Abstract
Latin America is a fast-growing region that currently faces unique challenges in the treatment of all forms of diabetes mellitus. The burden of this disease will be even greater in the coming years due, in part, to the large proportion of young adults living in urban areas and engaging in unhealthy lifestyles. Unfortunately, the national health systems in Latin-American countries are unprepared and urgently need to reorganize their health care services to achieve diabetic therapeutic goals. Stem cell research is attracting increasing attention as a promising and fast-growing field in Latin America. As future healthcare systems will include the development of regenerative medicine through stem cell research, Latin America is urged to issue a call-to-action on stem cell research. Increased efforts are required in studies focused on stem cells for the treatment of diabetes. In this review, we aim to inform physicians, researchers, patients and funding sources about the advances in stem cell research for possible future applications in diabetes mellitus. Emerging studies are demonstrating the potential of stem cells for β cell differentiation and pancreatic regeneration. The major economic burden implicated in patients with diabetes complications suggests that stem cell research may relieve diabetic complications. Closer attention should be paid to stem cell research in the future as an alternative treatment for diabetes mellitus.
Similar content being viewed by others
Background
Diabetes mellitus (DM), in all its forms, is a metabolic disorder that occurs due to deficient production of insulin by the pancreas. Physiological control of blood glucose levels can be restored in a number of ways: exogenous administration of insulin, medications that stimulate insulin, medications that decrease insulin resistance and/or replace the β cell mass (the producers of insulin) [1]. Pancreatic regeneration of the lost functional β cell mass is an attractive strategy for recovery from the disease. Current approaches utilized for pancreatic replacement of damaged β cells include cadaveric islet transplantation, induction of endogenous regeneration and administration of stem cell-derived β cells [2]. Transplantation of pancreatic islets has proven to be successful for functional replenishment of damaged islets [3, 4]. However, to achieve sustained metabolic control for 1 year, at least 2 million β cells per kg body weight need to be transplanted [5], resulting in a limited availability of healthy islets for this application. The increasing success rate of deriving glucose responsive β-like cells from human stem cells encouraged a new era of β cell replacement therapy, as stem cell therapy could potentially deliver 100–200 million β cells per graft. The current epidemiologic burden of diabetes, globally and especially in Latin America, urges the scientific community to target the key influencers of endogenous β cell regeneration that may be applied to increase the successful rate of differentiated functional β cells, with the hope that in the near future, the load of diabetic patients will be ameliorated through stem cell therapy. Latin America, although at a slow pace, has made important advances in stem cell research. The epidemiology and costs incurred by DM in the region are motivating local stem cell researchers to focus their efforts in developing optimal strategies for obtaining stem cell-derived β cells, which will be the topic of this review.
Epidemiology and costs of diabetes mellitus: Latin-American perspective
Globally, more than 415 million people were living with DM in 2015 [6]. A 31.1% increase in diabetes-related deaths was reported from 2006 to 2016, leading to 1.43 million deaths in 2016 [7]. In addition, between these years, disability-adjusted life years and years of life lost increased by 24.4 and 25.3%, respectively, reflecting a true global pandemic [8, 9].
According to estimates from the Global Burden of Disease, the burden of DM is greater than expected in Latin America and the Caribbean region [9]. This disease is estimated to affect 10–15% of the adult population in the Caribbean [10] and 9.4% in South and Central-American regions [6]. Recent estimates for DM in Latin America are summarized in Table 1. Furthermore, a strong relationship between socioeconomic deprivation and DM has been consistently reported in several studies, mirroring the social determinants of health in this metabolic disease [9, 11,12,13,14]. Recently, a population-based cross sectional study from the Southern Cone of Latin America reported prevalence estimates of DM between 8.4 and 14.3%, with 20% of these cases going undiagnosed [15].
Diabetes is a chronic disease with one of the highest costs to the healthcare system due to its multiple health hazards, high incidence of cardio-metabolic comorbidities, and disabilities that impair individual productivity [16, 17]. Approximately 7% of patients living with DM face costly long-term complications, many of which can be avoided or delayed [18, 19]. Currently, Latin America faces elevated out-of-pocket medical payments [20, 21]. In 2015, The Pan-American Health Organization reported that the average cost of diabetes care per year could range between US $1088 and US $1818, a high amount compared to the gross domestic profit in Latin-American countries [17]. The Prospective Urban and Rural Epidemiological Study revealed that the availability and affordability of essential diabetes medicines are insufficient in low-income and middle-income countries [22]. The current economic burden that diabetes represents prompts scrutiny of the clinical aspects of this pathology for the development of cost-effective treatment strategies.
Clinical aspects and treatment of diabetes mellitus
Diabetes is an endocrine disorder characterized by hyperglycemia resulting from variable degrees of insulin resistance and/or deficiency [23, 24]. Several forms of diabetes have been described (Table 2). Treatment strategies for diabetes depend on, among other factors, the type of diabetes diagnosed and the severity of the pathology.
Diabetic treatment encompasses an array of lifestyle and pharmaceutical interventions aimed at the prevention of disease progression, hyperglycemia control and mitigation of its micro and macrovascular complications. The treatment options in Latin America include lifestyle modification, hypoglycemic agents and insulin administration [25]. For the past 10 years, several new types of hypoglycemic agents have emerged in the market [26, 27], including metformin, alpha-glucosidase inhibitors, colesevelam, bromocriptine, sulfonylureas thiazolidinediones, dipeptidyl peptidase IV (DPP-4) inhibitors, meglitinide analogs, sodium-glucose cotransporter 2 (SGLT2) inhibitors, and a glucagon-like peptide-1 (GLP-1) receptor agonist. Insulin administration can comprise a simple injection or a more sophisticated insulin pump and closed loop system. However, none of these strategies are able to perfectly control blood glucose, eventually leading to complications. Advances in the development of new therapeutic options, through stem cells, will open the possibility of reversing hyperglycemia and alleviating the many debilitating complications of diabetes. A clear understanding of the pancreatic development and its mechanism of regeneration is critical for the discovery of appropriate treatment strategies for diabetes.
Insights into pancreatic regeneration: moving beyond conventional diabetes mellitus treatments through stem cell therapy
The pancreas is the major organ that systematically regulates glucose homeostasis. Pancreatic development involves the specific interplay of factors that, among other mechanisms, influence stem cell differentiation into pancreatic progenitor cells and the formation of the fully functional organ. Thus, most stem cell-based differentiation protocols are focused on the generation of mature, single hormone-expressing, glucose-responsive human β cells using information from studies of pancreatic development [28, 29]. Specific signals are involved in the programming of insulin-producing β cells. The transcription factors SRY (sex determining region Y)-box (Sox)17 and homeobox gene HB9 (Hlxb9) are involved in the formation of the endoderm during gastrulation. Following foregut formation, fibroblast growth factor (FGF)-10, retinoic acid, SOX9, and hedgehog signaling pathways induce development of the pancreas. Pancreatic specification and budding then occur through pancreas-specific transcription factor-1a (Ptf-1a), pancreatic and duodenal homeobox 1 (PDX-1), NK6 homeobox 1 (Nkx6.1), neurogenin-3 (Ngn-3), and mafA [30], enabling endocrine formation and consequent stimulation of ISL LIM homeobox 1 (Isl-1), NK2 homeobox 2 (Nkx2.2), neurogenic differentiation factor (NeuroD), paired box gene (Pax)4, and Pax6 signaling that form the islets of Langerhans. The transcription factors Sox17, hepatocyte nuclear factor (HNF)-6, and HNF-3beta (also known as forkhead box A2, Foxa2) are expressed throughout pancreatic development. Finally, FGF-10 and notch signaling-induced stem cell and pancreatic progenitor cell differentiation stimulate neogenesis to create β cells [31, 32].
