Abstract
Bagaza virus (BAGV; synonymous to Israel turkey meningoencephalomyelitis virus, ITV) is a relevant arthropod-borne epornitic flavivirus. In its first emergence in Europe (southern Spain, 2010) BAGV caused an outbreak, severely affecting red-legged partridges and common pheasants. The effects (pathogenicity, role as reservoir host) of BAGV in other European phasianids are unknown. To fill this gap, grey partridges were experimentally infected with BAGV. The clinical course of the disease was severe, with neurological signs, significant weight loss and 40% mortality. Low viral loads in the blood and the absence of contact transmission suggest a limited—if any—role on BAGV transmission for this European phasianid.
Similar content being viewed by others
Introduction, methods and results
Bagaza virus (BAGV), synonymous to Israel turkey meningoencephalomyelitis virus (ITV), is a mosquito-borne epornitic virus belonging to the Ntaya serocomplex within the Flavivirus genus of the family Flaviviridae. It causes a severe neurological disease in poultry and wild bird species [1, 2]. The first description of ITV was reported in 1959 from an outbreak in turkeys in Israel [3]. Seven years later, BAGV was isolated from a mosquito pool in the locality of Bagaza (Central African Republic). Subsequently, full genome sequence comparisons confirmed that both viruses are the same species [1, 4] and a new name for these viruses, “Avian meningoencephalomyelitis virus” (Acronym: AMEV), was proposed. Until recently, BAGV/ITV was restricted to sub-Saharan Africa (Central African Republic, Mauritania, Senegal and South Africa), Israel and India [5,6,7,8], but in 2010 it emerged for the first time in Europe causing an outbreak that affected red-legged partridges (Alectoris rufa) and common pheasants (Phasianus colchicus) in southern Spain [9]. Specific neutralizing antibodies were detected in the same area one year later in juvenile red-legged partridges, suggesting virus overwintering [10].
The emergence of BAGV in Spain in 2010 caused high mortality rates, in particular in red-legged partridges, demonstrating high virulence and spread ability in game birds and increasing the perception of risk associated with its introduction in Europe [9]. A study in game birds naturally infected in this outbreak showed differences in pathogenicity for red-legged partridges, ring-necked pheasants and common wood pigeons [11]. In addition, experimentally infected red-legged partridges showed high susceptibility to BAGV infection and the disease was spread by direct contact [12].
The grey partridge (Perdix perdix) is one of most widespread and popular game birds in Europe and western Asia. In Europe, in the last half-century the species has suffered an important demographic decline mainly due to the loss and degradation of breeding habitats and the related implications on demographic traits [13,14,15,16]. In many countries this species is bred in captivity and released into the wild for hunting and restocking purposes [14, 15, 17].
The grey partridge has an important ecological and economic value as a game bird in Europe. For this reason, the conservation concerns cited above and the pathogenic effects previously observed in BAGV-infected red-legged partridges, we studied to what extent BAGV might affect the grey partridge in terms of mortality and morbidity, as well as to define its potential role as a natural reservoir.
Thirty-six five-month-old grey partridges (Perdix perdix) were obtained from the Gibovendee breeding facility (Chambretaud, France). Previous exposure to WNV, USUV and BAGV was determined by analyzing specific antibodies by VNT and viral RNA in feathers using real-time RT-PCR [18, 19]. The grey partridges were distributed in homogenous experimental groups composed of 50% males and 50% females and housed in three separate cages in our BSL-3 animal facilities. An “inoculated group” (n = 10) and a “necropsy group” (n = 12), housed in two cages, received a subcutaneous inoculation in the neck of approximately 2 × 105 pfu/individual of BAGV (strain Spain RLP Hcc2/2010, GenBank: KR108245) diluted in PBS with 0.2% BSA, as previously described [12]. A third group, “in-contact group”, composed of 4 non-inoculated grey partridges, was kept together with the “inoculated group”. Finally, a “control group” (n = 10), housed in a third cage, was sham-inoculated with an equivalent volume of PBS with 0.2% BSA and handled as the infected group. All cages were placed in the same room.
After infection, the birds were observed daily to monitor clinical condition and weight for up to 18 dpi. In the inoculated and control groups, sampling of blood, immature rump feathers and oropharyngeal and cloacal swabs was carried out at 1, 3, 5, 7, 9, 11 and 15 dpi while at 18 dpi only feathers and blood were collected. As for the in-contact group, the same samples were collected at 5, 7, 9, 11, 15 and 18 dpi, following the previously described procedure [12]. Birds in the necropsy group were humanely euthanized by intravenous injection of embutramide (T61 ®, Intervet—Schering-Plough, Madrid, Spain) at 3, 4, 7 and 11 dpi (3 birds each day). Similarly, severely affected birds, showing irreversible clinical signs were euthanized in the same way for bioethical reasons. At the end of the experiment (18 dpi), survivors from the inoculated and contact groups were also euthanized as well as the control group. All birds were subjected to complete post-mortem examination and tissue samples from the brain, heart, kidney, spleen and liver were collected to evaluate viral tissue tropism by real-time RT-PCR. Samples yielding Ct values > 40 were considered negative.
The analysis of viral RNA loads in the blood, swabs, feathers and organs, and of specific antibodies in the serum, was performed as previously described [12]. Also, BAGV viremia levels were measured by a standard plaque formation assay [20]. Differences in survival curves of infected and control groups were calculated by the Kaplan–Meier method and analyzed by a log-rank test using SPSS15. Differences between groups in body weight along the experiment were analyzed using a general linear mixed model. Treatment (control or infected) and days post-infection (dpi) were entered as fixed factors whereas individual identity was included as a random term in the model. This analysis was run in R 3.4.1 (R Core Team 2017).
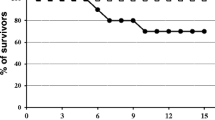
Most inoculated partridges developed a progressive neurological disorder showing weakness, apathy, unresponsiveness, ruffled feathers, ataxia and prostration. Some of them suffered severe neurological symptoms like partial or complete paralysis as well as ocular lesions. Four of them, showing irreversible clinical signs, were euthanized for bioethical reasons, yielding a mortality rate of 40%. These lethal cases occurred at 8 dpi (n = 2), 9 dpi (n = 1) and 15 dpi (n = 1) (Figure 1). The differences in survival curves between control and infected groups were statistically significant (p = 0.029).
BAGV infection strongly affected the body weight of the infected group as compared to the control group throughout the experiment (time × treatment interaction: F(1,165.9) = 117.8, p < 0.001). The weight loss started at 7 dpi and reached a maximum level at 15 dpi (13% loss of initial weight). At 18 dpi a slight recovery of body weight was observed in the survivors of the infected group but the average weight was still much lower than that of the control group (Figure 2).
Viral RNA in the blood was detectable in the inoculated individuals from day 1 to day 7 post-infection (at 9 dpi it was detected only in one bird very close to detection limits). Peak viral RNA load in the blood was observed in all infected birds at 3 dpi (Figure 3A). Viremia, as measured by standard plaque-formation, was only detected at 3 dpi with a mean titer of 2.29 × 104 pfu/mL. Infected partridges developed specific NtAb whereas there was no serocoversion of either in-contact or control birds. First NtAb were detected at 5 dpi and by day 7 post-infection all infected partridges had developed high NtAb titers (ranging from 1:160 to 1:640) that persisted until the end of the experiment (Figure 3B). Viral shedding through the oropharyngeal and cloacal routes reached a peak at 5 dpi. However, the viral RNA load was low in oropharyngeal and cloacal swabs throughout the experiment (Figure 3A). In contrast, the viral RNA load in immature feathers was present at much higher concentrations and could be detected up to 15 dpi (Figure 3A).
Viral RNA load and immune response in BAGV-inoculated grey partridges. A Viral genome load in blood, swabs (oropharyngeal, cloacal) and immature feathers. The course of the viral RNA load in each type of sample, measured by real-time RT-PCR, is represented at different dpi. The discontinuous line indicates viral RNA load in oral swabs; the dotted line the viral RNA load in cloacal swabs; the double line and open circles the viral RNA load in feathers; the continuous line the viral RNA load in blood. Error bars represent the standard deviation of the data. B Antibody response to BAGV in serum. Mean log titres of BAGV-NtAb (detected by VNT) measured on different dpi. Error bars represent the standard deviation of the data.
BAGV inoculation in grey partridges led to a systemic infection since the viral RNA was detected in all organs analyzed at different times post-infection (Table 1). The highest viral RNA loads were found in the heart, kidney, spleen and liver at 4 dpi, only 1 day after the viremia peak. However, the highest viral RNA load in the brain was observed later, at 7 dpi. At 11 dpi, the viral RNA load in all the organs was much lower than that found in birds euthanized at 3, 4 and 7 dpi. Those partridges that were euthanized for bioethical reasons (due to severe neurological symptoms) at 8–9 dpi showed very high viral RNA loads specifically in the heart, kidney and brain, while the partridge euthanized at 15 dpi had less viral RNA load. By the end of the experiment (at 18 dpi), the viral RNA load had decreased significantly in all the organs (Table 1).
In-contact and control groups remained healthy during the experiment. Remarkably, no evidence of BAGV infection (and hence of direct contact transmission) was observed in the in-contact group, based not only on the absence of clinical signs in this group, but also on the lack of virus in blood, feathers, swabs and organs and on the absence of seroconversion.
Discussion
This study demonstrates that the grey partridge is susceptible to BAGV infection showing severe neurological symptoms, including partial and complete paralysis. In contrast, BAGV infection in red-legged partridges does not cause severe neurological disease, but apathy and weakness probably related to the intense weight loss suffered (up to 23% of initial weight) [12]. In grey partridges, the infection also caused 100% morbidity but the weight loss was less severe (13% of initial weight). The mortality rates were similar in both species (30% for red-legged partridges and 40% for grey partridges).
Viral RNA loads in blood, feathers and swabs were lower and less persistent in grey partridges as compared to red-legged partridges. However, the viral RNA load in feathers in both species was high and persistent, indicating the usefulness of feather sampling in active surveillance programs for early detection of BAGV infection. A lower viremia in grey partridges, as compared to red-legged partridges, suggests a lower competence for transmission to mosquitoes, and hence the low host capacity for the grey partridge with regard to BAGV transmission.
One of the main differences between both species was the capacity to transmit the virus by direct contact. While non-vectored transmission has been demonstrated in red-legged partridges, this was not the case for grey partridges. Similarly, contact transmission was also unsuccessful in experimental infection attempts in turkeys [21, 22]. Possibly, the much higher viral RNA loads detected in swabs and feathers in red-legged partridges could explain their capacity to transmit the virus through close contact (by feather picking or water/food contamination).
The highest viral RNA loads in organs were detected in the heart, spleen and kidney in the early days of infection (3–6 dpi). However, the maximum viral RNA load in the brain was found a bit later, at 7 dpi. Between 7 and 9 dpi, viral RNA loads in the kidney and heart remained high, similar to those observed in the brain. The lowest viral RNA loads were consistently found in the liver, as previously observed in turkeys [22]. The consistent humoral immune response developed from day 5 post infection probably contributed to the progressive viral clearance in blood, swabs and organs of survivors.
In summary, the present study proves the high susceptibility of grey partridges to BAGV infection, suffering 100% morbidity and 40% mortality. Moreover, in natural conditions, an outbreak of BAGV could have a greater impact on this species since the clinical effects and the poor body condition caused by the virus could increase the mortality rate due to difficulties with feeding and/or escaping from predators. However, the viremia and viral shedding data indicate that the role of grey partridges in the epidemiology of BAGV is limited due to its low or no competence for mosquito-borne and direct contact transmission of the virus in our experimental conditions. Further studies on the potential interactions between the grey partridge and other flaviviruses that already circulate in Europe might contribute to the conservation efforts on this game species.
Abbreviations
- BAGV:
-
Bagaza virus
- ITV:
-
Israel turkey meningoencephalomyelitis virus
- WNV:
-
West Nile virus
- USUV:
-
Usutu virus
- VNT:
-
virus neutralization test
- RT-PCR:
-
reverse transcription polymerase chain reaction
- BSL-3:
-
biosafety level 3
- pfu:
-
plaque-forming unit
- PBS:
-
phosphate buffered saline
- BSA:
-
bovine serum albumin
- dpi:
-
days post-inoculation
- Ct:
-
threshold cycle value
- NtAb:
-
neutralizing antibodies
References
Fernández-Pinero J, Davidson I, Elizalde M, Perk S, Khinich Y, Jiménez-Clavero MA (2014) Bagaza virus and Israel turkey meningoencephalomyelitis virus are a single virus species. J Gen Virol 95:883–887
Ianconescu M (1976) Turkey meningo-encephalitis: a general review. Avian Dis 20:135–138
Komarov A, Kalmar E (1960) A hitherto undescribed disease-Turkey meningo encephalitis. Vet Rec 72:257–261
Kuno G, Chang GJ, Tsuchiya KR, Karabatsos N, Cropp CB (1998) Phylogeny of the genus Flavivirus. J Virol 72:73–83
Barnard BJ, Buys SB, Du Preez JH, Greyling SP, Venter HJ (1980) Turkey meningo-encephalitis in South Africa. Onderstepoort J Vet Res 47:89–94
Bondre VP, Sapkal GN, Yergolkar PN, Fulmali PV, Sankararaman V, Ayachit VM, Mishra AC, Gore MM (2009) Genetic characterization of Bagaza virus (BAGV) isolated in India and evidence of anti-BAGV antibodies in sera collected from encephalitis patients. J Gen Virol 90:2644–2649
Diallo M, Nabeth P, Ba K, Sall AA, Ba Y, Mondo M, Girault L, Abdalahi MO, Mathiot C (2005) Mosquito vectors of the 1998–1999 outbreak of Rift Valley Fever and other arboviruses (Bagaza, Sanar, Wesselsbron and West Nile) in Mauritania and Senegal. Med Vet Entomol 19:119–126
Digoutte J (1978) Bagaza (BAG) strain: Dak Ar B 209. Am J Trop Med Hyg 27:376–377
Agüero M, Fernández-Pinero J, Buitrago D, Sánchez A, Elizalde M, San Miguel E, Villalba R, Llorente F, Jiménez-Clavero MA (2011) Bagaza virus in partridges and pheasants, Spain, 2010. Emerg Infect Dis 17:1498–1501
Llorente F, Pérez-Ramírez E, Fernández-Pinero J, Soriguer R, Figuerola J, Jiménez-Clavero MA (2013) Flaviviruses in game birds, southern Spain, 2011–2012. Emerg Infect Dis 19:1023–1025
Gamino V, Gutiérrez-Guzman AV, Fernández-de-Mera IG, Ortiz JA, Duran-Martín M, de la Fuente J, Gortázar C, Höfle U (2012) Natural Bagaza virus infection in game birds in southern Spain. Vet Res 43:65
Llorente F, Pérez-Ramírez E, Fernández-Pinero J, Elizalde M, Figuerola J, Soriguer RC, Jiménez-Clavero MA (2015) Bagaza virus is pathogenic and transmitted by direct contact in experimentally infected partridges, but is not infectious in house sparrows and adult mice. Vet Res 46:93
Sotherton NW, Aebischer NJ, Ewald JA (2013) Research into action: grey partridge conservation as a case study. J Appl Ecol 51:1–5
Kuijper DPJ, Oosterveld E, Wymenga E (2009) Decline and potential recovery of the European grey partridge (Perdix perdix) population—a review. Eur J Wildlife Res 55:455–463
Aebischer NJ, Ewald JA (2004) Managing the UK grey partridge Perdix perdix recovery: population change, reproduction, habitat and shooting. Ibis 146:181–191
Gée A, Sarasa M, Pays O (2018) Long-term variation of demographic parameters in four small game species in Europe: opportunities and limits to test for a global pattern. Anim Biodiv Conserv 41.1:33–60
Buner FD, Browne SJ, Aebischer NJ (2011) Experimental assessment of release methods for the re-establishment of a red-listed galliform, the grey partridge (Perdix perdix). Biol Conserv 144:593–601
Buitrago D, Rocha A, Tena-Tomás C, Vigo M, Agüero M, Jiménez-Clavero MA (2012) Real-time fluorogenic reverse transcription polymerase chain reaction assay for the specific detection of Bagaza virus. J Vet Diagn Invest 24:959–963
Del Amo J, Sotelo E, Fernández-Pinero J, Gallardo C, Llorente F, Agüero M, Jiménez-Clavero MA (2013) A novel quantitative multiplex real-time RT-PCR for the simultaneous detection and differentiation of West Nile virus lineages 1 and 2, and of Usutu virus. J Virol Methods 189:321–327
Payne AF, Binduga-Gajewska I, Kauffman EB, Kramer LD (2006) Quantitation of flaviviruses by fluorescent focus assay. J Virol Methods 134:183–189
Ianconescu M, Aharonovici A, Samberg Y, Hornstein K, Merdinger M (1973) Turkey meningo-encephalitis: pathogenic and immunological aspects of the infection. Avian Pathol 2:251–262
Villalba R (2016) Experimental infection in turkeys with Bagaza virus. Proceedings of 10th annual meeting EPIZONE “Going Viral” Madrid, Spain
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
MAJC conceived this project, designed and supervised the animal experiment, and helped to draft the manuscript. CCG designed and performed the animal experiment, analyzed and interpreted the data and drafted the manuscript. EPR and FL designed and performed the animal experiment, analyzed and interpreted the data and revised the manuscript. RS and MS participated in the design and interpretation, and reviewed the manuscript. All authors read and approved the final manuscript.
Acknowledgements
The authors wish to thank María del Carmen Barbero Ameller for excellent technical support in the sample processing and molecular analysis, to Dr. Lorenzo Pérez-Rodríguez for statistical advice and to Denis Bourasseau (Gibovendée) who donated the grey partridges.
Ethics approval and consent to participate
This study and associated procedures were supervised and approved by the Ethics and Animal Welfare Committees of the Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria (INIA) in agreement with the rules in place in the EU (Directive 63/2010).
Funding
This work was financially supported by French National Hunting Federation (FNC PSN-PR1-2013 and FNC-PSN-PR1-B-2015).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Cano-Gómez, C., Llorente, F., Pérez-Ramírez, E. et al. Experimental infection of grey partridges with Bagaza virus: pathogenicity evaluation and potential role as a competent host. Vet Res 49, 44 (2018). https://doi.org/10.1186/s13567-018-0536-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13567-018-0536-8