Abstract
Introduction
The decline in fishery resources from the wild has led to an ever increasing focus on aquaculture in recent years. With increasing aquaculture of animal species, there is an increasing need for suitable microalgae in the production of these animals. However, cultivation of microalgae in expensive pure chemical media is one of the major challenges facing large-scale cultivation of microalgae.
Purpose
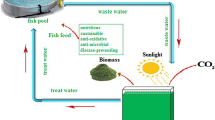
The present study investigated the suitability of aquaculture wastewater (AWW) supplemented with NPK (nitrogen:phosphorus:potassium) fertilizer as a cheap source of nutrient to cultivate a microalga Chlorella vulgaris (C. vulgaris).
Methods
C. vulgaris with an initial cell density of 0.8 × 106 cells/mL was batch cultured in AWW supplemented with NPK at 0.1, 0.5, 1.0 g/L and BBM for 20 days under laboratory conditions using 2000 mL Erlenmeyer flasks. The proximate composition, chlorophyll, minerals, and vitamins analysis of C. vulgaris biomass were done using standard analytical methods.
Results
The highest values in optical density (4.872 ± 0.025), dry cell weight (2.858 ± 0.015 g/L), specific growth rate (0.2097 ± 0.0038 day–1), and biomass productivity (0.1701 ± 0.0007 g/L/day) were obtained in C. vulgaris grown in AWW + 1.0 NPK medium. The total chlorophyll, protein, lipid, and carbohydrate content of the microalgae biomass were in the range of 0.05–0.862%, 44.062–57.089%, 17.064–23.260%, and 15.217–21.896%, respectively. Furthermore, microalgae grown in AWW + 1.0 NPK showed good vitamin and mineral content compared to BBM grown alga.
Conclusion
These findings indicated that the AWW + 0.1 NPK, AWW + 0.5 NPK, and AWW + 1.0 NPK are potential growth media for C. vulgaris cultivation and can replace the BBM medium, which is very expensive and less accessible to users.
Similar content being viewed by others
Introduction
With dwindling wild fishery catches since 2000, there is an ever increasing global effort in increasing aquaculture output (Ahmed et al. 2019), to ensure food security for all and keep up with the increasing per capita fish consumption. Since then, world aquaculture output (in tons) has more than doubled (32.4 × 106 in 2000 to 82.1 × 106 in 2018), with the output anticipated to increase by 60–100% over the next 20–30 years (FAO 2020). During the same period, Africa recorded over 5-fold increase in output from 399.9 × 103 to 2195 × 103 (FAO 2020). With increasing aquaculture of animal species, there is an increasing need for suitable microalgae in the production of these animals. Microalgae play a vital role in supplying energy, essential compounds, such as protein, lipid, carotene, vitamins, amino acids, polyunsaturated fatty acids, and essential minerals required for high-quality aquaculture production (Singh et al. 2014). Further on, microalgae in aquaculture are used as live food for rearing larvae and juveniles of many species of commercially important molluscs, crustaceans, fish species, and for zooplankton used in aquaculture food chains. Spolaore et al. (2006) demonstrated that the use of microalgae by fish larvae enhances their reproduction, giving the animal’s better immune system and health.
The microalga (C. vulgaris) is one such example widely used in aquaculture as a promising source of protein, carbohydrates, essential fatty acids, dietary fibers, and minerals (Ahmad et al. 2020). Apart from aquaculture, C. vulgaris has become a good candidate for biofuel production due to its rapid growth rate and high lipid content (Koller et al. 2014). It is also used as a healthy food, and nutrition supplements for animals and human consumption, because of the quality of proteins that they produce (Ramaraj et al. 2015). Besides the high levels of protein (51–58%), carbohydrates (12–17%), lipids (14–22%), and nucleic acids (4–5%), C. vulgaris contains appreciable amounts of valuable vitamins and minerals (Kim et al. 2010; Mata et al. 2010). This microalga can also accumulate pigments such as chlorophyll a and b, β-carotene, and xanthophylls which are used for enhancement of the pigmentation in fish and as a colorant for foods, drugs, and cosmetics (Guedes et al. 2011).
Despite of the several benefits from this microalga, its commercial scale production is still not cost-effective if algae are required in large amount (Di Caprio et al. 2015; Nayak et al. 2016b). Xia and Murphy (2016) reported that in large-scale production, nutrient requirements can be up to 50% of the total cost of biomass production. Its high production costs have triggered efforts to find cheaper and economically feasible approach for its culture. Studies by Nasir et al. (2015), Luo et al. (2016), and Hemalatha and Venkata Mohan (2016) have reported that the microalgae can be grown using aquaculture wastewater instead of the expensive synthetic medium often used as it contains all the necessary nutrients required for algal growth. Nevertheless, the composition of aquaculture wastewater depends on the nature and quantity of feed, the species being reared, stocking density, water replacement frequency, and the type of system in operation. The low or imbalanced nutrient concentration in wastewater may be a major challenge in cultivating microalgae using wastewater which often leads to low biomass yield and poor quality (Cai et al. 2013; Lu et al. 2015b). For example, De Lourdes et al. (2017) cultivated C. vulgaris by using real wastewater from a wastewater treatment plant and only recorded a maximum dry weight of 0.267 ± 0.031 g/L. Due to the low or inconsistency of nutrients compositions in aquaculture wastewater, some studies recommended the supplementation of nutrients as an alternative method to overcome the nutrient limitations in wastewater (Cai et al. 2013; Lu et al. 2015a).
According to the work of Ammar (2016) and Mahmood and Mohsin (2017), different agricultural fertilizers were used as a nutrient source as potential alternatives to reduce microalgal production costs. In that context, commercial fertilizers can be used as a nutrient source for cultivation and economically viable production of microalgae. Previous studies have mainly been focused on the culture of microalgae by using aquaculture wastewater (Ramanna et al. 2014; Caporgno et al. 2015; Hawrot-Paw et al. 2020). In the current study, commercial fertilizer NPK (20:20:10) purchased from the local market at a very low price of 1 US $ for 1 kg was added in aquaculture wastewater (AWW) to determine if these additions could enhance the biomass production of C. vulgaris under laboratory condition. The present study therefore aimed to develop inexpensive culture medium by using aquaculture wastewater and NPK fertilizer for mass production of C. vulgaris whose biomass composition was evaluated for its potential use as a feed ingredient in aquaculture.
