Abstract
In this study, we used RNA sequencing (RNA-seq) to analyze and compare bulk cell samples from wild-type (WT) dermal mesenchymal stem cells (DMSCs) (n = 3) and Prx II knockout DMSCs (n = 3). The purpose of the study was to elucidate the role of Prx II on allogeneic immune rejection of transplanted DMSCs. The results revealed differential expression of 472 genes (176 up-regulated and 296 down-regulated; p ≤ 0.05) between the PrxII+/+ (WT) and PrxII−/− sample groups. When highly regulated genes were categorized according to the Gene Ontology (GO) molecular function classification and the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis, the PrxII−/− samples showed a robust downward trend in allograft rejection. The study identified 43 all immunologically rejected differentially expressed genes, of which 41 showed lower expression in the PrxII−/− vs. PrxII+/+ (WT) samples. These findings suggest that Prx II gene knockout may down-regulate the allograft rejection that occurs during DMSCs transplantation and improve the survival rate of DMSCs in the host. This study provides a new perspective on the clinical treatment of stem cell transplantation.
Similar content being viewed by others
Introduction
Mesenchymal stem cells (MSCs) are pluripotent stem cells that exist in many tissues and can differentiate into several cell types [1, 2]. Therefore, they are widely used in transplantation and tissue regeneration. Dermal mesenchymal stem cells (DMSCs) are derived from skin and offer several advantages such as abundant source, easy collection, and low infection rate with pathogenic microorganisms, low immunogenicity, and secretion of a large number of cytokines. Consequently, DMSCs have broad application prospects in the clinical treatment of many diseases and the potential to regenerate organs and prolong life.
A large number of preclinical animal experiments have shown that MSCs can be implanted to treat myocardial infarction [3], spinal cord injury [4], diabetic foot [5] and other diseases. However, transplantation rejection is still an inevitable problem in the practical application of allogeneic mesenchymal stem cells and their derivatives. The major cause of immune rejection is the activation of T lymphocytes by the major histocompatibility complex (MHC), which results in a specific immune response [6, 7] with serious side effects that can render the treatment ineffective. Strategies to reduce organ rejection and improve the survival rate after transplantation will be crucial in the development of transplantation medicine.
The immune response includes innate immunity and acquired immunity, with the latter divided into humoral and cellular immune responses. The main contributor to immune rejection is the cellular immunity. The main function of the MHC is to activate T lymphocytes to produce specific immune responses. Cell surface antigens encoded by complex gene groups play a leading role in immune rejection [8], and MHC molecules are found on the cell surface of all vertebrates. Because T cell receptors recognize only a small fraction of antigenic molecule determinants, the T cell immune response requires the processing and presentation of antigens by antigen-presenting cells. There are two pathways involved in the process [9, 10]: the MHC-I and the MHC-II class pathways. In the MHC-I pathway, endogenous antigens in the cytoplasm form complexes with MHC-I molecules, which are transported to the cell surface for recognition by CD8+ T cells. In the MHC-II pathway, exogenous antigens form complexes with MHC-II molecules via the endoplasmic lysosomal pathway, which are then transported to the cell surface for recognition by CD4+ T cells. In addition to the cellular immune response, the humoral immune response and various regulatory factors also play an important role in the immune response.
Chemokines are a large cytokine superfamily composed of small secreted proteins. In recent years, chemokines have been found to play an important role in transplantation rejection, studies have shown that the role of chemokines in transplantation rejection is mainly related to the recruitment of T-lymphocytes [11]. It has been shown that the expression of CXCL9/MIG in allografted skin leads to complete rejection within a few days and that neutralization of CXCL9/MIG could inhibit the infiltration of T cells and delay rejection [12, 13]. Similarly, cardiac allograft rejection has a time pattern of chemokine expression. Both CCI3 and CCIA are expressed in the late phase of transplantation rejection, whereas CXCL10 and CXCL9 are highly expressed in the early and late phases of allograft rejection [14]. These patterns are also seen in human heart transplantation, indicating that chemokines do indeed play a key role in allograft rejection.
RNA sequencing (RNA-seq) using next-generation sequencing (NGS) platforms greatly improves the analysis of whole transcriptomes, allowing full annotation and quantification of a large number of genes in one run. This technique can detect known and non-characteristic transcripts and provide information about alternative and new splicing events [15]. Using the RNA-Seq technology, we analyzed the whole transcriptome of PrxII+/+ and PrxII−/− DMSCs samples. We found that a group of cytokines and chemokines was involved in the immune rejection process of both samples. Compared to the WT, the expression of most of these cytokines and chemokines was down-regulated in the Homo samples. These results suggest that reducing Prx II expression may be an effective way to reduce DMSCs rejection.
Materials and methods
Chemicals
Dulbecco’s Modified Eagle’s Medium (DMEM) and Dulbecco’s Modified Eagle Medium: Nutrient Mixture F-12 (DMEM/F-12) were purchased from Gibco (Gibco, CA, USA), fetal bovine serum (FBS) were purchased from Hyclone (General Electric Healthcare Life Sciences, Mississauga, Canada), penicillin streptomycin (P/S) was purchased from solarbio (Solarbio life sciences, Beijing, China), TRIzol was purchased from Sigma (Sigma, St. Louis, MO, USA).
Quality control chart
Clean reads were obtained after removal of the adaptor, the low-quality reads, and the ribosomal sequences (Fig. 1). If the probability of a read’s error at a certain position is p = 0.01, the y-coordinate value on the graph corresponds to − 10 * log 10 (p) = 20, red represents the median, yellow represents the 25–75% range, tentacle is the 10–90% range, and blue line is the average.
Cell culture
DMSCs were extracted from the skin of 129/SvJ mice. Cell cultures were maintained in DMEM/F12 medium supplemented with 10% (v/v) FBS and antibiotics (100 U/ml of penicillin and 100 µg/ml of streptomycin), and incubated at 37 °C and 5% CO2. Cells were sub-cultured once every 2 days.
Sample preparation
The six samples (WT 1-3 and Homo 1-3) were lysed from 129/SvJ mice using TRIzol Reagent according to the manufacturer’s manual (Invitrogen, Carlsbad, CA), recycled to independent 1.5 ml tubes and sent to the Gminix company for total RNA extraction and RNA sequencing.
RNA sequencing
The resulting RNA was randomly broken into fragments and reverse transcribed using random primers containing Poly (T) oligonucleotides and reverse transcriptase. We used Poly (T) oligonucleotides for reverse transcription PCR to extract all mature eukaryotic mRNAs, which contain Poly (A) tails as a post-transcriptional modification. Then, the ends of the resulting cDNA fragments were repaired and the adapter was connected to obtain the cDNA used for sequencing. Lastly, a new generation of high-throughput sequencing was used.
Identification of differentially expressed genes
To study the differences between the two sample groups (PrxII+/+ and PrxII−/−), gene expression in both groups was compared using DESeq2. In this analysis, a significant p-value and a fold-change (FC) between genes were obtained. The screening conditions were p < 0.05 and FC cut-offs of > 1.2 and < 0.83333, and yielded the identification of 472 differentially expressed genes between the two sample groups.
Reconstruction of co-expression network
The pathway analysis used was based on the KEGG database. Fisher exact test and Chi square test were used for differentially expressed genes. Then significance analysis is carried out for the pathway participated by the target gene, and the pathway with significance is screened following to p < 0.05. According to the significance level (p-value) of each differential gene pathway and the number of differential genes contained in the down-regulated significant differential gene pathway, a bubble chart was plotted for the top gene pathways that were significantly down-regulated (p < 0.05). For the construction of a co-expression network, the genes from the bubble chart were analyzed using ‘ClueGo’ from the Cytoscape v3.2 [16].
Results
Sample correlation analysis and principal component analysis
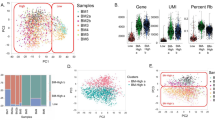
To determine whether the gene expression profiles presented different correlations between the two sample groups, we analyzed the RNA-seq data of the WT and Homo samples using the Pearson correlation coefficient (Fig. 2a). The results showed that both sample groups were very similar. Similar gene expression was also supported by the principal-component analysis (Fig. 2b).
