniai M, et al. Safety and efficacy of long-term tolvaptan therapy for decompensated liver cirrhosis. Hepatol Res. 2016;46:E194–200.
Kogiso T, Yamamoto K, Kobayashi M, Ikarashi Y, Kodama K, Taniai M, et al. Response to tolvaptan and its effect on prognosis in cirrhotic patients with ascites. Hepatol Res. 2017;47:835–44.
Tajiri K, Tokimitsu Y, Ito H, Atarashi Y, Kawai K, Minemura M, et al. Survival benefit of tolvaptan for refractory ascites in patients with advanced cirrhosis. Dig Dis. 2018;36:314–21.
Yan L, Xie F, Lu J, Ni Q, Shi C, Tang C, et al. The treatment of vasopressin V2-receptor antagonists in cirrhosis patients with ascites: a meta-analysis of randomized controlled trials. BMC Gastroenterol. 2015;15:65.
Sakaida I, Nakajima K, Okita K, Hori M, Izumi T, Sakurai M, et al. Can serum albumin level affect the pharmacological action of tolvaptan in patients with liver cirrhosis? A post hoc analysis of previous clinical trials in Japan. J Gastroenterol. 2015;50:1047–53.
Angeli P, Wong F, Watson H, Ginès P, Investigators C. Hyponatremia in cirrhosis: results of a patient population survey. Hepatology. 2006;44:1535–42.
Jia JD, Xie W, Ding HG, Mao H, Guo H, Li Y, et al. Utility and safety of tolvaptan in cirrhotic patients with hyponatremia: a prospective cohort study. Ann Hepatol. 2017;16:123–32.
Kogiso T, Kobayashi M, Yamamoto K, Ikarashi Y, Kodama K, Taniai M, et al. The outcome of cirrhotic patients with ascites is improved by the normalization of the serum sodium level by tolvaptan. Intern Med. 2017;56:2993–3001.
Ginès P, Arroyo V, Vargas V, Planas R, Casafont F, Panés J, et al. Paracentesis with intravenous infusion of albumin as compared with peritoneovenous shunting in cirrhosis with refractory ascites. N Engl J Med. 1991;325:829–35.
Tarantino G, Gentile A, Capone D, Basile V, Tarantino M, Di Minno MN, et al. Does protracted antiviral therapy impact on HCV-related liver cirrhosis progression? World J Gastroenterol. 2007;13:4903–8.
Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864–71.
Kogiso T, Yamamoto K, Ikarashi Y, Kodama K, Taniai M, Egawa H, Yamamoto M, Hashimoto E, Tokushige K. The safety of tolvaptan for liver transplantation candidates and its impact on renal function after transplantation. Open Access Journal of Gastroenterology and Hepatology. 2017.
Nakano Y, Mizuno T, Niwa T, Mukai K, Wakabayashi H, Watanabe A, et al. Impact of continuous administration of tolvaptan on preventing medium-term worsening renal function and long-term adverse events in heart failure patients with chronic kidney disease. Int Heart J. 2018;59:105–11.
Mori T, Ohsaki Y, Oba-Yabana I, Ito S. Diuretic usage for protection against end-organ damage in liver cirrhosis and heart failure. Hepatol Res. 2017;4:11–22.
Nakagawa A, Atsukawa M, Tsubota A, Kondo C, Okubo T, Arai T, et al. Usefulness of portal vein pressure for predicting the effects of tolvaptan in cirrhotic patients. World J Gastroenterol. 2016;22:5104–13.
Hiramine Y, Uojima H, Nakanishi H, Hiramatsu A, Iwamoto T, Kimura M, et al. Response criteria of tolvaptan for the treatment of hepatic edema. J Gastroenterol. 2018;53:258–68.
Acknowledgments
Not applicable.
Funding
Not applicable.
Availability of data and materials
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
Conception and design: TK and KT. Analysis and interpretation of data: TK. Drafting of the manuscript: TK. Patient care, follow-up, and data acquisition: TK, TS, KK, MT, and KT. All authors have read and have approved the final manuscript. All authors agree to be accountable for all aspects of the work; questions related to the accuracy or integrity of any part of the work will be appropriately investigated and resolved.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Research involving human participants. All procedures involving humans were performed in accordance with the ethical standards of our institutional and/or national research committee and those of the 1964 Helsinki declaration and later amendments thereof, or comparable ethical standards. The verbal informed consent was obtained and the study was disclosed the information on our web site. The study protocol (no. 3258-R) was approved by the Tokyo Women’s Medical University Hospital Institutional Review Board (TWMU, Tokyo, Japan).
Consent for publication
Not applicable.
Competing interests
KT has received research funding from Sumitomo Dainippon Pharma Co., Ltd.; Astellas Pharma Inc.; Eisai Co., Ltd.; TAIHO Pharmaceutical Co., Ltd.; Chugai Pharmaceutical Co. Ltd.; Daiichi Sankyo Pharmaceutical Co. Ltd.; AbbVie GK; Takeda Pharmaceutical Company Limited; Asahi Kasei Corporation; AJINOMOTO Co. Inc.;. and Otsuka Pharmaceutical Co. Ltd.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Kogiso, T., Sagawa, T., Kodama, K. et al. Impact of continued administration of tolvaptan on cirrhotic patients with ascites. BMC Pharmacol Toxicol 19, 87 (2018). https://doi.org/10.1186/s40360-018-0277-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40360-018-0277-3