Discovery of the key factors involved in β cell development has given rise to strategies for obtaining β cells, either by inducing the expression of pancreatic-related transcription factors in distinct types of stem cells or by supplementation of soluble factors during culture. Diverse stem cell models have been used for the successful differentiation of β cell in vitro, including embryonic stem cells, induced pluripotent stem cells, mesenchymal stem cells, and progenitor cells.
Embryonic stem cells
The best model for pancreatic regeneration studies has been obtained from the use of embryonic stem cells (ESCs). Transgenic expression of PDX-1 and Nkx6.1 was shown to induce the differentiation of ESCs into endocrine cells that are positive for insulin, somatostatin, and glucagon expression [33]. Growth and extracellular matrix factors, including laminin, nicotinamide and insulin, lead to the formation of ESC-derived C-peptide/insulin-positive islet-like cell clusters that release insulin upon glucose stimulation and express Pax4 [34]. Retinoic acid (RA) has important roles in pancreatic development and is widely used to induce pancreatic differentiation of ESCs. When RA is directly added to activin A-induced human ESCs expressing CXCR4, 95% of cells become positive for the pancreatic marker PDX-1 [35]. Animal studies have shown that human ESC-derived glucose-responsive mature β cells encapsulated in alginate and transplanted into a streptozotocin (STZ)-induced diabetic mouse model result in effective glycemic control [36]. However, the ethical implications involved with the use of ESCs have limited their further clinical application. In this respect, induced pluripotent stem cells have been proposed as a suitable alternative cell source with the same pluripotent characteristics as ESCs.
Induced pluripotent stem cells
Human induced pluripotent stem cells (iPSC) are obtained by reprogramming human somatic cells for the generation of stem cells with pluripotent properties. Human iPSCs have been shown to be an effective cell source for deriving glucose responsive β-like cells [37,38,39,40]. Given the complex processes involved in β cell development, it has been difficult to obtain an efficient and replicable β cell differentiation protocol. A potential solution has been proposed to start differentiation from human iPSC-derived PDX-1 and SOX9-expressing pancreatic progenitor cells, which have a prolonged proliferation potential and the ability to produce C-peptide positive β cells [41]. Another efficient differentiation protocol consisted of supplementation of factors involved in epidermal growth factor (EGF), transforming growth factor β (TGF-β), thyroid hormone, and RA signaling, as well as ɤ-secretase inhibition [38], resulting in β cells with the ability to induce Ca2+ flux in response to glucose, package insulin into secretory granules, and secrete insulin. It has recently been reported that supplementation of sodium cromoglicate in combination with a previously described protocol causes the induction rate of insulin-positive cells to increase from a mean ± SD of 5.9 ± 1.5% (n = 3) to 16.5 ± 2.1% (n = 3), with increased expression of Ngn-3-positive cells at a mean ± SD of 32.6 ± 4.6% (n = 3) compared to 14.2 ± 3.6% (n = 3) for the non-supplemented control group [42]. Utilization of iPSCs for therapeutic applications involves other major challenges, including recurrent autoimmune attacks in type 1 diabetes, the inherent risk of placing foreign tissue in the body, and potential tumor formation from cells that are not fully differentiated [2]. Fortunately, the use of mesenchymal stem cells has the potential to overcome these barriers.
Mesenchymal stem cells
Another attractive strategy for obtaining β cells is adult stem cells. Mesenchymal stem cells (MSCs) are considered the most attractive cell source for regenerative medicine. MSCs have been highlighted because of their multi-potentialities, including self-renewal ability, pluripotency, low antigenicity, reduced toxicity, and ease of culture and expansion in vitro to obtain sufficient cells for treatment. These cells are localized in diverse parts of our body, including the bone marrow, adipose tissue, amniotic fluid, umbilical cord blood, and placenta. We have demonstrated that adipose and placenta-derived MSCs (PDMSCs) can be expanded for several passages without losing their self-renewal capacity [43, 44]. The International Society for Cellular Therapy has provided criteria for defining MSCs. As has been previously demonstrated, MSC populations are composed of multipotent cells that are able to adhere to plastic in culture; express the cell surface markers CD105, CD73, and CD90 [45]; lack expression of CD45, CD34, CD14 or CD11b, CD79a, or CD19 and HLADR surface molecules [46]; and have the ability to differentiate into osteoblasts, adipocytes, or chondrocytes [44, 45]. MSCs have also been shown to be able to differentiate into cell types of endodermal and ectodermal lineages [47], including renal tubular cells [48], skin [49], neural cells [50], hepatocytes [51], and insulin-producing cells (IPCs) [52].
MSCs are being extensively investigated in the clinical setting for their immunomodulatory and tissue regenerative properties, as well as their feasibility in the context of islet transplantation [53, 54], demonstrating improved engraftment of pancreatic islets through the suppression of inflammatory damage and immune-mediated rejection. MSC immunomodulatory properties may be mediated through cell–cell interactions and/or secretion of soluble factors [55]. The cell-mediated immune response in MSCs induces T cell activation and leukocyte recruitment to the inflammatory site through CD106. PDMSCs isolated from the chorionic villi have been shown to contain a population of CD106+ cells with unique immunoregulatory properties that activate T helper cells and induce tumor necrosis factor (TNF)-α/interleukin (IL)-1b-mediated MSC expansion [56]. PDMSCs secrete soluble factors that mediate immunosuppressive functions through inhibition of lymphocyte proliferation by TGF-β1, hepatocyte growth factor (HGF), prostaglandin E2 (PGE2), and IL-1β and through inhibition of monocyte differentiation into macrophages or dendritic cells through IL-6, IL-10, and macrophage colony stimulating factor [57]. Umbilical cord-derived MSCs (UC-MSCs) cocultured with a hepatoma cell line effectively alleviated palmitic acid and lipopolysaccharide-induced insulin resistance by blocking NLRP3 inflammasome activation and inflammatory agents [58]. When UC-MSCs were infused into type 2 diabetic rats, hyperglycemia was significantly ameliorated, and inflammatory activity was reduced, resulting in improved insulin sensitivity in insulin target tissues. Similarly, in adipose-derived MSCs (AD-MSCs), infusion into diabetic NOD mice reversed hyperglycemia through inducing higher serum insulin, amylin, and glucagon-like peptide 1 levels compared to untreated controls. AD-MSC treatment also reduced CD4+ T helper (Th) 1 cells, interferon-γ, and inflammatory cell infiltration, as well as expanded Tregs in a cell contact-dependent manner in vitro and within the pancreas [59]. Administration of bone marrow-MSC-derived extracellular vesicles into mice resulted in the inhibition of antigen-presenting cell activation and suppression of Th1 and Th17 cell development, inhibiting the onset of type 1 diabetes [60]. Other studies have reported the immunomodulatory properties of bone marrow-derived MSCs (BM-MSCs) in islet xenotransplantation, as evidenced by reduced inflammatory markers and increased immune tolerance markers, demonstrating the potential of this strategy in solving transplantation issues of immune-related graft rejection, as described in more detail in the following sections [61]. Interestingly, a recent study in a UC-MSC model demonstrated that MSC-derived IPCs exhibited hypo-immunogenic characteristics in vitro but became immunogenic after transplantation to the host, possibly due to activation from the immune microenvironment [62].
In addition to their immunomodulatory effects, MSCs provide a supportive micro-environmental niche by secreting paracrine factors and depositing extracellular matrix [63]. Evidence has suggested a supportive role for MSCs in the regeneration of endogenous β cells. Studies have demonstrated that BM-MSCs from mice can differentiate in vitro into IPCs and that the differentiated cells express pancreas-specific marker genes [64]. Through genetic manipulation, overexpression of PDX-1 in human BM-MSCs results in differentiation into IPCs [65]. Human BM-MSCs transfected with three genes, PDX-1, Neuro D, and Ngn-3, differentiate into insulin-expressing cells in vitro but lack glucose-responsive insulin expression. However, transplantation of these differentiated cells reduced blood glucose levels in diabetic mice. Interestingly, differentiated IPCs from AD-MSCs intraportally infused into patients exhibited a 30–50% decrease in their insulin requirement, with a 4- to 26-fold increase in serum C-peptide levels [66]. Umbilical cord blood derived embryonic stem cell-like cells that express stage specific antigen 4 (SSEA4) and octamer 4 (Oct4) can differentiate into insulin-producing islet-like cells that express insulin and C-peptide protein [67].
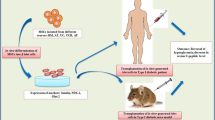
Despite the success of the differentiation protocols described in this review, none of these protocols are reproducible for the production of fully functional mature β cells yet. Additional research for the development of more sophisticated differentiation protocols is still required to apply these strategies clinically. Nonetheless, the successful generation of glucose-responsive IPCs through supplementation of crucial factors to the cell-culture medium gives hope for a diabetes treatment derived through stem cell-based cell therapy in the future (Fig. 1). Hence, due to their ease of isolation, immunomodulatory and tissue regenerative properties and the supportive niche they provide by secreting micro-environmental factors and depositing of extracellular matrix, MSCs are suggested to be a suitable stem cell resource for deriving in vitro β cells and for immunomodulation that may prevent graft rejection and autoimmune destruction of β cells.
Factors that promote stem cells to induce pancreatic regeneration. Inducible factors that promote ESCs, iPSCs, MSCs, and pancreatic progenitor cells differentiation into β cells, includes among others, PDX-1, NGN-3, Laminin, retinoic acid, among others. iPSCs induced pluripotent stem cells, ESCs embryonic stem cells, MSCs mesenchymal stem cells, PPCs pancreatic progenitor cells
Progenitor cells
Identification of progenitor cells in the adult pancreas has received increasing attention due to their pancreatic lineage characteristics that enable them to generate new functional β cells. When pancreatic progenitor cells were induced to differentiate into islets in vitro and transplanted into STZ-induced mice, progenitor cells directly migrated into the injured pancreas, rapidly differentiating into IPCs that decreased glucose levels towards normoglycemia [68]. A recent study demonstrated that progenitor cells expressing Ngn-3, which is expressed at extremely low levels in normal postnatal pancreatic tissues, exists in the ducts of adult mouse pancreas. Ectopic expression of Ngn-3 in pancreatic ductal cells converted them into IPCs, and treatment of human ductal and acinar cells with a combination of epidermal growth factor and gastrin induced neogenesis of islet β cells from the ducts, increasing the functional β cell mass [69]. In other studies, co-transplantation of purified human non-endocrine pancreatic epithelial cells with human fetal pancreatic tissue under the kidney capsule of immuno-deficient mice resulted in their differentiation into endocrine cells. Fetal cells seem to provide factors that support the survival and differentiation of epithelial cells. Stem cell-like cells with the ability to be expanded and form clones ex vivo have also been reported. These cells have the ability to proliferate and form cellular aggregates that display the capacity for endocrine and exocrine differentiation [70]. These results suggest that stem/progenitor cells exist within the pancreas and that these cells might be a source for new islets. However, identification of specific markers is urgently needed for isolation of these cell populations.
Transplantation of stem cell-derived pancreatic cells
Several types of stem cell-derived pancreatic cells have been proposed for transplantation into diabetic models, including pancreatic progenitors and insulin-secreting cells. As endocrine progenitors differentiate, they migrate cohesively and form bud-like islet precursors. Increasing evidence indicates that proper glucose regulation requires coordination between various islet cell types; therefore, it may be advantageous to produce whole islets in vitro rather than differentiating cells into a specific cell type. A recent study demonstrated obtaining islet precursors from embryonic stem cells, proposing this model to be optimal for obtaining whole islet populations [71].
When conditioned to mature in vivo, transplanted pancreatic progenitor cells produce insulin-secreting cells that prevent or reverse diabetes after transplantation. Transplantation of stem cell-derived pancreatic progenitors on scaffolds that release exendin-4 has been reported to promote the engraftment of stem cell-derived pancreatic progenitors and their maturation toward insulin producing β cells, significantly increasing C-peptide levels and reducing blood glucose in STZ-induced mice [72]. Chronic hyperglycemia and an immunodeficient environment accelerate the maturation of transplanted progenitor cells under the kidney capsule in mice [73, 74]. Pancreatic progenitor cell-to-cell contact before transplantation is crucial for maturation into IPCs in vivo [75]. Nevertheless, in vivo maturation remains a critical issue to be resolved. It is expected that mature endocrine cells generated in vitro would reverse diabetes more rapidly than pancreatic progenitor cells after transplantation. The development of novel techniques is required for in vitro differentiation protocols that may efficiently direct progenitor cells further down the β cell development pathway.
Use of TMP269, a histone inhibitor, in endocrine precursor-like cells derived from Wharton jelly-derived MSCs significantly improved differentiation toward IPCs, as evidenced by the increased expression of PAX4, β- and Δ cells-related genes, and increased secretion of insulin at the end of maturation [76]. Another report describes an improved iPSC-generated endocrine pancreas precursor differentiation protocol that generated populations of greater than 60% insulin-expressing cells that secrete insulin in response to glucose and are capable of reversing diabetes in rodents [77]. In vitro administration of GLP-1 and perGLP-1, the native form of GLP-1, stimulate the differentiation of β cell precursors isolated from mice with diabetes into insulin-producing β cells [78, 79]. Preadipocyte factor 1 (Pref-1) is involved in the proliferation and differentiation of various precursor cells. Overexpression of Pref-1 activates MAPK/AKT signaling, which induces the differentiation of human pancreatic ductal cells into β-like cells with increased insulin synthesis and secretion, and improves glucose homeostasis by accelerating pancreatic ductal and β cell regeneration after injury in a pancreatectomized diabetic animal model [80]. When isolated MSC-like cells from adult mouse pancreas were exposed to an islet differentiation serum-free medium, significant upregulation of the pancreatic markers, Nkx2.2, Nkx6.1, Pdx1, insulin, and somatostatin was observed accompanied by increased insulin secretion over days in culture after glucose exposure [81]. Similarly, incubation of human pancreatic anlage cells with mature β cells resulted in the differentiation of pancreatic anlage into mature β cells. Induced cells acquired the features of mature cells, including increased expression of glucose transporter-2, insulin secretion in response to glucose in vitro, and corrected hyperglycemia in vivo when co-transplanted with vascular cells [82]. Studies have also demonstrated the immunoprophylactic effects of precursors on IPCs. Culture of MSCs in high-glucose media resulted in the generation of precursors to β-like cells with the ability to further differentiate into mature IPCs [12]. Precursor to IPC differentiation seems to more efficiently arrest the autoimmune response in type 1 diabetes when administered before the onset of the disease in NOD mice compared to differentiated IPCs [83].
Stem cells treatment for diabetic complications
In addition to the generation of IPCs from renewable stem cells, their immunomodulatory, self-renewal, and differentiation properties also suggests MSCs as potentially new therapeutic candidates in the treatment of diabetic-related complications [84]. Retinopathy, critical limb ischemia, and nephropathy are the most common and deleterious diabetic-related complications. Advances in stem cell research have shown the reversal of these complications through stem cell transplant, which will be reviewed below.
Critical limb ischemia
Peripheral arteriopathy in diabetic patients remains a serious health problem, despite enormous clinical and surgical advances over the last few decades. Stem cell therapy may be a good alternative to major amputation for restoring blood flow and attenuating ischemic disease. Induction of diabetic animals with streptozotocin is a well-defined methodology for the study of peripheral arteriopathy in diabetic models. Our laboratory has established this methodology and demonstrated that a formulated matrix gel induces regenerative neovasculogenesis in the ischemic region (unpublished). AD-MSCs secrete angiogenic and cell survival factors and have been shown to be effective in the treatment of both coronary disease and complications of diabetes in animal and human models [85, 86]. MSC transplantation improves diabetic neuropathy via promoting direct peripheral nerve angiogenesis, neurotrophic effects, and restoration of myelination [87]. A recently reported GMP-compatible protocol described the generation of human ESC-derived endothelial cell products that improve ischemic limb perfusion and local angiogenesis [88]. PDMSC injection into STZ-treated mice demonstrated newly formed capillaries, increased arterioles, and the secretion of proangiogenic factors that promoted ischemic recovery [89].
Retinopathy
Diabetic retinopathy (DR) is a microvascular complication caused by hyperglycemia in which the retinal blood vessels weaken and rupture due to the chronic degeneration of retinal nerve tissue. Retinal glial cells and pericytes are the earliest-damaged cells with the highest rate of cell death during disease progression, for which cell replacement therapy will be more effective compared to conventional localized treatments. Stem cells have been studied for nerve regeneration in the retina [90]. Recent studies have demonstrated that intravitreal transplantation of neural stem cells originating from human UC-MSCs in DR rats resulted in the long-term preservation of retinal function and significantly delayed the progression of DR for up to 8 weeks with the restoration of vision [91]. It was previously demonstrated that stem cells have protective effects against retinal vasculopathy by preventing capillary loss and retinal capillary dropout in an STZ-induced rodent model of DR [92]. A single intravitreal dose of adipose-derived stem cells along with its secreted paracrine factors was shown to therapeutically improve retina damage through neurovascular repair that led to improved vision [93]. An efficient protocol was reported for generating highly purified human ESC-derived perivascular progenitor cells that demonstrated pericyte marker expression, neural differentiation potential, and improved damaged retinal vasculature after transplantation into a STZ-induced rodent model [94]. In recent clinical studies, autologous BM-MSCs intravenously infused into patients’ eyes exhibited reduced fasting blood glucose, decreased macular thickness reduction, and improved the best corrected visual acuity [95]. Taken together, these studies demonstrate the potential of stem cell-based therapies in the treatment of retinovascular diseases.
Nephropathy
Diabetic nephropathy is the most common cause of end-stage renal disease and is characterized by alterations of the renal structure and function, including changes in renal tubules, stromal cells, and the incidence of glomerular filtration. MSCs have been shown to relieve diabetic nephropathy through renal tissue repair, modulation of the immune response, and exertion of anti-fibrotic effects [96]. The paracrine effects of MSCs have been shown to increase the regeneration speed of renal tissue during diabetic nephropathy compared to the ability of MSCs to differentiate into renal cells [97]. MSC-derived exosomes improved renal function and repaired renal tissue through autophagic mechanisms in an STZ-induced rat model [98, 99]. In addition, when MSCs are infused in combination with microRNA-124, the results demonstrate the attenuation of renal impairment as well as the inhibition of nephrocyte apoptosis during diabetic nephropathy [100]. Injections of BM-MSCs at the early stages of diabetic nephropathy suppress renal macrophage and cytokine infiltration in diabetic rats, which prevented kidney dysfunction and glomerular defects [101].
Advances in stem cell research for diabetes treatment in Latin America
Stem cell research in Latin America, although moving at a slow pace, has produced important advances in the establishment of well-equipped laboratories led by specialized local scientists and physicians. Significant advances have been made in stem cell research in Latin America for DM. Potential application of MSCs in the treatment of diabetic neuropathy was demonstrated after observing that preconditioning of human AD-MSCs with increasing concentrations of an iron chelator, deferoxamine, increased the abundance of hypoxia inducible factor 1 alpha, leading to upregulation of pro-angiogenic and neuroprotective factors [102]. On the other hand, studies on diabetic nephropathy from Chile demonstrated that intravenously administered BM-MSCs allowed for pancreatic islet recovery, improved insulin secretion and reversed hyperglycemia in low dose STZ-induced diabetic mice [103]. Further Chilean collaborations with Argentina demonstrated that administration of MSCs to diabetic-induced mice restored pro-regenerative factors, increased the renal proliferation index and anti-inflammatory cytokines levels, and reduced the renal apoptotic index, macrophage infiltration, and oxidative stress damage, resulting in preserved renal function and structure in mice with severe DM. MSC administration completely prevented retinal ganglion cell loss, improving diabetic retinopathy [104]. Donor cells remained in the vitreous cavity and did not differentiate into neural or perivascular-like cells. Nevertheless, they increased the intraocular levels of several potent neurotrophic factors (nerve growth factor, basic fibroblast growth factor and glial cell line-derived neurotrophic factor) and reduced oxidative damage in the retina. MSC administration in diabetic-induced mice showed significant improvement in the functional parameters of kidneys with diabetic nephropathy, with an improved renal proliferation index, decreased renal apoptotic index and restoration of pro-regenerative factors and anti-inflammatory cytokine levels [105, 106]. By contrast, intravenous administration of BM-MSCs neither improved nor impaired diabetic cardiomyopathy in an obesity-induced mouse model [107]. Another research collaboration between Chile and Colombia showed that administration of allogenic BM-MSCs was insufficient for wound healing in diabetic mice, resulting in a delayed therapeutic effect, potentially explained by trophic factors secreted by MSCs being critical for skin regeneration and not the cells per se, suggesting that MSCs may require time to secrete these factors after their administration [108]. In this respect, they showed that MSCs have the capability of restoring the balance between Th1 and Th2 immunological responses along with modification of the pancreatic microenvironment [109].
Pre-clinical animal studies in Brazil demonstrated that betacellulin (BTC), a ligand of the epidermal growth factor receptor, promotes the growth and differentiation of pancreatic β cells and improves glucose metabolism in experimental diabetic rodent models [110]. When MSC-BTC was transplanted into STZ diabetic rats, BTC-transfected cells ameliorated hyperglycemia from over 500 to approximately 200 mg/dL at 35 days post-cell transplantation. Administration of BM-MSCs into diabetic mice reversed hyperglycemia, improved β cell function, and modulated pancreatic cytokine levels [111]. Transplantation of stem cells obtained from murine dental pulp into STZ-induced type 1 diabetic mice improved pancreatic damage and renal function during diabetic neuropathy [112]. AD-MSC treatment reversed hyperglycemia in early-onset diabetes in 78% of diabetic NOD mice, and this effect was associated with higher serum insulin, amylin, and glucagon-like peptide 1 levels compared to untreated controls. In addition, AD-MSC treatment ameliorated autoimmune diabetes pathogenesis in diabetic NOD mice by attenuating the Th1 immune response concomitant with the expansion/proliferation of Tregs, thereby contributing to the maintenance of functional β cells [59]. Co-transplantation of rat-derived BM-MSCs with pancreatic islets into mice resulted in reduced expression of inflammatory markers, such as TNFs, chemoattractant protein 1, and IL-1b, along with increased immune tolerance markers, IL-4, IL-10, and forkhead box P3, demonstrating the immunomodulatory actions of BM-MSC [61].
Brazil launched clinical trials testing the ability of autologous BM-MSCs to reverse diabetes, stroke and heart conditions. Seventeen clinical trials in progress are utilizing AD-MSCs, especially in cardiology, orthopedics, diabetes and neurology. High-dose immunosuppression and hematopoietic stem cell (HSCs) transplant performed with acceptable toxicity in a small number of patients with newly diagnosed type 1 DM has shown increased β cell function in all but 1 of 18 patients with prolonged insulin independence in the majority of patients [113]. Administration of autologous HSCs into 21 type 1 diabetic patients resulted in all patients becoming insulin independent for a period of 43 months with induced immunoregulation that consisted of lower CD3+CD4+ T cell numbers and consistent CD3+CD8+ T-cell levels, resulting in a CD4/CD8 ratio inversion. Memory cytotoxic T cells comprised the majority of T cells detected, and B cells returned to baseline levels 2–3 months post-transplantation. Baseline islet-specific T-cell autoreactivity persisted after transplantation, but regulatory T cell counts increased. Patients with lower frequencies of autoreactive islet-specific T cells remained insulin-free longer and presented greater C-peptide levels than those with lower frequencies of these cells [114].
Mexico reported that administration of autologous HSCs demonstrated increased C-peptide synthesis and insulin independence in most type 1 DM patients, with a decrease in HbA1c levels [115]. In addition, a protocol to isolate PDX-1-expressing IPCs from MSCs was developed by inducing expression of Nestin in MSCs, followed by a short incubation of 24 h in low glucose medium, and finally, a longer incubation of 168 h in high glucose medium [116]. Another study reported full ulcer recovery of a patient with chronic foot ulcer after MSC transplantation [117].
Findings from Argentina demonstrated that the combined therapy of intra-pancreatic AD-MSC infusion and hyperbaric oxygen improved metabolic control and reduced insulin requirements in patients with type 2 DM [118]. In general, Argentina’s governmental support is strong and a driving force in stem cell research. Currently, 0.65% of the country’s GDP is invested in science and technology, which is a model that should be followed in additional Latin-American countries.
The latest strategy for the restoration of the β cell mass is through the generation and transplantation of stem cell-derived β cells [119], indicating that related research will be beneficial to Latin America (Table 3). Our shared vision is that these countries will maintain their effort in promoting innovative excellent research, establishing or improving regulations to the highest international level, increasing regional and international cooperation and identifying country- or region-specific opportunities to collaborate worldwide without diminishing identity or sovereignty.
Conclusion
Diabetes is a global health and economic burden in which the disability-adjusted life years and years of life lost represent a problematic issue in Latin America. A unique set of challenges exist for DM treatment, as the diabetic prevalence has increased over the years. Latin America urgently needs to reorganize its health care services to optimize diabetes therapeutic goals. Recent breakthroughs in deriving glucose responsive β-like cells from human stem cells has provided encouragement for β cell replacement therapy. More evidence is demonstrating the potential for embryonic stem cells, adult stem cells, and progenitor cells to produce β cells with the ability to produce insulin, reduce glucose levels in animal models, and to some extent, reverse diabetes symptoms through pancreatic regeneration. Stem cell research groups in Latin America have focused their efforts and provided important contributions to the DM field. Success in the generation of glucose-responsive IPCs and MSC-induced immunomodulation gives hope for the development of improved diabetic treatments through stem cell-based cell therapy in the near future.
Abbreviations
- DM:
-
diabetes mellitus
- MODY:
-
maturity-onset diabetes of the young
- LADA:
-
latent autoimmune diabetes of the adult
- DPP-4:
-
dipeptidyl peptidase IV
- SGLT2:
-
sodium-glucose cotransporter 2
- GLP-1:
-
glucagon-like peptide-1
- NPH:
-
neutral protomaine hagedorn
- Sox:
-
SRY (sex determining region Y)-box
- Hlxb9:
-
homeobox gene HB9
- FGF-10:
-
fibroblast growth factor 10
- Ptf-1a:
-
pancreas-specific transcription factor-1a
- PDX-1:
-
pancreatic and duodenal homeobox 1
- Nkx6.1:
-
NK6 homeobox 1
- Ngn-3:
-
neurogenin-3
- Isl-1:
-
ISL LIM homeobox 1
- Nkx2.2:
-
NK2 homeobox 2
- NeuroD:
-
neurogenic differentiation factor
- Pax:
-
paired box gene
- HNF:
-
hepatocyte nuclear factor
- Foxa2:
-
forkhead box A2
- ESC:
-
embryonic stem cells
- RA:
-
retinoic acid
- STZ:
-
streptozotocin
- EGF:
-
epidermal growth factor
- TGF-β:
-
transforming growth factor beta
- iPSC:
-
induced pluripotent stem cells
- MSC:
-
mesenchymal stem cell
- PDMSCs:
-
placenta-derived MSCs
- IPCs:
-
insulin-producing cells
- TNF:
-
tumor necrosis factor
- IL:
-
interleukin
- HGF:
-
hepatocyte growth factor
- PGE2:
-
prostaglandin E2
- UC-MSC:
-
umbilical cord-derived MSCs
- Th:
-
T helper
- BTC:
-
betacellulin
- BMSC:
-
bone marrow-derived MSC
- SSEA4:
-
stage specific antigen 4
- Oct4:
-
octamer 4
- DR:
-
diabetic retinopathy
- NSC:
-
neural stem cell
- HSC:
-
hematopoietic stem cell
- PPC:
-
pancreatic progenitor cell
References
Weir GC, Bonner-Weir S, Leahy JL. Islet mass and function in diabetes and transplantation. Diabetes. 1990;39:401–5.
Aguayo-Mazzucato C, Bonner-Weir S. Pancreatic β cell regeneration as a possible therapy for diabetes. Cell Metab. 2018;27(1):57–67.
Ryan EA, Lakey JRT, Rajotte RV, Korbutt GS, Kin T, Imes S, et al. Clinical outcomes and insulin secretion after islet transplantation with the edmonton protocol. Diabetes. 2001;50:710–9.
Shapiro AMJ, Lakey JRT, Ryan EA, Korbutt GS, Toth E, Warnock GL, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–8.
Keymeulen B, Gillard P, Mathieu C, Movahedi B, Maleux G, Delvaux G, et al. Correlation between β cell mass and glycemic control in type 1 diabetic recipients of islet cell graft. Proc Natl Acad Sci. 2006;103:17444–9.
Rodriguez F, Cuero C, Delgado E, Camargo I, Tuñon R. Diagnóstico de la enfermedad renal crónica y factores de riesgo asociados en áreas seleccionadas de la provincia de Coclé, Panamá. Rev Med Panamá. 2014;34:31–8.
The Lancet. Life, death, and disability in 2016. Lancet. 2017;390:1083.
GBD 2016 Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the global burden of disease study 2016. Lancet. 2017;390:1151–210.
GBD 2016 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet. 2017;390:1260–344.
Sobers-Grannum N, Murphy MM, Nielsen A, Guell C, Samuels TA, Bishop L, et al. Female gender is a social determinant of diabetes in the Caribbean: a systematic review and meta-analysis. PLoS ONE. 2015;10:e0126799.
Sacerdote C, Ricceri F, Rolandsson O, Baldi I, Chirlaque MD, Feskens E, et al. Lower educational level is a predictor of incident type 2 diabetes in European countries: the EPIC-InterAct study. Int J Epidemiol. 2012;41:1162–73.
Schneiderman N, Llabre M, Cowie CC, Barnhart J, Carnethon M, Gallo LC, et al. Prevalence of diabetes among Hispanics/Latinos from diverse backgrounds: the Hispanic community health study/study of Latinos (HCHS/SOL). Diabetes Care. 2014;37:2233–9.
Mc Donald Posso AJ, Meza RAB, Morales EAM, Jaen Y, Ortega AC, Posada EJM. Diabetes in Panama: epidemiology, risk factors, and clinical management. Ann Glob Health. 2015;81:754–64.
Motta JA, Ortega-Paz LG, Gordón CA, Gómez B, Castillo E, Herrera Ballesteros V, et al. Diabetes mortality in Panama and related biological and socioeconomic risk factors. Revista Panamericana de Salud Pública. 2013;34:114–20.
Irazola V, Rubinstein A, Bazzano L, Calandrelli M, Chung-Shiuan C, Elorriaga N, et al. Prevalence, awareness, treatment and control of diabetes and impaired fasting glucose in the Southern Cone of Latin America. PLoS ONE. 2017;12:e0183953.
Fowler MJ. Microvascular and macrovascular complications of diabetes. Clin Diabetes. 2008;26:77–82.
Barcelo A, Arredondo A, Gordillo-Tobar A, Segovia J, Qiang A. The cost of diabetes in Latin America and the Caribbean in 2015: evidence for decision and policy makers. J Glob Health. 2017;7:020410.
Bertoldi AD, Kanavos P, França GVA, Carraro A, Tejada CAO, Hallal PC, et al. Epidemiology, management, complications and costs associated with type 2 diabetes in Brazil: a comprehensive literature review. Glob Health. 2013;9:62.
Chatterjee S, Davies MJ, Heller S, Speight J, Snoek FJ, Khunti K. Diabetes structured self-management education programmes: a narrative review and current innovations. Lancet Diabetes Endocrinol. 2018;6:130–42.
Pauly MV, Zweifel P, Scheffler RM, Preker AS, Bassett M. Private health insurance in developing countries. Health Aff. 2006;25:369–79.
Smith-Spangler CM, Bhattacharya J, Goldhaber-Fiebert JD. Diabetes, its treatment, and catastrophic medical spending in 35 developing countries. Diabetes Care. 2012;35:319–26.
Chow CK, Ramasundarahettige C, Hu W, AlHabib KF, Avezum A Jr, Cheng X, et al. Availability and affordability of essential medicines for diabetes across high-income, middle-income, and low-income countries: a prospective epidemiological study. Lancet Diabetes Endocrinol. 2018;6:798–808.
Association AD. Standards of medical care in diabetes-2014. Diabetes Care. 2014;37:S14–80.
Association AD. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37:S81–90.
Hippisley-Cox J, Pringle M. Prevalence, care, and outcomes for patients with diet-controlled diabetes in general practice: cross sectional survey. Lancet. 2004;364:423–8.
Bennett WL, Maruthur NM, Singh S, Segal JB, Wilson LM, Chatterjee R, et al. Comparative effectiveness and safety of medications for type 2 diabetes: an update including new drugs and 2-drug combinations. Ann Intern Med. 2011;154:602–13.
Fradkin JE, Rodgers GP. Glycemic therapy for type 2 diabetes: choices expand, data lag behind. Ann Intern Med. 2017;166:309–10.
Jorgensen MC, Ahnfelt-Ronne J, Hald J, Madsen OD, Serup P, Hecksher-Sorensen J. An illustrated review of early pancreas development in the mouse. Endocr Rev. 2007;28:685–705.
Murtaugh LC. Pancreas and beta-cell development: from the actual to the possible. Development. 2007;134:427–38.
Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129:2447–57.
Bhushan A, Itoh N, Kato S, Thiery JP, Czernichow P, Bellusci S, et al. Fgf10 is essential for maintaining the proliferative capacity of epithelial progenitor cells during early pancreatic organogenesis. Development. 2001;128:5109–17.
Hald J, Hjorth JP, German MS, Madsen OD, Serup P, Jensen J. Activated Notch1 prevents differentiation of pancreatic acinar cells and attenuate endocrine development. Dev Biol. 2003;260:426–37.
Ida H, Akiyama T, Ishiguro K, Goparaju SK, Nakatake Y, Chikazawa-Nohtomi N, et al. Establishment of a rapid and footprint-free protocol for differentiation of human embryonic stem cells into pancreatic endocrine cells with synthetic mRNAs encoding transcription factors. Stem Cell Res Ther. 2018;9:277.
Schroeder IS, Rolletschek A, Blyszczuk P, Kania G, Wobus AM. Differentiation of mouse embryonic stem cells to insulin-producing cells. Nat Protoc. 2006;1:495–507.
Cai J, Yu C, Liu Y, Chen S, Guo Y, Yong J, et al. Generation of homogeneous PDX1+ pancreatic progenitors from human ES cell-derived endoderm cells. J Mol Cell Biol. 2010;2:50–60.
Vegas AJ, Veiseh O, Gürtler M, Millman JR, Pagliuca FW, Bader AR, et al. Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice. Nat Med. 2016;22:306.
Korytnikov R, Nostro MC. Generation of polyhormonal and multipotent pancreatic progenitor lineages from human pluripotent stem cells. Methods. 2016;101:56–64.
Pagliuca Felicia W, Millman Jeffrey R, Gürtler M, Segel M, Van Dervort A, Ryu JH, et al. Generation of functional human pancreatic β cells in vitro. Cell. 2014;159:428–39.
Rezania A, Bruin JE, Arora P, Rubin A, Batushansky I, Asadi A, et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat Biotechnol. 2014;32:1121–33.
Russ HA, Parent AV, Ringler JJ, Hennings TG, Nair GG, Shveygert M, et al. Controlled induction of human pancreatic progenitors produces functional beta-like cells in vitro. EMBO J. 2015;34:1759–72.
Trott J, Tan EK, Ong S, Titmarsh DM, Denil SLIJ, Giam M, et al. Long-term culture of self-renewing pancreatic progenitors derived from human pluripotent stem cells. Stem Cell Rep. 2017;8:1675–88.
Kondo Y, Toyoda T, Ito R, Funato M, Hosokawa Y, Matsui S, et al. Identification of a small molecule that facilitates the differentiation of human iPSCs/ESCs and mouse embryonic pancreatic explants into pancreatic endocrine cells. Diabetologia. 2017;60:1454–66.
Chen PY, Huang LLH, Hsieh HJ. Hyaluronan preserves the proliferation and differentiation potentials of long-term cultured murine adipose-derived stromal cells. Biochem Biophys Res Commun. 2007;360:1–6.
Wong TY, Chang CH, Yu CH, Huang LLH. Hyaluronan keeps mesenchymal stem cells quiescent and maintains the differentiation potential over time. Aging Cell. 2017;16:451–60.
Solis MA, Wei Y-H, Chang C-H, Yu C-H, Kuo P-L, Huang LLH. Hyaluronan upregulates mitochondrial biogenesis and reduces adenoside triphosphate production for efficient mitochondrial function in slow-proliferating human mesenchymal stem cells. Stem Cells. 2016;34:2512–24.
Liu CM, Chang CH, Yu CH, Hsu CC, Huang L. Hyaluronan substratum induces multidrug resistance in human mesenchymal stem cells via CD44 signaling. Cell Tissue Res. 2009;336:465–75.
Figliuzzi M, Bonandrini B, Silvani S, Remuzzi A. Mesenchymal stem cells help pancreatic islet transplantation to control type 1 diabetes. World J Stem Cells. 2014;6:163–72.
Morigi M, Imberti B, Zoja C, Corna D, Tomasoni S, Abbate M, et al. Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J Am Soc Nephrol. 2004;15:1794–804.
Nakagawa H, Akita S, Fukui M, Fujii T, Akino K. Human mesenchymal stem cells successfully improve skin-substitute wound healing. Br J Dermatol. 2005;153:29–36.
Munoz-Elias G, Marcus AJ, Coyne TM, Woodbury D, Black IB. Adult bone marrow stromal cells in the embryonic brain: engraftment, migration, differentiation, and long-term survival. J Neurosci. 2004;24:4585–95.
Schwartz RE, Reyes M, Koodie L, Jiang Y, Blackstad M, Lund T, et al. Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. J Clin Invest. 2002;109:1291–302.
Jun H-S, Park E-Y. Adult stem cells as a renewable source of insulin-producing cells. Int J Stem Cells. 2009;2:115–21.
Sakata N, Chan NK, Chrisler J, Obenaus A, Hathout E. Bone marrow cell cotransplantation with islets improves their vascularization and function. Transplantation. 2010;89:686–93.
Zang L, Hao H, Liu J, Li Y, Han W, Mu Y. Mesenchymal stem cell therapy in type 2 diabetes mellitus. Diabetol Metab Syndr. 2017;9:36.
Lu LL, Liu YJ, Yang SG, Zhao QJ, Wang X, Gong W, et al. Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis-supportive function and other potentials. Haematologica. 2006;91:1017–26.
Yang ZX, Han ZB, Ji YR, Wang YW, Liang L, Chi Y, et al. CD106 identifies a subpopulation of mesenchymal stem cells with unique immunomodulatory properties. PLoS ONE. 2013;8:e59354.
Abumaree MH, Abomaray FM, Alshabibi MA, AlAskar AS, Kalionis B. Immunomodulatory properties of human placental mesenchymal stem/stromal cells. Placenta. 2017;59:87–95.
Sun X, Hao H, Han Q, Song X, Liu J, Dong L, et al. Human umbilical cord-derived mesenchymal stem cells ameliorate insulin resistance by suppressing NLRP3 inflammasome-mediated inflammation in type 2 diabetes rats. Stem Cell Res Ther. 2017;8:241.
Bassi EJ, Moraes-Vieira PM, Moreira-Sa CS, Almeida DC, Vieira LM, Cunha CS, et al. Immune regulatory properties of allogeneic adipose-derived mesenchymal stem cells in the treatment of experimental autoimmune diabetes. Diabetes. 2012;61:2534–45.
Shigemoto-Kuroda T, Oh JY, Kim D-K, Jeong HJ, Park SY, Lee HJ, et al. MSC-derived extracellular vesicles attenuate immune responses in two autoimmune murine models: type 1 diabetes and uveoretinitis. Stem Cell Rep. 2017;8:1214–25.
Corradi-Perini C, Santos TM, Camara NOS, Riella MC, Aita CAM. Co-transplantation of xenogeneic bone marrow-derived mesenchymal stem cells alleviates rejection of pancreatic islets in non-obese diabetic mice. Transplant Proc. 2017;49:902–5.
Yang XF, Chen T, Ren LW, Yang L, Qi H, Li FR. Immunogenicity of insulin-producing cells derived from human umbilical cord mesenchymal stem cells. Exp Ther Med. 2017;13:1456–64.
Hematti P, Kim J, Stein AP, Kaufman D. Potential role of mesenchymal stromal cells in pancreatic islet transplantation. Transplant Rev. 2013;27:21–9.
Tang D-Q, Cao L-Z, Burkhardt BR, Xia C-Q, Litherland SA, Atkinson MA, et al. In vivo and in vitro characterization of insulin-producing cells obtained from murine bone marrow. Diabetes. 2004;53:1721–32.
Karnieli O, Izhar-Prato Y, Bulvik S, Efrat S. Generation of insulin-producing cells from human bone marrow mesenchymal stem cells by genetic manipulation. Stem Cells. 2007;25:2837–44.
Trivedi HL, Vanikar AV, Thakker U, Firoze A, Dave SD, Patel CN, et al. Human adipose tissue-derived mesenchymal stem cells combined with hematopoietic stem cell transplantation synthesize insulin. Transplant Proc. 2008;40:1135–9.
Sun B, Roh KH, Lee SR, Lee YS, Kang KS. Induction of human umbilical cord blood-derived stem cells with embryonic stem cell phenotypes into insulin producing islet-like structure. Biochem Biophys Res Commun. 2007;354:919–23.
Srivastava A, Dadheech N, Vakani M, Gupta S. Pancreatic resident endocrine progenitors demonstrate high islet neogenic fidelity and committed homing towards diabetic mice pancreas. J Cell Physiol. 2018. https://doi.org/10.1002/jcp.27568.
Rooman I, Bouwens L. Combined gastrin and epidermal growth factor treatment induces islet regeneration and restores normoglycaemia in C57Bl6/J mice treated with alloxan. Diabetologia. 2004;47:259–65.
Rovira M, Scott SG, Liss AS, Jensen J, Thayer SP, Leach SD. Isolation and characterization of centroacinar/terminal ductal progenitor cells in adult mouse pancreas. Proc Natl Acad Sci USA. 2010;107:75–80.
Sharon N, Chawla R, Mueller J, Vanderhooft J, Whitehorn LJ, Rosenthal B, et al. A Peninsular structure coordinates asynchronous differentiation with morphogenesis to generate pancreatic islets. Cell. 2019;176:790–804.
Kasputis T, Clough D, Noto F, Rychel K, Dye B, Shea LD. Microporous polymer scaffolds for the transplantation of embryonic stem cell derived pancreatic progenitors to a clinically translatable site for the treatment of type i diabetes. ACS Biomater Sci Eng. 2018;4:1770–8.
Bruin JE, Saber N, Braun N, Fox JK, Mojibian M, Asadi A, et al. Treating diet-induced diabetes and obesity with human embryonic stem cell-derived pancreatic progenitor cells and antidiabetic drugs. Stem Cell Rep. 2015;4:605–20.
Bruin JE, Asadi A, Fox JK, Erener S, Rezania A, Kieffer TJ. Accelerated maturation of human stem cell-derived pancreatic progenitor cells into insulin-secreting cells in immunodeficient rats relative to mice. Stem Cell Rep. 2015;5:1081–96.
Beattie GM, Rubin JS, Mally MI, Otonkoski T, Hayek A. Regulation of proliferation and differentiation of human fetal pancreatic islet cells by extracellular matrix, hepatocyte growth factor, and cell-cell contact. Diabetes. 1996;45:1223–8.
Belame Shivakumar S, Bharti D, Baregundi Subbarao R, Park J-M, Son Y-B, Ullah I, et al. Pancreatic endocrine-like cells differentiated from human umbilical cords Wharton’s jelly mesenchymal stem cells using small molecules. J Cell Physiol. 2019;234:3933–47.
Southard SM, Kotipatruni RP, Rust WL. Generation and selection of pluripotent stem cells for robust differentiation to insulin-secreting cells capable of reversing diabetes in rodents. PLoS ONE. 2018;13:e0203126.
Skurikhin EG, Ermakova NN, Khmelevskaya ES, Pershina OV, Krupin VA, Ermolaeva LA, et al. Differentiation of pancreatic stem and progenitor beta-cells into insulin secreting cells in mice with diabetes mellitus. Bull Exp Biol Med. 2014;156:726–30.
Skurikhin EG, Pakhomova AV, Epanchintsev AA, Stronin OV, Ermakova NN, Pershina OV, et al. Role of β cell precursors in the regeneration of insulin-producing pancreatic β cells under the influence of glucagon-like peptide 1. Bull Exp Biol Med. 2018;165:644–8.
Rhee M, Lee S-H, Kim J-W, Ham D-S, Park H-S, Yang HK, et al. Preadipocyte factor 1 induces pancreatic ductal cell differentiation into insulin-producing cells. Sci Rep. 2016;6:23960.
Gopurappilly R, Bhat V, Bhonde R. Pancreatic tissue resident mesenchymal stromal cell (MSC)-like cells as a source of in vitro islet neogenesis. J Cell Biochem. 2013;114:2240–7.
Chen W, Begum S, Opare-Addo L, Garyu J, Gibson TF, Bothwell AL, et al. Promotion of beta-cell differentiation in pancreatic precursor cells by adult islet cells. Endocrinology. 2009;150:570–9.
Sharma A, Rani R. Do we really need to differentiate mesenchymal stem cells into insulin-producing cells for attenuation of the autoimmune responses in type 1 diabetes: immunoprophylactic effects of precursors to insulin-producing cells. Stem Cell Res Ther. 2017;8:167.
Katuchova J, Harvanova D, Spakova T, Kalanin R, Farkas D, Durny P, et al. Mesenchymal stem cells in the treatment of type 1 diabetes mellitus. Endocr Pathol. 2015;26:95–103.
Kim SW, Han H, Chae GT, Lee SH, Bo S, Yoon JH, et al. Successful stem cell therapy using umbilical cord blood-derived multipotent stem cells for Buerger’s disease and ischemic limb disease animal model. Stem Cells. 2006;24:1620–6.
Williams AR, Trachtenberg B, Velazquez DL, McNiece I, Altman P, Rouy D, et al. Intramyocardial stem cell injection in patients with ischemic cardiomyopathy: functional recovery and reverse remodeling. Circ Res. 2011;108:792–6.
Xia N, Xu JM, Zhao N, Zhao QS, Li M, Cheng ZF. Human mesenchymal stem cells improve the neurodegeneration of femoral nerve in a diabetic foot ulceration rats. Neurosci Lett. 2015;597:84–9.
MacAskill MG, Saif J, Condie A, Jansen MA, MacGillivray TJ, Tavares AAS, et al. Robust revascularization in models of limb ischemia using a clinically translatable human stem cell-derived endothelial cell product. Mol Ther. 2018;26:1669–84.
Liang L, Li Z, Ma T, Han Z, Du W, Geng J, et al. Transplantation of human placenta-derived mesenchymal stem cells alleviates critical limb ischemia in diabetic nude rats. Cell Transplant. 2017;26:45–61.
Ezquer F, Ezquer M, Arango-Rodriguez M, Conget P. Could donor multipotent mesenchymal stromal cells prevent or delay the onset of diabetic retinopathy? Acta Ophthalmol. 2014;92:e86–95.
Chen S, Zhang W, Wang JM, Duan HT, Kong JH, Wang YX, et al. Differentiation of isolated human umbilical cord mesenchymal stem cells into neural stem cells. Int J Ophthalmol. 2016;9:41–7.
Zhang W, Wang Y, Kong J, Dong M, Duan H, Chen S. Therapeutic efficacy of neural stem cells originating from umbilical cord-derived mesenchymal stem cells in diabetic retinopathy. Sci Rep. 2017;7:408.
Elshaer SL, Evans W, Pentecost M, Lenin R, Periasamy R, Jha KA, et al. Adipose stem cells and their paracrine factors are therapeutic for early retinal complications of diabetes in the Ins2Akita mouse. Stem Cell Res Ther. 2018;9:322.
Kim JM, Hong KS, Song WK, Bae D, Hwang IK, Kim JS, et al. Perivascular progenitor cells derived from human embryonic stem cells exhibit functional characteristics of pericytes and improve the retinal vasculature in a rodent model of diabetic retinopathy. Stem Cells Transl Med. 2016;5:1268–76.
Gu X, Yu X, Zhao C, Duan P, Zhao T, Liu Y, et al. Efficacy and safety of autologous bone marrow mesenchymal stem cell transplantation in patients with diabetic retinopathy. Cell Physiol Biochem. 2018;49:40–52.
Li Y, Liu J, Liao G, Zhang J, Chen Y, Li L, et al. Early intervention with mesenchymal stem cells prevents nephropathy in diabetic rats by ameliorating the inflammatory microenvironment. Int J Mol Med. 2018;41:2629–39.
Nagaishi K, Mizue Y, Chikenji T, Otani M, Nakano M, Saijo Y, et al. Umbilical cord extracts improve diabetic abnormalities in bone marrow-derived mesenchymal stem cells and increase their therapeutic effects on diabetic nephropathy. Sci Rep. 2017;7:8484.
Ebrahim N, Ahmed I, Hussien N, Dessouky A, Farid A, Elshazly A, et al. Mesenchymal stem cell-derived exosomes ameliorated diabetic nephropathy by autophagy induction through the mTOR signaling pathway. Cells. 2018;7:226.
Rashed LA, Elattar S, Eltablawy N, Ashour H, Mahmoud LM, El-Esawy Y. Mesenchymal stem cells pretreated with melatonin ameliorate kidney functions in a rat model of diabetic nephropathy. Biochem Cell Biol. 2018;96:564–71.
Sun J, Zhao F, Zhang W, Lv J, Lv J, Yin A. BMSCs and miR-124a ameliorated diabetic nephropathy via inhibiting notch signalling pathway. J Cell Mol Med. 2018;22:4840–55.
Li H, Rong P, Ma X, Nie W, Chen C, Yang C, et al. Paracrine effect of mesenchymal stem cell as a novel therapeutic strategy for diabetic nephropathy. Life Sci. 2018;215:113–8.
Oses C, Olivares B, Ezquer M, Acosta C, Bosch P, Donoso M, et al. Preconditioning of adipose tissue-derived mesenchymal stem cells with deferoxamine increases the production of pro-angiogenic, neuroprotective and anti-inflammatory factors: potential application in the treatment of diabetic neuropathy. PLoS ONE. 2017;12:e0178011.
Ezquer FE, Ezquer ME, Parrau DB, Carpio D, Yañez AJ, Conget PA. Systemic administration of multipotent mesenchymal stromal cells reverts hyperglycemia and prevents nephropathy in type 1 diabetic mice. Biol Blood Marrow Transplant. 2008;14:631–40.
Ezquer M, Urzua CA, Montecino S, Leal K, Conget P, Ezquer F. Intravitreal administration of multipotent mesenchymal stromal cells triggers a cytoprotective microenvironment in the retina of diabetic mice. Stem Cell Res Ther. 2016;7:42.
Ezquer F, Giraud-Billoud M, Carpio D, