Abstract
In this study, Insect tea primary leaf (Malus sieboldii (Regal) Rehd.) was used as the research object to investigate the protective effect of Insect tea primary extract (ITPLE) on hydrogen peroxide (H2O2)-induced oxidative damage in human embryonic kidney 293T cells (HEK 293T cells) and the mechanism of action of the main active components. The 3-(4,5-dimethyl-2-thiazolyl)- 2,5-diphenyl-2-H-tetrazolium bromide (MTT) assay was used to determine the toxicity of ITPLE to HEK 293T cells in vitro as well as its protective effect against (H2O2)-induced oxidative damage in HEK 293T cells. In addition, various assay kits were used to measure oxidation-related indicators in HEK 293T cells, and quantitative polymerase chain reaction (qPCR) analysis was used to determine the mRNA expression levels of oxidation-related genes in HEK 293T cells. High performance liquid chromatography (HPLC) analysis was used to characterize active components in ITPLE. The experimental results revealed that the ITPLE had no toxic effect on cells in the range of 0–200 μg/mL, and, in this range, exhibited a concentration-dependent protective effect against H2O2-induced oxidative damage in HEK 293T cells. It was also found that the ITPLE can reduce the malondialdehyde (MDA) level and increase the levels of superoxide dismutase (SOD), glutathione (GSH), glutathione peroxidase (GSH-Px), and catalase (CAT)in oxidative damage HEK 293T cells. The qPCR analysis results also showed that the ITPLE upregulated the mRNA expression levels of SOD, CAT, GSH and GSH-Px in HEK 293T cells damaged by H2O2-induced oxidative stress. The HPLC analysis identified 7 bioactive components in the ITPLE, including neochlorogenic acid, cryptochlorogenic acid, rutin, kaempferin, isochlorogenic acid B, isochlorogenic acid A and hesperidin. This study reveals that ITPLE is rich in active compounds and has good antioxidant effect in vitro, thus it has the potential to be developed into a traditional Chinese medicine and functional drinks.
Similar content being viewed by others
Introduction
Insect tea is a traditional health drink in southern China. The production process of Insect tea is very special, in particular, the Insect tea primary leaves are fed to insect larvae, and then the excreta of the larvae are processed into tea like drinks [1]. Besides the influence of insects, the species of raw primary leaves (material leaves) also play an important role in the quality and function of Insect tea. The plant leaves used to make Insect tea mainly include Kuding tea (Ligustrum robustum (Roxb.) Bl.), Liubao tea (Camellia sinensis) and toringo (Malus sieboldii (Regal) Rehd.), etc. [2]. The fruit and leaf of toringo are both used in traditional Chinese medicine to help digestion and reduce inflammation [2]. It has been shown that the leaves of toringo contains abundant amino acids, polysaccharides, flavonoids and saponins [3]. However, the toringo leaves used for preparing Insect tea need to go through certain processing procedures. The leaves are first steamed, dried, and then added to rice soup for natural fermentation and ripening [4]. Afterwards, the composition and bioactivity of the leaves of toringo will change, but the research on the composition and function of the processed leaves is still lacking.
Reactive oxygen species (ROS) produced by aerobic metabolism in humans, mainly include superoxide anion radical (O•−2 ), peroxide anion (O22−), hydroxyl radical (·OH), hydroxyl ion (OH−), hydrogen peroxide (H2O2), etc. ROS can cause oxidative stress (OS) in human cells. OS is an imbalance between oxidation and antioxidation in vivo, which tends to oxidize, resulting in inflammatory infiltration of neutrophils, increase of protease secretion and production of a large number of oxidation intermediates. OS is a negative effect produced by free radicals in the cells, and is considered to be an important factor leading to aging and diseases [5]. OS can damage cell structures and affect function in human cells, resulting in molecular degradation, apoptosis, inflammation and ultimately leading to various types of chronic diseases, such as cardiovascular disease, neurodegenerative diseases, kidney disease and cancer [6, 7]. The development of these diseases is closely related to the oxidative changes of key physiological molecules in the body, including the regulation of proteins, lipids, carbohydrates, nucleic acids, gene expression and inflammatory response [8].