Materials and methods
Isolation of microalgae
Chlorella vulgaris was isolated from freshwater fish ponds with pH of 8.5 at Fisheries Education and Training Agency (FETA) in Mwanza, Tanzania, using a standard plating method. Twenty grams of agar was mixed with 1 l of autoclaved Bold’s Basal Medium (BBM) in a conical flask and boiled for 20 min. After boiling, the agar solution was poured on sterilized glass petri dishes (100 × 15 mm) and allowed to cool for 2 h. Five milliliters of algal sample was transferred into media plate and spread uniformly across the surface; the plates were then placed at a light intensity of 5000 lux under room temperature (27–30 °C). After 15 days, cell colonies were observed to grow on the surface, and the best individual colonies were picked up by using a sterile syringe needle and transferred to the culture tubes containing liquid BBM and placed at the same conditions. After 14 days, when the color change was observed in the culture tube, a sample of 5 mL was taken and checked under the light microscope to see the isolated algal strain. Identification was carried out using a guide provided by (Shubert and Gärtner 2015).
Preparation of microalgal growth media
Aquaculture wastewater (AWW) was collected from African catfish pond at the University of Dar es Salaam, Department of Aquatic Science and Fisheries at Kunduchi, Dar es Salaam. Immediately after collection, the AWW was sterilized by autoclaving for 15 min at 121 °C and stored in a refrigerator (4 ± 2 °C) for 2 days for sedimentation of any visible solid particles (Zhu et al. 2013). The supernatant was collected and used as the microalgae culture medium. Water quality parameters such as temperature (T), dissolved oxygen (DO), and pH were analyzed at the time of wastewater collection using a multiparameter equipment (multi-3430 WTW, Germany). Ammonium (NH4+) and phosphorus (P) were analyzed using indophenol blue and ascorbic acid method respectively, following the procedures described by (Allen 1989), whereas nitrate (NO3) was analyzed using cadmium reduction method as described by (Emteryd 1989). The calcium (Ca), sodium (Na), magnesium (Mg), iron (Fe), manganese (Mn), zinc (Zn), and copper (Cu) were determined using Atomic Absorption Spectrophotometer (AA240 Varian, USA).
Experimental setup
The microalgae were cultured using AWW in the laboratory. The inoculum of C. vulgaris was pre-cultured in 1000 mL conical flasks using BBM at a light intensity of 5000 lux, temperature (28 ± 1 °C) and constantly mixed (150 rpm). The nutrient composition and costs for 1 l of BBM medium is depicted in Table 1. At the exponential growth phase, the microalgae cells were collected and cultured in five different media. The first and second media were BBM (control) and AWW, respectively. Third, fourth, and fifth media were AWW supplemented with NPK fertilizer at concentrations of 0.1, 0.5, and 1.0 g/L, respectively. All media components were sterilized by autoclaving at 121 °C for 15 min. The microalgae were batch cultured in 1000 mL Erlenmeyer flask containing 800 mL of medium and 200 mL of C. vulgaris with the initial cell density of C. vulgaris of 0.8 × 106 cells/mL in all treatments. All experiments were conducted at a controlled environment of temperature (28 ± 1 °C) maintained using air condition, illumination intensity (5000 ± 10 lux) measured using vertex VXLM-636 light meter, photoperiod (16:8 light:dark cycle) adjusted by electricity timer and continuous aeration was provided by aerators. The pH values in all treatments were measured using a pH meter (Fisher Scientific AB 15 Accumet Basic, Singapore) and maintained at the pH of 9–10 by adding 5 M sodium hydroxide (NaOH) or 3 M hydrochloric acid (HCL). The microalgae were cultured for 20 days and all treatments were carried out in triplicate.
Estimation of microalgae culture media cost
The estimation of cultivation media costs was done by considering only the concentration and the price of each reagent used to make 1 l of the medium. Other expenses like taxation, electricity consumption, and transport cost were not considered. The prices of all reagents and commercial fertilizer used were obtained from https://www.alibaba.com and https://www.sigmaaldrich.com. The AWW was obtained for free.
Determination of microalgae growth
Microalgal growth was monitored by measuring dry cell weight, optical density, and photosynthetic pigments (total chlorophyll and carotenoids). The optical density (as an indicator of cell density) was determined daily using a UV–Vis spectrophotometer (UV 6305, Genway, UK) at a wavelength of 688 nm. Microalgae dry weight (biomass concentration, g/L) was determined every 2 days, where a sample of 10 mL was taken from each treatment and filtered using a pre-weighed glass fiber filter (Whatman GF/F) and oven dried at 105 °C for 24 h. The biomass (dry weight) was weighed by electronic analytical balance XPE 105 (Mettler-Toledo, Switzerland). The specific growth rate, SGR (μ, day−1) of algal culture is a measure of the increase in biomass over time and it was calculated according to (Liang et al. 2013)
Where W2 and W1 are the biomass concentrations (g/L) at T2 and T1 respectively.
The biomass productivity, PB (g/L/day) was calculated by the method of (Liang et al. 2013)
Where B0 and B1 are the mean dry biomass concentration at the times T0 and T1, respectively.
The determination of total chlorophyll content in C. vulgaris cells was done using a spectrophotometric technique, as described by (Quarmby and Allen 1989). The chlorophyll content within the algal samples was extracted by dissolving well grinded 1 g of C. vulgaris biomass into 50 mL aqueous acetone (85% v/v) and stored at 4 °C for 24 h. Twenty-five milliliters aliquot of the extract was added to 50 mL of diethyl ether in a separating funnel and mixed well. The ether layer was washed with distilled water until all the chlorophyll passed into the ether layer. The water layer was then decanted, and the ether phase was transferred into a volumetric flask and anhydrous sodium sulfate (Na2SO4) was added for drying out the water. The absorbance of ether containing chlorophyll was measured at 660 and 643 nm using the UV–Vis spectrophotometer (Shimadzu) and the total chlorophyll content of the C. vulgaris was calculated using Eq. 3.
Where C = chlorophylls in ether solution = 7.12 × OD660 + 16.8 × OD643: whereby OD = optical density
Biochemical composition analysis
Due to sample size requirements, the biomass composition analysis was done only for the growth media which showed good results in growth parameters analyzed. At the end of the experiment, the C. vulgaris biomass was harvested by centrifugation at 978.02×g for 10 min. The collected biomass was then dried at 100 °C to constant weight for protein, lipid, carbohydrate, minerals, and vitamin analyses.
Microalgae lipid extraction
Total lipids from microalgae biomass were extracted based on the procedure described by (Bligh and Dyer 1959). Accurately weighed 5 g of C. vulgaris biomass was mixed with chloroform, methanol and water in the proportion of 1:1:0.8 respectively and homogenized for 2 min under oxygen-free nitrogen (OFN) with cooling. The chloroform and water were then added to give a final solvent ratio of chloroform:methanol:water solvent ratio of 2:2:1.8. The mixture was filtered to remove biomass residues and transferred to a graduated cylinder where the volume of the chloroform layer was recorded. The solvent was separated into two layers (chloroform and aqueous methanol layers) using a separating funnel. An aliquot of the lower chloroform layer was pipetted and weighed using a pre-weighed, clean, and dry evaporation dish. The solvent was then oven dried at 40 °C for 30 min for lipid recovery and the remaining lipids were cooled in a desiccator and weighed. The percentage of lipid in C. vulgaris biomass was calculated using the equation below:
Determination of protein contents
The total protein content in the C. vulgaris biomass was determined using semi-micro Kjeldahl digestion method (Emteryd 1989; Quarmby and Allen 1989). Here, 4 g of C. vulgaris biomass was digested with a strong sulfuric acid in the presence of selenium catalyst to convert nitrogen compounds into ammonium form. The ammonium concentration was then determined by using indophenol-blue colorimetric method and the nitrogen content was calculated using the Eq. (5) below. The percentage crude protein present in algal biomass was calculated by multiplying the nitrogen content by the conventional factor of 6.25.
Determination of carbohydrate contents
The total soluble carbohydrate (CHO) was determined in the algal biomass by using Anthrone method as described by (Allen 1989). Five grams of C. vulgaris biomass was mixed with 30 mL of distilled water in 100 mL conical flask and boiled at boiling point of water for 2 h. The sample was allowed to cool at room temperature and filtered through a Whatman filter paper No. 44. An aliquot of a clear sample solution was placed into the test tube and the anthrone reagent was added. The solution was allowed to cool, and its absorbance was measured at 625 nm. The percentage soluble carbohydrate in the C. vulgaris biomass was calculated based on the formula below.
Whereby C = mg glucose obtained from the graph
Vitamins composition analysis
Extraction of vitamins from C. vulgaris biomass was done by mixing 0.5 g of sample with 100 mL of 95% ethanol in a conical flask. The mixture was vigorously shaken for 15 min to ensure complete extraction. The extract was centrifuged for 10 min and filtered using Whatman No. 1 filter paper. To remove ethanol and obtain clear extracts, the sample was placed in a rotary evaporator (Gmbh & Co.KG, Germany) under reduced pressure at 40 °C. The obtained extract was then kept at 4 °C until analyses. Vitamin A (as beta- carotene) was determined according to the method of (Nagata and Yamashita 1992). One hundred milligrams of dried extract was vigorously stirred with 10 mL of acetone-hexane mixture (4:6) for 1 min and filtered through Whatman No. 4 filter paper. The absorbance of the filtrate was measured at 453, 505, and 663 nm. Content of beta carotene was calculated according to the following equation:
Determination of vitamin B complex present in microalgae extracts was done according to the method of (Rajput et al. 2011). The working standard solution for the standard vitamins and microalgae samples were prepared by dissolving a known weight of the standard vitamin and extracts in a known volume of distilled water into a conical flask. Vitamins B1, B2, B3, B6, B12, and C were determined from riboflavin, nicotinamide, pyridoxine hydrochloride, cyanocabalamin, and ascorbic acid stock solutions, respectively. The absorbance of the working solutions, C. vulgaris samples, and blank were read at 430 nm for vitamin B1, 444 nm for vitamin B2, 450 nm for vitamin B3, 650 nm for vitamin B6, 530 nm for vitamin B12, and 450 for vitamin C using a UV-visible spectrophotometer (Jenway 6305).
Mineral composition analysis
To determine the mineral composition of C. vulgaris biomass, the biomass was digested using nitric perchloric acid method as described by (Jones 1984). Approximately 0.5 g (dry weight) of C. vulgaris biomass from each treatment was weighed into a beaker. To the samples, a mixture of 5 mL of concentrated nitric acid (HNO3) and 1 mL of per-chloric acid (HCIO4) in the ratio of 5:1 was added. The solution was heated at 120 ̊ C until the disappearance of the brown fumes which indicated the complete digestion of the organic matter. The solution was then cooled and diluted with distilled water up to 100 mL solution. The Ca, Mg, Fe, K, Mn, Na, and Zn concentration of the digested biomass were determined using Atomic Absorption Spectrophotometer (AA240 Varian, USA).
Statistical analysis
The data were presented as mean ± standard error (SE). Statistical analysis was carried out by using R software (Version 3.6.3). Normally and not normally distributed data were tested using one-way analysis of variance (ANOVA) and Kruskal-Wallis respectively. Tukey’s (ANOVA) and Dunn (Kruskal-Wallis) post hoc test were used to check the significant difference among the treatment means. A p value of less than 0.05 was considered statistically significant.
Results
Microalgae growth
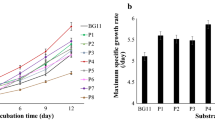
The physico-chemical composition of the AWW and the growth media cultivation costs are shown in Tables 2 and 3, respectively. The statistical analysis showed significant variation in the optical density (χ2 = 66.824, df = 4, p = 0.000, Fig. 1), dry weight (χ2 = 31.404, df = 4, p = 0.000; Fig. 2), biomass productivity (F = 887.660, df = 4, p = 0.000; Fig. 3), specific growth rate (F = 158.600, df = 4, p = 0.000; Fig. 3) and total chlorophyll content (F = 46.612, df = 4, p = 0.000; Fig. 4) of C. vulgaris for different growth media. All the microalgae growth parameters analyzed increased with the increase of NPK concentration in AWW. The optical density and dry weight of C. vulgaris cultured in BBM, AWW + 0.1 NPK, AWW + 0.5 NPK, and AWW + 1.0 NPK were significantly higher than that grown in AWW (p = 0.000). The highest values of optical density (4.872 ± 0.025) and dry weight (2.858 ± 0.015 g/L) were recorded in microalgae cultured in AWW + 1.0 NPK, followed by AWW + 0.5 NPK, AWW + 0.1 NPK, BBM, and finally AWW. Additionally, significantly higher amount of biomass productivity (0.170 ± 0.001 g/L/day) and specific growth rate (0.210 ± 0.004 μ, day-1) were found in C. vulgaris grown in AWW + 1.0 NPK than those raised in all other media (p = 000). The statistical analysis also showed significantly higher amount of chlorophyll content (0.862 ± 0.087%) in microalgae cultured in AWW + 1.0 NPK than those cultivated in the rest of the media including BBM (p < 0.05).
Biochemical composition
The protein, lipid, and carbohydrate contents of C. vulgaris biomass are shown in Fig. 5. The statistical analysis showed that there was a significant effect of the cultivation media on the protein (F = 337.140, df = 3, p = 0.000), lipid (F = 38.674, df = 3, p = 0.000), and carbohydrate (F = 216.830, df = 3, p = 0.000) content of microalgae. Cells grown in AWW + 1.0 NPK medium had significantly higher protein content (57.089 ± 0.186%) than those grown in the other media (p = 0.000). On the other hand, a significantly higher percentages of lipid (23.260 ± 0.484%) and carbohydrate (21.896 ± 0.169%) contents were observed in C. vulgaris biomass cultivated in AWW + 0.1 NPK than those grown in the rest of the media (p = 0.000).
Furthermore, the growth media had a significant impact on vitamins A, C, B2, B1, B3, and B6 content of the microalgae biomass tested (p < 0.05; Table 4). C. vulgaris cultured in BBM, AWW + 0.5 NPK and AWW + 1.0 NPK had significantly higher vitamin A content than those grown in AWW + 0.1 NPK medium (p < 0.05). Moreover, significantly higher vitamin C, B1, and B2 content of C. vulgaris was recorded in AWW + 1.0 NPK when compared with those cultured in other cultivation media (p < 0.05). A significantly higher amount of vitamin B3 content was found in AWW + 0.1 NPK, AWW + 0.5 NPK, and AWW + 1.0 NPK (p < 0.05) than in BBM. The vitamin B6 of microalgae grown in AWW + 1.0 NPK was significantly higher than those cultured in BBM (p = 0.013) but no significant difference was observed with those cultured in AWW + 0.1 NPK and AWW + 0.5 NPK (p > 0.05). In addition, the cultivation media did not show any significant difference in vitamin B12 content of the microalgae investigated (p > 0.05; Table 4).
Mineral components of C. vulgaris are given in Table 5. We observed significant variations in the calcium, iron, magnesium, potassium and zinc content of C. vulgaris biomass for the different cultivations medium used (p < 0.05). Cells grown in BBM medium had a statistically higher amount of calcium, iron and magnesium content than those grown in AWW + 0.1 NPK (p < 0.05); however, no significant variation was recorded with the other growth media (p > 0.05). Significantly lower amount of sodium content in BBM than in AWW + 0.5 NPK was observed (p = 0.013), though no significant variation was found with AWW + 0.1 NPK and AWW + 1.0 NPK (p > 0.05). Microalgae raised in AWW + 0.1 NPK had statistically higher potassium content than those cultured in BBM (p = 0.386). Also, potassium value for C. vulgaris grown in BBM did not show significant difference with those cultivated in AWW + 0.5 NPK and AWW + 1.0 NPK (p > 0.05). Zinc content of C. vulgaris cultured in BBM was significantly higher than that grown in AWW + 1.0 NPK (p = 0.013). But no significant variation was found with the rest of the media (p > 0.05). In addition, the growth media did not show any significant difference in manganese content of C. vulgaris biomass (p = 0.057).
Discussion
Microalgae growth
Nutrient is among the key factor that greatly affects the microalgae growth rate and their production of various bio-chemicals (George et al. 2014; Mahmood and Mohsin, 2017). However, for large-scale production, it poses an economical challenge to provide excess nutrients (Di Caprio et al. 2015; Nayak et al. 2016a). The present study was done to investigate the effect of AWW supplemented with NPK fertilizer as a low-cost growth medium for production of C. vulgaris. The growth parameter analyzed were optical density, biomass content, biomass productivity, total chlorophyll content, and specific growth rate. The specific growth rate is the most important parameter for assessing algal growth. It determines both the time required for achieving the maximum algal biomass concentration during the batch culture and the hydraulic retention time required for high biomass productivity during a continuous culture (Ruiz et al. 2013). In the current study, the highest specific growth rate of 0.209 day−1 was recorded in microalgae cultured in AWW + 1.0 NPK. This value is within those reported in the literature for C. vulgaris. Gao et al. (2014) observed specific growth rates of 0.277 day−1 and 0.108 day−1 when C. vulgaris was grown in membrane and conventional photobioreactor respectively, using treated sewage medium. On the other hand, De Lourdes et al. (2017) reported a growth rate of 0.205 day−1 on their study of tolerance and nutrients consumption of C. vulgaris growing in mineral medium and real wastewater under laboratory condition.
The chlorophyll content of microalgae is an important parameter as it indicates the physiological status of the culture (Martínez-Roldán et al. 2014). In our study, media with high nutrient concentrations were found to support the accumulation of pigments which indicate that the physiological activity in C. vulgaris was affected by nutrient concentrations. The highest total chlorophyll content was observed in microalgae cultured in AWW + 1.0 NPK (0.862 ± 0.090%). These results are in accordance with that of Paes et al. (2016) who reported values ranging from 0.72 ± 0.07% to 1.05 ± 0.06 for Chlorella sp under nitrogen starvation culture condition.. Contrary to our results, Ribeiro et al. (2019) obtained optical density of 0.519 ± 0.002 and 0.849 ± 0.003 when cultured Chlorella sorokiniana in NPK and BBM media respectively. These values are much less than the present findings (0.629 ± 0.059 − 4.872 ± 0.025), probably due to differences in media composition and concentration used (Richmond 2004).
Furthermore, the maximum biomass productivity of C. vulgaris was recorded in AWW + 1.0 NPK (0.17g/L/day). This value is higher than that of Gao et al. (2016) who obtained the biomass productivity of 0.043 g/L/day when cultivated C. vulgaris in a membrane photobiorector, using aquaculture wastewater as a nutrient medium. Nevertheless, Tan and Zhang (2016) in their study on C. pyrenoidosa growth in diluted anaerobically digested activated sludge in outdoors obtained highest dry weight of 2.430 g/L against 2.858 g/Lin our study. Furthermore, De Lourdes et al. (2017) recorded a maximum dry weight of 0.267 ± 0.031 g/L in cells of C. vulgaris cultured in real wastewater from a wastewater treatment plant. This observation can be largely attributed to nutrients differences resulting from the addition of NPK fertilizer in AWW (Cai et al. 2013; Lu et al. 2015a). The results of this work agree with those reported by Cai et al. (2013) and Lu et al. (2015a), where they emphasized that supplementation of nutrient in wastewater can enhance microalgae growth; however, it depends on the initial value of the nutrient concentrations of the wastewater used and the requirements of the selected microalgal strain. Results presented here agree with those reported by Bhatnagar et al. (2011) where they found that the addition of 1% w/v glucose and 250 mg/L of N as NaNO3 into wastewater yielded the highest biomass concentration compared to BG11 addition and without any supplementation. In another study, Ramanna et al. (2014) supplemented wastewater with urea (1.5 g/L) as a cheap N source for the cultivation of Chlorella sorokiniana and found 23% increase in biomass production when compared to the control wastewater without any supplementation. Furthermore, Ansari et al. (2017) grew Chlorella sorokiniana in aquaculture wastewater with sodium nitrate supplementation and found comparable biomass yields to the synthetic medium. The authors also observed high-nutrient removal and proposed that treated water can be used for aquaculture. The current results indicated that the AWW + 0.1 NPK, AWW + 0.5 NPK, and AWW + 1.0 media are ideal for C. vulgaris culture and can be used as substitute for the BBM medium which is expensive and less accessible to poor aquaculture farmers.
Biochemical composition
One of the most noteworthy nutritional characteristics of Chlorella is its high protein content which contains all essential amino acids, demonstrating that Chlorella could be used as healthy food for humans and feed for fish and other livestock (Becker 2007). It has been reported that the nitrogen concentration in the medium can influence the protein content of microalgae, whereby having a higher amount of nitrogen in the cultivation medium can induce protein synthesis in microalgae (Martínez et al. 2000). Our data show that cells grown in AWW + 1.0 NPK had a higher percentage of protein content (57.400%) in their biomass, and this could be due to its composition, as this medium was rich in nitrogen and had higher algal biomass. Such protein content was higher than that of C. vulgaris (50.640%) cultivated in the 100-fold diluted Monosodium glutamate wastewater (MSGW) by (Ji et al. 2014) and lower than the value reported (about 63.500%) by Abreu et al. (2012).
Lipid accumulation by microalgae depends on the species and is affected by medium composition and cultivation conditions (Richmond 2004; Chen et al. 2011). Kim et al. (2010) and Mata et al. (2010) reported the average lipid content of C. vulgaris of approximately 14–22% under common conditions. In this study, the total lipid content ranged from 16.400–23.300% and the highest value was detected in the biomass cultivated in AWW + 0.1 NPK medium. These values were much lower than that found in C. vulgaris (42%) cultivated using industrial dairy waste as organic carbon source (Abreu et al. 2012). Furthermore, the present finding of lipid content was higher than that of Song et al. (2013) who cultivated C. vulgaris in BG11 medium and obtained a value of 20.820 ± 1.020% of dry weight. The difference in lipid contents of microalgae biomass cultured under different growth media in our study may be attributed to the variation in nitrogen contents in the cultivation medium. It is known that under nitrogen deficiency or limitations, the metabolic pathway of carbon assimilation diverts from protein synthesis to lipid or carbohydrate production as carbon and energy storage (Chandra et al. 2014; Kim et al. 2014). Similar results have been reported in the other green algae Chlorella sp. and Chlamydomons sp. (e.g., Shen et al. 2015).
Carbohydrate is one of the major components in C. vulgaris biomass, and its production can vary as a result of changes in growth conditions, such as temperature and light intensity and nutrient media characteristics including the concentration of nitrogen, phosphates, and iron (Illman and Scragg 2000; Liu et al. 2008). The carbohydrate content of algae in the present study ranged from 15 to 22% which is within the range of carbohydrate contents in Chlorella sp. reported in the literature (Batista et al. 2013; Beuckels et al. 2015; Lu et al. 2015b). The highest content of carbohydrate (22%) was in the biomass cultivated in AWW + 0.5 NPK medium, which are also characterized by a lower concentration of nitrogen, especially in the form of nitrate. Our results are agreeing with the literature data, which indicate that under limited nutrient conditions, cell division slows down but carbon uptake continues (through photosynthesis) which leads to a higher concentration of carbon-rich metabolites such as carbohydrates or lipids in the biomass of algae (González-Fernández and Ballesteros 2012; Vitova et al. 2015)
Apart from main compounds such as proteins, lipid, and carbohydrates, living organisms require several micro-nutrients for survival. These micronutrients act as either co-enzymes or as active electron/proton carriers in the breakdown process of the macro-nutrients. One such group of important micro-nutrients is vitamins (Koyande et al. 2019). C. vulgaris is a very rich source of nearly all the important vitamins such as vitamin A (in the form of beta-carotene), vitamin C, vitamin E, and vitamin B such as thiamine (B1), riboflavin (B2), niacin (B3), pantothenic acid (B5), pyridoxine (B6), folic acid (B9), and cobalamin (B12) (Safi et al. 2014). These vitamins have great potential benefits to animal and human health; since they are used to nourish the body, detoxify, and normalize intestinal function, as well as stimulate the immune system and regenerate cells. Even though vitamins are required in a small amount to maintain good health, lack of them can cause serious diseases in humans (Heudi et al. 2005). The cells grown in AWW + 1.0 NPK had highest content of all vitamins analyzed when compared to other cultivation media. The highest vitamin B1, B3, B6, B12, and C obtained in our study are in accordance with those achieved in past studies for C. vulgaris (Maruyama et al. 1997; Panahi et al. 2012; Andrade et al. 2018). The vitamin A content recorded in this study was much lower when compared with the study by (Panahi et al. 2012) who obtained the vitamin A content of 180 mg/100g in their study investigating the effects of Chlorella vulgaris as adjunctive therapy for dyslipidemia. This variation could be a result of different composition and concentrations of media used as it was reported that environmental factors, particularly nutrient status, light, and temperature not only affect photosynthesis and productivity of cell biomass, but also influence the pattern, pathway, and activity of cellular metabolism and thus dynamic cell composition (Richmond 2004)
As illustrated in Table 5, C. vulgaris is a good source of important minerals such as Ca, K, Mg, Na, Fe, Zn, and Mn. These minerals are considered to be essential for cellular metabolism in all animals including fish (Paul and Mukhopadhyay 2016). For instance, iron is essential for the production of hemoglobin, myoglobin, cytochromes, and many other enzyme system and its deficiency has been reported to induce anemia in trout and carp (Paul and Mukhopadhyay 2016). Further on, zinc is an essential component of enzymes which participate in many metabolic processes including synthesis of carbohydrate, lipid, and protein synthesis and it is also a cofactor of the superoxide dismutase enzyme, which is involved in protection against oxidative processes (Mann and Truswell 2009). Potassium is associated with intracellular fluid balance and volume, carbohydrate metabolism, protein synthesis, and nerve impulses (Safi et al. 2014). Magnesium is important in maintaining normal and constant nervous activity and muscle contraction; hence, magnesium deficiency in human organism can lead to depression and symptoms of suicidal behavior (Diehl 2002). The mineral components obtained in this study were within the range of reported elemental composition of Chlorella biomass (Tokuşoglu and üUnal, 2003; Panahi et al. 2012).
Conclusion
The present study was done to formulate a low-cost culture medium by using aquaculture wastewater and NPK fertilizer for cultivation of C. vulgaris. The best growth performance in respect of optical density, dry weight, specific growth rate, biomass productivity, total chlorophyll content, and biochemical composition were observed in C. vulgaris grown in AWW + 1.0 NPK medium. Also, the C. vulgaris grown in AWW + 0.1 NPK, AWW + 0.5 NPK showed good results in biomass composition when compared with C. vulgaris grown in BBM. These results indicated that it is feasible to use AWW supplemented with NPK fertilizer medium in place of the synthetic medium (BBM) to cultivate C. vulgaris for feed production in aquaculture. More importantly, these media are inexpensive since the NPK fertilizer is widely available in the local market and the AWW is found at a lower or no cost. Therefore, the use of these media will reduce the mass production costs of highly nutritive C. vulgaris and improve the aquaculture sector.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abreu AP, Fernandes B, Vicente AA, Teixeira J, Dragone G (2012) Mixotrophic cultivation of Chlorella vulgaris using industrial dairy waste as organic carbon source. Bioresour Technol 118:61–66
Ahmad MT, Shariff M, Yusoff F, Goh YM, Banerjee S (2020) Applications of microalga Chlorella vulgaris in aquaculture. Rev Aquac 12:328–346
Ahmed N, Thompson S, Glaser M (2019) Global aquaculture productivity, environmental sustainability, and climate change adaptability. Environ Manage 63:159–172
Allen S (1989) Analysis of vegetation and other organic materials. Chemical analysis of ecological materials. Blackwell. Scientific Publications, Oxford London Edinburgh, Boston Melbourne, pp 46–60
Ammar SH (2016) Cultivation of microalgae for biomass production. Al-Khwarizmi Eng J 12:90–99
Andrade LM, Andrade CJ, Dias M, Nascimento CAO (2018) Chlorella and spirulina microalgae as sources of functional foods , nutraceuticals , and food supplements ; an overview, vol 6, pp 45–58
Ansari FA, Singh P, Guldhe A, Bux F (2017) Microalgal cultivation using aquaculture wastewater: Integrated biomass generation and nutrient remediation. Algal Res 21:169–177
Batista AP, Gouveia L, Bandarra NM, Franco JM, Raymundo A (2013) Comparison of microalgal biomass profiles as novel functional ingredient for food products. Algal Res 2:164–173
Becker EW (2007) Micro-algae as a source of protein. Biotechnol Adv 25:207–210
Beuckels A, Smolders E, Muylaert K (2015) Nitrogen availability influences phosphorus removal in microalgae-based wastewater treatment. Water Res 77:98–106
Bhatnagar A, Chinnasamy S, Singh M, Energy KD-A (2011) U (2011) Renewable biomass production by mixotrophic algae in the presence of various carbon sources and wastewaters. Appl Energy 88:3425–3431
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Cai T, Park SY, Li Y (2013) Nutrient recovery from wastewater streams by microalgae: Status and prospects. Renew Sustain Energy Rev 19:360–369
Caporgno MP, Taleb A, Olkiewicz M, Font J, Pruvost J, Legrand J, Bengoa C (2015) Microalgae cultivation in urban wastewater: Nutrient removal and biomass production for biodiesel and methane. Algal Res 10:232–239
Chandra R, Rohit MV, Swamy YV, Venkata Mohan S (2014) Regulatory function of organic carbon supplementation on biodiesel production during growth and nutrient stress phases of mixotrophic microalgae cultivation. Bioresour Technol 165:279–287
Chen CY, Yeh KL, Aisyah R, Lee DJ, Chang JS (2011) Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: A critical review. Bioresour Technol 102:71–81
De Lourdes FMM, Dolores Josefina RRM, Ulises MMC, De Jesús MRA (2017) Tolerance and nutrients consumption of Chlorella vulgaris growing in mineral medium and real wastewater under laboratory conditions. Open Agric 2:394–400
Di Caprio F, Altimari P, Pagnanelli F (2015) Integrated biomass production and biodegradation of olive mill wastewater by cultivation of Scenedesmus sp. Algal Res 9:306–311
Diehl JF (2002) Nuts shown to offer health benefits. International News on Fats. Oils Relat Mater 13:134–113
Emteryd O (1989) Chemical and physical analysis of inorganic nutrients in plant, soil, water and air. Department of Forest Site Research, Stencil No,10, UMEA
FAO (2020) The State of World Fisheries and Aquaculture (SOFIA). FAO,
Gao F, Li C, Yang ZH, Zeng GM, Feng LJ, Zhi LJ, Liu M, Wen CH (2016) Continuous microalgae cultivation in aquaculture wastewater by a membrane photobioreactor for biomass production and nutrients removal. Ecol Eng 92:55–61
Gao F, Yang ZH, Li C, Jie WY, Hong JW, Bing DY (2014) Concentrated microalgae cultivation in treated sewage by membrane photobioreactor operated in batch flow mode. Bioresour Technol 167:441–446
George B, Pancha I, Desai C, Chokshi K, Paliwal C, Ghosh T, Mishra S (2014) Effects of different media composition, light intensity and photoperiod on morphology and physiology of freshwater microalgae Ankistrodesmus falcatus - a potential strain for bio-fuel production. Bioresour Technol 171:367–374
González-Fernández C, Ballesteros M (2012) Linking microalgae and cyanobacteria culture conditions and key-enzymes for carbohydrate accumulation. Biotechnol Adv 30:1655–1661
Guedes AC, Amaro HM, Malcata FX (2011) Microalgae as sources of carotenoids. Mar Drugs 9:625–644
Hawrot-Paw M, Koniuszy A, Gałczynska M, Zajac G, Szyszlak-Bargłowicz J (2020) Production of microalgal biomass using aquaculture wastewater as growth medium. Water 12:106
Hemalatha M, Venkata Mohan S (2016) Microalgae cultivation as tertiary unit operation for treatment of pharmaceutical wastewater associated with lipid production. Bioresour Technol 215:117–122
Heudi O, Kilinc T, Fontannaz P (2005) Separation of water-soluble vitamins by reversed-phase high performance liquid chromatography with ultra-violet detection : Application to polyvitaminated premixes. J Chromatogr A 1070:49–56
Illman AM, Scragg AH (2000) Enzyme and microbial technology : increase in chlorella strains calorific values when grown in low nitrogen medium. Enzyme Microb Technol 27:631–635
Ji Y, Hu W, Li X, Ma G, Song M, Pei H (2014) Mixotrophic growth and biochemical analysis of Chlorella vulgaris cultivated with diluted monosodium glutamate wastewater. Bioresour Technol 152:471–476
Jones JJB (1984) Plants. In: Williams S (ed) Official methods of analysis of the association of official analytical chemists. Association of Official Analytical Chemists, Arlington 22209, USA, pp 38–64
Kim G, Mujtaba G, Rizwan M, Lee K (2014) Environmental stress strategies for stimulating lipid production from microalgae for biodiesel. Appl Chem Eng 25:553–558
Kim J, Lingaraju BP, Rheaume R, Lee JY, Siddiqui KF (2010) Removal of ammonia from wastewater effluent by chlorella vulgaris. Tsinghua Sci Technol 15:391–396
Koller M, Muhr A, Braunegg G (2014) Microalgae as versatile cellular factories for valued products. Algal Res 6:52–63
Koyande AK, Chew KW, Rambabu K, Tao Y, Chu D-T, Show P-L (2019) Microalgae: a potential alternative to health supplementation for humans. Food Sci Hum Wellness 8:16–24
Liang F, Wen X, Geng Y, Ouyang Z, Luo L, Li Y (2013) Growth rate and biomass productivity of Chlorella as affected by culture depth and cell density in an open circular photobioreactor. J Microbiol Biotechnol 23:539–544
Liu ZY, Wang GC, Zhou BC (2008) Effect of iron on growth and lipid accumulation in Chlorella vulgaris. Bioresour Technol 99:4717–4722
Lu Q, Zhou W, Min M, Ma X, Ceria Chandra YTTD, Yiwei M, Zheng H, Cheng S, Griffith R, Chen P, Chen C, Urriola P, Shurson GC, Gislerød RR HR (2015a) Growing Chlorella sp. on meat processing wastewater for nutrient removal and biomass production. Bioresour Technol 198:189–197
Lu W, Wang Z, Wang X, Yuan Z (2015b) Cultivation of Chlorella sp. using raw diary wastewater for nutrient removal and biodiesel production: characteristics comparison of indoor bench-scale and outdoor pilot-scale cultures. Bioresour Technol 192:382–388
Luo L, He H, Yang C, Wen S, Zeng G, Wu M, Zhou Z, Lou W (2016) Nutrient removal and lipid production by Coelastrella sp. in anaerobically and aerobically treated swine wastewater. Bioresour Technol 216:135–141
Mahmood AKH, Mohsin KE (2017) Experimental study for commercial fertilizer NPK (20:20:20+TE N: P: K) in microalgae cultivation at different aeration periods. Iraqi J Chem Pet Eng 18:99–110
Mann J, Truswell A (2009) Essentials of Human Nutrition. Oxford University Press, New York
Martínez ME, Sánchez S, Jiménez JM, El Yousfi F, Muñoz L (2000) Nitrogen and phosphorus removal from urban wastewater by the microalga Scenedesmus obliquus. Bioresour Technol 73:263–272
Martínez-Roldán AJ, Perales-Vela HV, Cañizares-Villanueva RO, Torzillo G (2014) Physiological response of Nannochloropsis sp. to saline stress in laboratory batch cultures. J Appl Phycol 26:115–121
Maruyama I, Nakao T, Shigeno I, Ando Y, Hirayama K (1997) Application of unicellular algae Chlorella vulgaris for the mass-culture of marine rotifer Brachionus. Hydrobiologia 358:133–138
Mata TM, Martins AA, Caetano NS (2010) Microalgae for biodiesel production and other applications: A review. Renew Sustain Energy Rev 14:217–232
Nagata M, Yamashita I (1992) Method Tomato Masayasu * National NAGATA * and Ichiji YAMASHITA * of Vegetables rnamental Plants and Tea , Ministry of Agriculture , Forestry and Fisheries. Forestry 39:1–4
Nasir NM, Bakar NSA, Lananan F, Abdul Hamid SH, Lam SS, Jusoh A (2015) Treatment of African catfish, Clarias gariepinus wastewater utilizing phytoremediation of microalgae, Chlorella sp. with Aspergillus niger bio-harvesting. Bioresour Technol 190:492–498
Nayak M, Karemore A, Sen R (2016a) Performance evaluation of microalgae for concomitant wastewater bioremediation, CO2 biofixation and lipid biosynthesis for biodiesel application. Algal Res 16:216–223
Nayak M, Thirunavoukkarasu M, Mohanty RC (2016b) Cultivation of freshwater microalga Scenedesmus sp. using a low-cost inorganic fertilizer for enhanced biomass and lipid yield. J Gen Appl Microbiol 62:7–13
Paes CRPS, Faria GR, Tinoco NAB, Castro DJFA, Barbarino E, Lourenço SO (2016) Growth, nutrient uptake and chemical composition of Chlorella sp. and Nannochloropsis oculata under nitrogen starvation. Lat Am J Aquat Res 44:275–292
Panahi Y, Pishgoo B, Jalalian HR, Mohammadi E, Taghipour HR, Sahebkar A, Abolhasani E (2012) Investigation of the effects of Chlorella vulgaris as an adjunctive therapy for dyslipidemia: Results of a randomised open-label clinical trial. Nutr Diet 69:13–19
Paul BN, Mukhopadhyay PK (2016) Importance of trace minerals in aquaculture nutrition. Fish Chimes 21:34–36
Quarmby C, Allen SE (1989) Organic constituents. In: Allen SE (ed) Chemical analysis of ecological materials. Blackwell Scientific Publications, Oxford London Edinburgh, Boston Melbourne, pp 160–199
Rajput G, Kumar A, Kumar A, Res GS-IJDF (2011) U (2011) To develop a simple (UV-Vis spectrometric) method for the estimation of water soluble vitamins. Int J Drug Res 2:232–239
Ramanna L, Guldhe A, Rawat I, Bux F (2014) The optimization of biomass and lipid yields of Chlorella sorokiniana when using wastewater supplemented with different nitrogen sources. Bioresour Technol 168:127–135
Ramaraj R, Dussadee N, Whangchai N, Unpaprom Y (2015) Microalgae Biomass as an Alternative Substrate in Biogas Production. Int J Sustain Green Energy Spec Issue Renew Energy Appl Agric F Nat Resour Technol 4:13–19
Ribeiro DM, Zanetti GT, Heloisa M, Julião M, Masetto TE, Mary J, Neves L, Fonseca GG (2019) Effect of different culture media on growth of Chlorella sorokiniana and the influence of microalgal effluents on the germination of lettuce seeds. J Appl Biol Biotechnol 7:6–10
Richmond A (2004) Handbook of Microalgal Culture. Biotechnology and Applied Phycology. Blackwell Science, Oxford
Ruiz J, Álvarez-Díaz PD, Arbib Z, Garrido-Pérez C, Barragán J, Perales JA (2013) Performance of a flat panel reactor in the continuous culture of microalgae in urban wastewater: Prediction from a batch experiment. Bioresour Technol 127:456–463
Safi C, Zebib B, Merah O, Pontalier PY, Vaca-Garcia C (2014) Morphology, composition, production, processing and applications of Chlorella vulgaris: a review. Renew Sustain Energy Rev 35:265–278
Shen QH, Gong YP, Fang WZ, Bi ZC, Cheng LH, Xu XH, Chen HL (2015) Saline wastewater treatment by Chlorella vulgaris with simultaneous algal lipid accumulation triggered by nitrate deficiency. Bioresour Technol 193:68–75
Shubert E, Gärtner G (2015) Nonmotile Coccoid and Colonial Green Algae. In: Wehr DJ, Kociolek PJ, Sheath (eds) Freshwater Algae of North America. Ecology and Classification, Academic Press, Elsevier Inc, pp 265- 313
Singh B, Guldhe A, Rawat I, Bux F (2014) Towards a sustainable approach for development of biodiesel from plant and microalgae. Renew Sustain Energy Rev 29:216–245
Song M, Pei H, Hu W, Ma G (2013) Evaluation of the potential of 10 microalgal strains for biodiesel production. Bioresour Technol 141:245–251
Spolaore P, Joannis-Cassan C, Duran E, Isambert A (2006) Commercial applications of microalgae. J Biosci Bioeng 101:87–96
Tan XB, Zhang YL, Bin YL, Chu HQ, Guo J (2016) Outdoor cultures of Chlorella pyrenoidosa in the effluent of anaerobically digested activated sludge: the effects of pH and free ammonia. Bioresour Technol 200:606–615
Tokuşoglu Ö, üUnal MK (2003) Biomass nutrient profiles of three microalgae. J Food Sci 68:1144–1148
Vitova M, Bisova K, Kawano S, Zachleder V (2015) Accumulation of energy reserves in algae: From cell cycles to biotechnological applications. Biotechnol Adv 33:1204–1218
Xia A, Murphy JD (2016) Microalgal Cultivation in Treating Liquid Digestate from Biogas Systems. Trends Biotechnol 34:264–275
Zhu L, Wang Z, Shu Q, Takala J, Hiltunen E, Feng P, Yuan Z (2013) Nutrient removal and biodiesel production by integration of freshwater algae cultivation with piggery wastewater treatment. Water Res 47:4294–4302
Acknowledgements
The authors are grateful to the Institute of Marine Sciences, University of Dar es Salaam, for financial support through the Sida (Swedish International Development Cooperation Agency) supported Bilateral Marine Sciences Program.
Involvement of human and animals in the study
N/A
Funding
The study was funded by the Swedish International Cooperation Agency (Sida) through the Bilateral Marine Science Program of 2015-2020 (grant no. 51170071).
Author information
Authors and Affiliations
Contributions
Conceptualization, Kulwa Mtaki, Margareth S. Kyewalyanga and Matern S.P. Mtolera; experimental design, Kulwa Mtaki, Margareth S. Kyewalyanga and Matern S.P. Mtolera; data analysis, Kulwa Mtaki; manuscript drafting and writing, Kulwa Mtaki, Margareth S. Kyewalyanga and Matern S.P. Mtolera; funding acquisition, Margareth S. Kyewalyanga and Matern S.P. Mtolera; supervision, Margareth S. Kyewalyanga and Matern S.P. Mtolera. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
N/A
Competing interests
The authors declare to have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mtaki, K., Kyewalyanga, M.S. & Mtolera, M.S.P. Supplementing wastewater with NPK fertilizer as a cheap source of nutrients in cultivating live food (Chlorella vulgaris). Ann Microbiol 71, 7 (2021). https://doi.org/10.1186/s13213-020-01618-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13213-020-01618-0