The general results of the RNAseq results. a Analysis of the degree of correlation between the PrxII+/+ and PrxII−/− samples using the Pearson correlation coefficient. When R2 > 0.92, the two samples were considered correlated. b Principal-component analysis (PCA) performed in the transcriptome of the two different sample groups. The different color circles indicate the two different samples
Gene expression profiling of samples by RNA sequencing analysis
In this study, I analyzed the differentially expressed (DE) genes between the WT and Homo sample groups. Using p < 0.05 and FC > 1.2 or < 0.83333 as the screening conditions, we identified 472 DE genes (176 up-regulated and 296 down-regulated) between the WT and Homo sample groups (Fig. 3a). All DE genes were plotted in a heat map (Fig. 3b).
Differentially expressed genes among the PrxII+/+ and PrxII−/− samples. a Volcano plot of differentially expressed (DE) genes in WT and Homo samples. The log2 fold change difference between samples and the negative log of the p-value are represented on the x- and y-axis, respectively. Each point represents one single gene having detectable expression in both samples. Down-regulated and up-regulated genes in the Homo samples compared to the WT samples are plotted in blue and red, respectively, and non-significant genes are shown as gray points. b Clusters of genes showing representative expression patterns. All of the DE genes between any two samples were selected. Sample names within the 2 groups and the DE genes are represented on the x- and y-axis, respectively. High and low expression values of DE genes in group samples are represented by red and green colors, respectively
Functional enrichment analysis of highly regulated genes
To investigate the biological role of the DE genes at the transcriptional level, we categorized the 472 DE genes into enriched categories according to the GO molecular function classification and the KEGG pathway analysis.
There were 20 top GO categories that were significantly enriched with a p < 0.05, which included the following: TAP binding; peptide antigen binding; T cell receptor binding; antigen binding. The complete list of genes included in each category is shown in Table 1. The results show that most of the DE genes were related to immune function. We found both up-regulated and down-regulated genes within the GO enriched categories (Table 1). In this respect, we found that many genes encoding potentially secreted proteins are down-regulated in the Homo sample, whereas the Cx3cl1 and Cxcr6 genes were up-regulated.
To evaluate the enrichment in signaling transduction pathways, we analyzed the 472 genes with the KEGG pathway analysis. In addition, the top 24 gene pathways that were significantly (p < 0.05) down-regulated were plotted in a bubble chart (Fig. 4, Table 2). These results suggest that the most enriched pathways were related to antigen processing and presentation, which are known to be associated with allograft rejection.
Functional enrichment analysis of the down-regulated DE genes. a Bubble chart of KEGG pathway enrichment analyses for down-regulated DE genes. Different bubble color represents the negative log of the p-value. Red indicates highly statistically significant. (p < 0.05) Bubble size indicates gene numbers enriched in a pathway
Network reconstruction using down-regulated genes in the bubble chart
In the first analysis, the WT samples were compared to the Homo samples. This analysis found five pathways associated with allograft rejection that consisted of down-regulated genes in the bubble chart (Table 3). These DE genes were used to reconstruct the co-expression network of high co-expression genes. We used the ClueGo in the Cytoscape (v3.2.0) to analyze the relationship between the pathways and the DE genes, using a parameter of p < 0.05. The results show that the expression of allograft rejection-associated genes (41/43) in the Homo samples was lower than in the WT samples (Fig. 5).
Cytoscape analysis on the DE gene datasets. Graphic depiction of a bubble chart (allograft rejection) using the function of ClueGo in Cytoscape. The colors in the network show the DE genes. Red shows down-regulated genes in the Homo samples compared to the WT samples. Green shows up-regulated genes in the Homo samples compared to the WT samples
Discussion
MSCs are pluripotent cells that exist in a variety of tissues (bone marrow, cord blood and cord tissue, placental tissue, etc.) [17]. MSCs have the potential for multi-directional differentiation, and a unique cytokine secretion function. These properties make them ideal seed cells for gene and cell therapy. They have a broad application prospect in the treatment of nervous system injury [18], premature ovarian failure [19], bone defect [20] and other diseases. It is believed that MSCs used in the treatment of several diseases can replace and replenish damaged cells through their own proliferation and differentiation [21]. Recent studies have shown that transplanted MSCs have a relatively low survival rate in the damaged area and rarely differentiate into other types of cells, However, the transplanted MSCs were able to repair the site of injury even though it was remote from the actual injury, suggesting that the stem cells could exert their therapeutic effects in a paracrine manner [22, 23]. Regardless of the therapeutic mechanism, the main problem after MSCs transplantation is still the host immune rejection.
Peroxiredoxins (Prxs) are a highly conserved superfamily of peroxidases. Prxs play an important role in gene expression, internal immunity and inflammation-related physiological reactions such as tissue repair, parasite infection and tumor formation [24]. Prx II is a member of the Prx family with a molecular weight of about 22 kDa, which is localized in the cytoplasm and expressed in a variety of human tissues. Prx II can inhibit the replication of HIV in human T cells [25, 26]. Moreover, the Prx II oligomer (300–400 kDa) is an active natural killer (NK) cell enhancer (NKEF-B) [27, 28]. Treatment of Prx II with dithiothreitol inhibited its oligomerization process and eliminated virtually all its NK cell enhancing activity. Furthermore, the sulfhydryl group of Prx II was blocked by n-ethylmaleimide. This activity was completely lost after the formation of dimer and oligomer was inhibited [29]. In summary, these results suggests a potential link between Prx II and the immune system.
Immune rejection is a process by which the body destroys the grafts (allogeneic cells, tissues, or organs) through a specific immune response. In general, after transplantation, the recipient can recognize and respond to the graft antigen, and the immune cells in the graft can also recognize and respond to the recipient antigen tissue. The main function of the MHC is to activate T lymphocytes to produce specific immune responses. Because the T cell receptor (TCR) can only recognize a small part of the determinant of antigen molecules, the immune response process needs the processing and presentation of antigens by antigen-presenting cells (APCs). In addition, T cell recruitment and the complement activation pathway also play important roles in the immune response.
The present study used bulk cell samples for transcriptome sequencing. The results show 472 differentially expressed (DE) genes in the two samples, with down-regulated genes more prominent than up-regulated genes. Therefore, we carried out GO and KEGG analyses on the down-regulated genes. In the down-regulated signaling pathways, we found five signaling pathways related to immune rejection: allograft rejection; antigen processing and presentation; proteasome; the chemokine signaling pathway; and complement and coagulation cascades. In this study, we found that after knockout of Prx II, the expression of Psmb8, Psmb10, Psmb9, Psme1, Tapbp, Tap2, Tap1, B2m, H2-T24, H2-Q7, H2-K1, H2-T23, H2-Q6, H2-D1, H2-T10, and H2-Q1 genes were down-regulated in DMSCs. Consequently, antigen processing, transport and MHC-I molecular assembly, and formation and presentation of peptide-MHC-I complex, were all inhibited. Moreover, the expression of Ccl8, Stat1, Cxcl9, Rasgrp2, Cxcl10, Ccl5, Stat2, and Cxcl11 were down-regulated, rendering the DMSCs unable to recruit T cells and inhibiting T cell infiltration. In addition, we also found that the expression of Serping1, C3, C2, C1s1, C1ra, C1s2, and Cfb were down-regulated, thus inhibiting the complement activation pathway. Based on the above results, we believe that Prx II knockout can reduce the allograft rejection of DMSCs.
It has been shown that DMSCs do not express MHC-II molecules and present low expression of MHC-I molecules [30]. However, after allograft DMSCs were transplanted to recipients, immune rejection still took place, resulting in low survival rate and short survival time [31]. In this study, we found that the DMSCs with Prx II knockout expressed lower MHC-I molecules and weakened T cell-specific immune responses. The specific regulatory mechanism needs further study.
In conclusion, immune rejection is the most difficult problem in the field of transplantation. Finding out how to induce the recipients to develop immune tolerance to the graft would be a turning point in this field. In this study, high-throughput sequencing technology shows that Prx II gene knockout may reduce immune rejection after DMSCs transplantation and prolong the survival time of DMSCs in vivo, providing a theoretical basis for allogeneic DMSC transplantation.
Availability of data and materials
The datasets used and analyzed in this study are available from the corresponding author upon reasonable request.
References
Kobolak J, Dinnyes A, Memic A, Khademhosseini A, Mobasheri A (2016) Mesenchymal stem cells: identification, phenotypic characterization, biological properties and potential for regenerative medicine through biomaterial micro-engineering of their niche. Methods 99:62–68
D’souza N, Rossignoli F, Golinelli G, Grisendi G, Spano C, Candini O, Osturu S, Catani F, Paolucci P, Horwitz EM (2015) Mesenchymal stem/stromal cells as a delivery platform in cell and gene therapies. BMC Med 13(1):186
Lu D-f, Yao Y, Su Z-z, Zeng Z-h, Xing X-w, He Z-y, Zhang C (2014) Downregulation of HDAC1 is involved in the cardiomyocyte differentiation from mesenchymal stem cells in a myocardial microenvironment. PLoS ONE 9(4):e93222
Watanabe S, Uchida K, Nakajima H, Matsuo H, Sugita D, Yoshida A, Honjoh K, Johnson WEB, Baba H (2015) Early transplantation of mesenchymal stem cells after spinal cord injury relieves pain hypersensitivity through suppression of pain-related signaling cascades and reduced inflammatory cell recruitment. Stem Cells 33(6):1902–1914
Kato J, Kamiya H, Himeno T, Shibata T, Kondo M, Okawa T, Fujiya A, Fukami A, Uenishi E, Seino Y (2014) Mesenchymal stem cells ameliorate impaired wound healing through enhancing keratinocyte functions in diabetic foot ulcerations on the plantar skin of rats. J Diabetes Complic 28(5):588–595
Vyas JM, Van der Veen AG, Ploegh HL (2008) The known unknowns of antigen processing and presentation. Nat Rev Immunol 8(8):607
Sivanathan KN, Gronthos S, Rojas-Canales D, Thierry B, Coates PT (2014) Interferon-gamma modification of mesenchymal stem cells: implications of autologous and allogeneic mesenchymal stem cell therapy in allotransplantation. Stem Cell Rev Rep 10(3):351–375
Amadou C, Younger RM, Sims S, Matthews LH, Rogers J, Kumnovics A, Ziegler A, Beck S (2003) Fischer Lindahl K (2003) Co-duplication of olfactory receptor and MHC class I genes in the mouse major histocompatibility complex. Hum Mol Genet 12(22):3025–3040
Vugmeyster Y, Glas R, Perarnau B, Lemonnier FA, Eisen H, Ploegh H (1998) Major histocompatibility complex (MHC) class I KbDb−/− deficient mice possess functional CD8+ T cells and natural killer cells. Proc Natl Acad Sci 95(21):12492–12497
Sayegh MH, Turka LA (1998) The role of T-cell costimulatory activation pathways in transplant rejection. N Engl J Med 338(25):1813–1821
Tan Y, Abdulreda MH, Cruz-Guilloty F, Cutrufello N, Shishido A, Martinez RE, Duffort S, Xia X, Echegaray-Mendez J, Levy RB (2013) Role of T cell recruitment and chemokine-regulated intra-graft T cell motility patterns in corneal allograft rejection. Am J Transplant 13(6):1461–1473
Koga S, Auerbach MB, Engeman TM, Novick AC, Toma H, Fairchild RL (1999) T cell infiltration into class II MHC-disparate allografts and acute rejection is dependent on the IFN-gamma-induced chemokine Mig. J Immunol 163(9):4878–4885
Fahy O, Porte H, Senechal S, Vorng H, McEuen AR, Buckley MG, Walls AF, Wallaert B, Tonnel AB, Tsicopoulos A (2001) Chemokine-induced cutaneous inflammatory cell infiltration in a model of Hu-PBMC-SCID mice grafted with human skin. Am J Pathol 158(3):1053–1063
Horiguchi K, Kitagawa-Sakakida S, Sawa Y (2002) Selective chemokine and receptor gene expressions in allografts that develop transplant vasculopathy. J Heart Lung Transplant 21(10):1090–1100
Wang Z, Gerstein M, Snyder M (2009) RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet 10(1):57
Bindea G, Mlecnik B, Hackl H, Charoentong P, Tosolini M, Kirilovsky A, Fridman W-H, Pagès F, Trajanoski Z, Galon J (2009) ClueGO: a Cytoscape plug-into decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 25(8):1091–1093
Monteiro BS, Neto NMA, Carlo RJD (2016) Mesenchymal stem cell infusion on skin wound healing of dexamethasone immunosuppressed wistar rats. Ciência Rural 40(1):238–245
Nairz M, Sonnweber T, Schroll A, Theurl I, Weiss G (2012) The pleiotropic effects of erythropoietin in infection and inflammation. Microbes Infect 14(3):238–246
Oku R, Oda S, Nakada T-a, Sadahiro T, Nakamura M, Hirayama Y, Abe R, Tateishi Y, Ito M, Iseki T (2013) Differential pattern of cell-surface and soluble TREM-1 between sepsis and SIRS. Cytokine 61(1):112–117
Jirí Z, Michal F (2012) Changes in the serum levels of clusterin in children with sepsis. Pediatr Pol 88(1):6–13
Eggenhofer E, Benseler V, Kroemer A, Popp F, Geissler E, Schlitt H, Baan C, Dahlke M, Hoogduijn MJ (2012) Mesenchymal stem cells are short-lived and do not migrate beyond the lungs after intravenous infusion. Front Immunol 3:297
Dormady SP, Bashayan O, Dougherty R, Zhang X-M (2001) Basch RS (2001) Immortalized multipotential mesenchymal cells and the hematopoietic microenvironment. J Hematother Stem Cell Res 10(1):125–140
Fox JM, Chamberlain G, Ashton BA (2007) Middleton J (2007) Recent advances into the understanding of mesenchymal stem cell trafficking. Br J Haematol 137(6):491–502
Ishii T, Warabi E, Yanagawa T (2012) Novel roles of peroxiredoxins in inflammation, cancer and innate immunity. J Clin Biochem Nutr 50(2):91–105
Geiben-Lynn R, Kursar M, Brown NV, Addo MM, Shau H, Lieberman J, Luster AD, Walker BD (2003) HIV-1 antiviral activity of recombinant natural killer cell enhancing factors, NKEF-A and NKEF-B, members of the peroxiredoxin family. J Biol Chem 278(3):1569–1574
Asmal M, Letvin NL, Geiben-Lynn R (2013) Natural killer cell-dependent and non-dependent anti-viral activity of 2-Cys peroxiredoxin against HIV. Int Trends Immun 1(4):69–77
Shau H, Gupta RK, Golub SH (1993) Identification of a natural killer enhancing factor (NKEF) from human erythroid cells. Cell Immunol 147(1):1–11
Yin G, Li C, Shan B, Wang W, Chen H, Zhong Y, Di J, Lin Q, Lin Y (2011) Insufficient peroxiredoxin-2 expression in uterine NK cells obtained from a murine model of abortion. J Cell Biochem 112(3):773–781
Sauri H, Ashjian PH, Kim AT, Shau H (1996) Recombinant natural killer enhancing factor augments natural killer cytotoxicity. J Leukoc Biol 59(6):925–931
Majumdar MK, Keane-Moore M, Buyaner D, Hardy WB, Moorman MA, McIntosh KR, Mosca JD (2003) Characterization and functionality of cell surface molecules on human mesenchymal stem cells. J Biomed Sci 10(2):228–241
Ding Y, Liang X, Zhang Y (2018) Rap1 deficiency-provoked paracrine dysfunction impairs immunosuppressive potency of mesenchymal stem cells in allograft rejection of heart transplantation. Cell Death Dis 9(3):386
Acknowledgements
This study was supported by grants from the Korean Research Institute of Bioscience and Biotechnology Research Initiative Program (KRIBB) (KGM5162021). This research was supported by the Basic Science Research Program from the National Research Foundation of Korea (NRF) and funded by the Ministry of Education (2018R1A6A3A11051196, 2020R1I1A2052417).
Funding
This work was supported by the Project of Sanzong (ZRCPY201816) and University Nursing Program for Young Scholars with Creative Talents in Heilongjiang Province (CXRC2017016) and the scientific research team support plan of Heilongjiang Bayi Agricultural University (TDJH201904).
Author information
Authors and Affiliations
Contributions
Y-HH, Y-YM and N-NY writing the manuscript, M-HJ, Y-HJ and G-NS, Y-QZ performed the experiments, A-GW, Y-DC, L-YY, Y-JJ, D-SL and H-NS performed the data analysis and funding supplying, Y-HH, JK and TK made substantial contributions to conception and design. All Authors read and approved the final article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Han, YH., Mao, YY., Yu, NN. et al. RNA sequencing reveals that Prx II gene knockout can down-regulate the allograft rejection of dermal mesenchymal stem cells. Appl Biol Chem 63, 30 (2020). https://doi.org/10.1186/s13765-020-00515-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13765-020-00515-z