In this study, the components of the processed leaves of toringo (Insect tea primary leaf) extract (ITPLE) were analyzed. In addition, since the available evidence indicates that H2O2 is the main form of ROS in vivo, which can generate·OH through Fenton reaction and intracellular Fe2+ and then cause chain reaction, H2O2 is used in this study as an inducer of OS. Also, an in vitro human embryonic kidney 293T (HEK 293T) cell injury model was established and used to investigate the inhibitory effect of ITPLE on OS-induced cell injury, and to preliminarily analyze its potential active components, so as to provide certain reference basis for the protective effect of Insect tea primary leaf on human chronic diseases.
Materials and methods
Extraction of insect tea primary leaf
After the Insect tea primary leaf was freeze-dried, 100 g of the Insect tea primary leaf was taken out and crushed, and extracted in 2 L of 80% aqueous methanol solution (v/v). Then, the filtrate of the extract was evaporated and the evaporated drying extract (ITPLE) was used for subsequent cellular assays.
Cell culture
After thawing, HEK 293T cells (Shanghai Institute of Biochemistry and Cell Biology, Shanghai, China) were resuspended in high glucose Eagle’s Minimum Essential Medium (EMEM) medium (Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing 10% fetal bovine serum and 1% penicillin–streptomycin double antibody solution, and cultured in a Heracell 150i CO2 incubator (Thermo Fisher Scientific Inc.) with a saturated humid environment at 37 °C and 5% CO2.
Assay of ITPLE toxicity on HEK 293T cells
HEK 293T cells were suspended in EMEM medium at a concentration of 1 × 104 cells/mL. Then, 200 μL of this cell suspension was seeded into a 96-well cell culture plate and cultured for 24 h at 37 °C. After the cells adhered to the plate, the original culture medium was suctioned off, discarded, and replaced with fresh medium containing ITPLE in the range of 0–300 μg/mL, and adherent cells were further cultured for 48 h. Afterwards, the medium was suctioned off, discarded, and replaced with medium containing the MTT reagent (5 mg/mL) and cells were cultured for 4 h. Subsequently, the medium was discarded, and 200 μL of dimethyl sulfoxide (DMSO) was added and reacted in the dark for 30 min. Eventually, the optical density (OD) value was measured at 494 nm om an Evolution™ 350 UV–Vis spectrophotometer (Thermo Fisher Scientific Inc.), and the cell proliferation rate was determined [9].
Effect of ITPLE on H2O2-induced oxidative damage in HEK 293T cells
After the cells were attached to the plate as described in ITPLE toxicity determination section, the medium was suctioned off, discarded, and replaced with fresh medium containing 0.3 mmol/L H2O2 and cells were cultured for 4 h to induce oxidative damage in HEK 293T cells. Subsequently, the culture medium was discarded, and fresh medium containing ITPLE as described in sub-Sect. “Assay of ITPLE toxicity on HEK 293T cells”. Eventually, after treatment with ITPLE, the absorbance value was measured and the cell proliferation rate was determined [9].
Determination of MDA, SOD, GSH, GSH-Px and CAT in HEK 293T cells subjected to oxidative damage
As described in ITPLE toxicity determination section, after treating the cells with ITPLE for 48 h, cells in the culture plate were washed with phosphate-buffered saline (PBS). Then cells were detached from the plate by adding 200 μL of trypsin solution per well, resuspended in complete medium and centrifuged at 4000 rpm for 15 min. Afterwards, the supernatant was discarded, and the process was repeated once. Then, the 800 μL lysate (solarbio, Beijing, China) was added to the cell, and after full lysis, the lysate cells were centrifuged for 5 min (8000 rpm), and the supernatant was taken out. The levels of MDA, SOD, GSH, GSH-Px and CAT in the cell homogenate were determined according to the instructions of the corresponding kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, Jiangsu, China).
qPCR analysis of mRNA expression levels in HEK 293T cells subjected to oxidative damage
Total RNA was isolated from HEK 293T cells treated as described in sub-Sect. “Determination of MDA, SOD, GSH, GSH-Px and CAT in HEK 293T cells subjected to oxidative damage”, using Trizol reagent (Thermo Fisher Scientific Inc.). Briefly, 1 μL of oligo (dT) 18 primer (500 ng/μL) and 1.0 μL of total RNA (1.0 μg/μL) were added to 10.0 μL of nuclease free water and heated at 65 °C for 5 min. Then, a reagent mixture containing 4.0 μL of 5× reaction buffer, 1.0 μL of ribolock RNase inhibitor (20 U), 2.0 μL of 10 mM dNTP mix and 1.0 μL of 200 U/μL reversible reverse transcriptase (Thermo Fisher Scientific Inc.) was added to the total RNA system, and the cDNA was synthesized at 42 °C for 60 min and 700 ℃ for 5 min. Then, 1.0 μL cDNA, 1.0 μL each (10 μm/μL) of forward and reverse primers (Thermo Fisher Scientific), 10.0 μL premix and 7.0 μL sterile double-distilled water were mixed for cDNA amplification. The amplification conditions were as follows: denaturation at 95 °C for 3 min, annealing at 60 °C for 30 s, extension at 95 °C for 1 min, for 40 cycles, performed on an ABI StepOne Plus qPCR system (Thermo Fisher Scientific Inc.). The mRNA expression of SOD, CAT, GSH and GSH Px were detected by qPCR analysis. The primer sequences are shown in Table 1. The cDNA of each gene was amplified three times in parallel, and the average CT value was used. The housekeeping gene Actin was used as an internal reference and the related gene was calculated according to 2−ΔΔCT method [10].
Determination of the composition of ITPLE by HPLC analysis
A 10 mg/mL stock solution of ITPLE was prepared in DMSO t, and then diluted with an aqueous 50% methanol solution to obtain a liquid sample with a final concentration of 2 mg/mL. The diluted ITPLE solution was tested (injection volume: 10 μL) on the Ultimate 3000 high performance liquid chromatography (HPLC) system (Thermo Fisher Scientific) after passing a 0.22 μm organic filter membrane from Standard products (Shanghai Yuanye Biotechnology Co., Ltd., Shanghai, China). The chromatographic conditions were as follows: Hypersep C18 column (4.6 mm × 150 mm, 5 μm); mobile phase A, 0.5% acetic acid water; mobile phase B, acetonitrile; flow rate, 0.5 mL/min; column temperature: 25 °C; detection wavelength, 280 nm; gradient elution conditions were as shown in Table 2.
Results
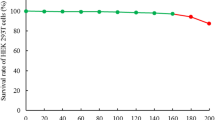
Toxicity of ITPLE to HEK 293T cells
As shown in Fig. 1, after ITPLE treatment, the proliferation of HEK 293T cells was not inhibited in the concentration range of 0–200 μg/mL, with cells basically maintaining normal proliferation. However, when the concentration exceeded 200 μg/mL, the cell proliferation was inhibited. Thus, these results indicate that ITPLE has no toxic effect on HEK 293T cells in the concentration range of 0–200 μg/mL. Accordingly, the concentrations of 50, 100 and 200 μg/mL were selected for subsequent experiments.
Protective effect of ITPLE against oxidative damaged in HEK 293T cells
As shown in Fig. 2, compared with normal HEK 293T cells, the proliferation rate (28.3%) of HEK 293T cells subjected to H2O2-induced oxidative damage decreased significantly (P < 0.05). Compared with the HEK 293T cells subjected to oxidative damage without ITPLE treatment, the proliferation rate of HEK 293T cells similarly subjected to oxidative damage, but treated with 50 (51.8%) or 100 (40.6%) or 200 μg/mL (73.9%) of the ITPLE increased significantly (P < 0.05), and the increased proliferation was positively correlated with the concentration of action.
SOD, GSH, GSH-Px, CAT and MDA levels in HEK 293T cells subjected to oxidative damaged and treated with the ITPLE
The results shown in Fig. 3 reveal that the levels of SOD, GSH, GSH Px and CAT in normal HEK 293T cells were the highest, while the level of MDA was the lowest. The ITPLE significantly (P < 0.05) inhibited in a concentration-dependent manner the H2O2-induced changes in the levels of SOD, GSH, GSH Px, CAT and MDA in HEK 293T. Clearly, the results showed that the ITPLE can regulate the oxidation related indicators in HEK 293T cells, thus attenuating the oxidative damage induced by H2O2.
SOD, GSH, GSH-Px and CAT mRNA expression levels in HEK 293T cells subjected to oxidative damaged and treated with the ITPLE
The results of the qPCR analysis (Fig. 4) revealed that the mRNA expression levels of SOD, CAT, GSH and GSH-Px decreased significantly (P < 0.05) in HEK 293T cells subjected to H2O2-induced oxidative injury. The mRNA expression levels of SOD, CAT, GSH and GSH-Px increased significantly (P < 0.05) in HEK 293T cells subjected to oxidative damage.
Constituents of ITPLE
As shown in Fig. 5, the results of the HPLC analysis revealed that the ITPLE was composed of neochlorogenic acid, cryptochlorogenic acid, rutin, kaempferin, quercetin, isochlorogenic acid A and isochlorogenic acid B. In particular, the content of kaempferin and isochlorogenic acid B were higher than that of the other chemical components.
Discussion
Oxidative stress can cause lipid peroxidation of polyunsaturated fatty acids in biofilm, and then cell oxidative damage, resulting in a series of diseases [11]. In order to avoid the damage caused by oxidative stress, human beings can resist oxidative stress through their own antioxidant system and intake of exogenous antioxidants. In particular, the regulation enzyme system including SOD, CAT, GSH-Px and so on plays a role in resisting oxidative stress [12]. It has been proved that many plants have the effect of improving the damage caused by oxidative stress, and have been used in practical application [13,14,15]. However, many application cases are based on the inheritance of experience and traditional medicine, and lack of in-depth study on the active components of plants and their mechanism of action. The purpose of this study was to analyze the components of Insect tea primary leaf (Malus sieboldii (Regal) Rehd.) that had been used in traditional Chinese medicine, and to studied the role of inhibiting oxidative stress, and to accumulated theoretical basis for the application of this plant in other diseases in the future.
MDA is the final metabolite of membrane lipid peroxidation in vivo, which can reflect the degree of tissue peroxidation. MDA released from cell membrane can react with protein and nucleic acid, cause cross-linking polymerization, inhibit protein synthesis, damage cell membrane structure and function, change its permeability, and affect normal physiological activities of the body [16, 17]. Therefore, the level of MDA can be used to monitor the degree of lipid peroxidation and indirectly reflect the degree of cell and body damage. Both human endogenous antioxidant enzymes of CAT, SOD, GSH-Px and non enzyme GSH can effectively improve oxidative stress injury [18]. SOD can catalyze superoxide anion radical disproportionation to produce oxygen and hydrogen peroxide, which plays an important role in the balance of oxidation and oxidation resistance, and is closely related to the occurrence and development of many diseases [19]. The biological function of catalase is to promote the decomposition of hydrogen peroxide in cells, so that it will not further produce toxic free radicals of hydrogen and oxygen, so as to protect the function of antioxidant enzyme system, which is also of great significance for human growth and metabolism [20]. GSH is characterized by active sulfhydryl (-SH), which is the most important functional group. It can participate in various important biochemical reactions of the body, protect the sulfhydryl group of important enzyme protein from oxidation and inactivation, and ensure energy metabolism and cell utilization. Meanwhile, it can directly reduce the free radicals to acidic substances by the combination of sulfhydryl and free radicals in the body, so as to accelerate the excretion of free radicals and fight against the damage of free radicals to important organs [21]. GSH-Px can reduce toxic peroxides to nontoxic hydroxyl compounds, and promote the decomposition of H2O2, so as to protect the structure and function of cell membrane from the interference and damage of oxides [22]. SOD can convert the excess O2− of the body into H2O2, which is then converted into H2O by CAT and GSH-Px [22]. CAT has a high affinity for H2O2 and can reduce the toxic H2O2 to H2O [23]. In addition, GSH can react directly with ROS, reduce it, and act as the substrate of GSH-Px, catalyze GSH to GSSG, reduce alkane hydroperoxides to hydroxyl compounds, and promote the decomposition of H2O2 [24]. The cooperation of CAT, SOD, GSH-Px and GSH can play the role of resisting oxidative stress in the body and protect the body together [25]. In this study, through the detection of kits and mRNA analysis, it was observed that the ITPLE could increase the SOD, CAT, GSH, GSH-Px levels and reduce the MDA level of HEK 293T cells, thus reducing the oxidative damage caused by H2O2.
Neochlorogenic acid, cryptochlorogenic acid, isochlorogenic acid A and isochlorogenic acid B are chlorogenic acid compounds, all of which have good antioxidant effects [26]. The research also showed that they played the role of antibacterial, antiviral, increasing leukocyte, protecting liver and choleretic, anti-tumor, lowering blood pressure, reducing blood lipid, clearing free radicals and stimulating central nervous system through their antioxidant abilities [27,28,29,30,31]. In addition, in vitro experiments also show that isochlorogenic acid B has a good scavenging effect on hydroxyl radicals, hydrogen peroxide is easy to produce hydroxyl radicals and cause oxidative damage [32], which suggests that isochlorogenic acid B should have a good inhibitory effect on hydrogen peroxide induced oxidative damage. Rutin, as a commonly used antioxidant, has a strong ability of scavenging free radicals. In pharmacology, it has the functions of anti-bacterial and anti-inflammatory, anti radiation, regulating the permeability and brittleness of capillary wall, preventing vascular rupture, hemostasis, etc. [33]. Kaempferoside is also an antioxidant. Preliminary studies have also shown that kaempferoside has been detected in a variety of traditional Chinese medicine ingredients. It can be inferred that kaempferoside has certain efficacy in treating inflammation and improving immunity [34]. It has been shown that the phenolic hydroxyl of kaempferoside can scavenge hydrogen peroxide, so as to play the role of kaempferoside in inhibiting the oxidative damage caused by peroxidation [35]. Quercetin is also used as an antioxidant in the food industry. At the same time, research shows that quercetin has good expectorant and antitussive effects [36]. These seven substances have good antioxidant effect, these ingredients are also detected in traditional Chinese medicine and functional food, they also have a variety of disease prevention and treatment effects [26,27,28,29,30,31,32,33,34,35,36]. In this study, the ITPLE contained these active substances, their own functions and synergistic effects constituted the inhibition of oxidative damaged HEK 293T cells, which might play the preventive or therapeutic effect on some diseases.
In conclusion, in this study, we evaluated the protective effect of the ITPLE against OS at the cellular level and preliminarily examined the relationship between the active compounds and their efficacy. The results of biochemical and molecular biological experiments showed that the ITPLE had excellent inhibitory effects on oxidative damage in HEK 293T cells. In particular, ITPLE could enhance the production of antioxidant enzymes and other antioxidant substances in HEK 293T cells induced by OS, thereby protecting cells from oxidative damage. Through the analysis of the composition of ITPLE, we found that it contains several antioxidant compounds, whose mutual synergistic effects have a cytoprotective effect. Therefore, it can be concluded that ITPLE showed excellent inhibitory effects against oxidative damage in HEK 293T cells. However, this study had certain limitations, for instance, this study was carried out in an in vitro cell model of oxidative injury, and further animal experiments are needed in the future. Additionally, the synergistic mechanism between the active components of ITPLE needs further study.
Availability of data and materials
Datasets used and/or analysed during the current study that are not included in the manuscript are available from the corresponding author on reasonable request.
References
Zhao X, Song JL, Yi R, Li G, Sun P, Park KY, Suo H (2018) Comparison of antioxidative effects of Insect tea and its raw tea (Kuding tea) polyphenols in Kunming mice. Molecules 23:204
Liu JF, Yang MF, Shang XL, Hu JF, Wang CC (2013) Morphological characters of three main kinds of insect tea in Hunan-Guizhou area. J Mount Agri Biol 32:407–410
Wang Z, Meng TB, Mei SM, Xiao CY (2006) The biological characteristics of Malus sieboldii (Regel) Rehd-Kudingcha and its exploitation. Guiding J TCM 12:90–91
Wen LZ, Shen ZR, Zang XB, Hu YX (2004) Toxicological assessment on safety of Chinese Sanye Chongcha. J Chinese Inst Food Sci Technol 4:83–87
Baud L, Ardaillou R (1986) Reactive oxygen species: production and role in the kidney. Am J Physiol 251:765–776
Ratliff BB, Abdulmahdi W, Pawar R, Wolin MS (2016) Oxidant mechanisms in renal injury and disease. Antioxid Redox Signal 25:119–146
Quiñonez-Flores CM, González-Chávez SA, Del Río Nájera D, Pacheco-Tena C (2016) Oxidative stress relevance in the pathogenesis of the rheumatoid arthritis: a systematic review. Biomed Re Int 2016:6097417
Rowen RJ (2019) Ozone and oxidation therapies as a solution to the emerging crisis in infectious disease management: a review of current knowledge and experience. Med Gas Res 9:232–237
Sun C, Jin W, Shi H (2017) Oligomeric proanthocyanidins protects A549 cells against H2O2-induced oxidative stress via the Nrf2-ARE pathway. Int J Mol Med 39:1548–1554
Yi R, Tan F, Liao W, Wang Q, Mu J, Zhou X, Yang Z, Zhao X (2019) Isolation and identification of Lactobacillus plantarum HFY05 from natural fermented yak yogurt and its effect on alcoholic liver injury in mice. Microorganisms 7:530
Aviram M (2000) Review of human studies on oxidative damage and antioxidant protection related to cardiovascular diseases. Free Radic Res 33:85–97
Cong X, Zhang Q, Li H, Jiang Z, Cao R, Gao S, Tian W (2017) Puerarin ameliorates heat stress-induced oxidative damage and apoptosis in bovine Sertoli cells by suppressing ROS production and upregulating Hsp72 expression. Theriogenology 88:215–227
Luna C, Estévez M (2018) Oxidative damage to food and human serum proteins: radical-mediated oxidation vs. glyco-oxidation. Food Chem 267:111–118
Ghobadi S, Dastan D, Soleimani M, Nili-Ahmadabadi A (2019) Hepatoprotective potential and antioxidant activity of Allium tripedale in acetaminophen-induced oxidative damage. Res Pharm Sci 14:488–495
Zhu K, Zeng X, Tan F, Li W, Li C, Song Y, Zhao X (2019) Effect of Insect tea on D-galactose-induced oxidation in mice and its mechanisms. Food Sci Nutr 7:4105–4115
Marnett L (1999) Lipid peroxidation-DNA damage by malondialdehyde. Mutat Res 424:83–95
Ni CX, Gong H, Liu Y, Qi Y, Jiang CL, Zhang JP (2017) Green tea consumption and the risk of liver cancer: a meta-analysis. Nutr Cancer 69:211–220
Bowler C (1982) Superoxide dismutase and stress tolerance. Annu Rev Plant Biol 43:83–116
Poprac P, Jomova K, Simunkova M, Kollar V, Rhodes CJ, Valko M (2017) Targeting free radicals in oxidative stress-related human diseases. Trends Pharmacol Sci 38:592–607
Glorieux C, Zamocky M, Sandoval JM, Verrax J, Calderon PB (2015) Regulation of catalase expression in healthy and cancerous cells. Free Radic Biol Med 87:84–97
Wu G, Fang YZ, Yang S, Lupton JR, Turner ND (2004) Glutathione metabolism and its implications for health. J Nutr 134:489–492
Lubos E, Loscalzo J, Handy DE (2011) Glutathione peroxidase-1 in health and disease: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal 15:1957–1997
Glorieux C, Calderon PB (2017) Catalase, a remarkable enzyme: targeting the oldest antioxidant enzyme to find a new cancer treatment approach. Biol Chem 398:1095–1108
Wilke BC, Vidailhet M, Favier A, Guillemin C, Ducros V, Arnaud J, Richard MJ (1992) Selenium, glutathione peroxidase (GSH-Px) and lipid peroxidation products before and after selenium supplementation. Clin Chim Acta 207:137–142
Zhou Y, Tan F, Li C, Li W, Liao W, Li Q, Qin G, Liu W, Zhao X (2019) White Peony (fermented Camellia sinensis) polyphenols help prevent alcoholic liver injury via antioxidation. Antioxidants 8:524
Xu XK, Chen ZY, Liao LP, Zhang ZJ, Wang ZT (2015) Determination of scopolin, chlorogenic acid, scopoletin, isochlorogenic acid A, isochlorogenic acid B and isochlorogenic acid C in plants of Erycibe. Zhongguo Zhong Yao Za Zhi 40:1119–1122
Wang XM, Xi Y, Fan XH, Cao JK, Jiang WB (2019) Research progress on bioavailability and antioxidant activity of chlorogenic acid. J Chin Inst Food Sci Technol 19:271–279
Zhang XH, Wang YF (2007) A comparative study on bioactivities of tea polyphenols and chlorogenic acid. J Tea Sci 27:39–44
Thurow T, Lee S (2012) Effect of chlorogenic acid and neochlorogenic acid on human colon cancer cells. Dis Stud J Dale Bump Coll Agri Food Life Sci 13:86–93
Liu X, Huang K, Niu Z, Mei D, Zhang B (2019) Protective effect of isochlorogenic acid B on liver fibrosis in non-alcoholic steatohepatitis of mice. Basic Clin Pharmacol Toxicol 124:144–153
Wang J, Wang H, Peng Y, Wang GJ, Hao HP (2016) Isochlorogenic acid A affects P450 and UGT enzymes in vitro and in vivo. Chin J Nat Med 14:865–870
Hu JW, Wu L, Tu ZX, Xie X, Huang BH (2019) Extraction and antioxidant activity of chlorogenic acids and isochlorogenic acids from Gynura procumbens (Lour.) Merr. Nat Prod Res Dev 31:38–43
Sasikala V, Rooban BN, Sahasranamam V, Abraham A (2013) Rutin ameliorates free radical mediated cataract by enhancing the chaperone activity of α-crystallin. Graefes Arch Clin Exp Ophthalmol 251:1747–1755
Chaipech S, Morikawa T, Ninomiya K, Yoshikawa M, Pongpiriyadacha Y, Hayakawa T, Muraoka O (2012) Structures of two new phenolic glycosides, kaempferiaosides A and B, and hepatoprotective constituents from the rhizomes of Kaempferia parviflora. Chem Pharm Bull (Tokyo) 60:62–69
Chen JW, Zhu ZQ, Hang K, Yang XN (2002) Relationship between structure and activity of eight natural flavonoids against oxidation. J East China Nor Univ (Nat Sci) 2002:90–95
Marunaka Y, Marunaka R, Sun H, Yamamoto T, Kanamura N, Inui T, Taruno A (2017) Actions of quercetin, a polyphenol, on blood pressure. Molecules 22:209
Funding
This research was supported by the Science and Technology Research Program of Chongqing Municipal Education Commission of China (KJQN201804504) and the Scientific Research Foundation for Returned Overseas Chinese Scholars, and the State Education Ministry [Jiaowaisiliu (2014)1685], China.
Author information
Authors and Affiliations
Contributions
JZ and HW performed the majority of the experiments and wrote the manuscript; SY, ZG, YH and WL contributed to the data analysis; HL and XZ designed and supervised the study, and checked the final manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
All authors declare that there is no competing of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, J., Wang, H., Yi, S. et al. Protective effect of Insect tea primary leaf (Malus sieboldii (Regal) Rehd.) extract on H2O2-induced oxidative damage in human embryonic kidney 293T cells. Appl Biol Chem 63, 32 (2020). https://doi.org/10.1186/s13765-020-00516-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13765-020-00516-y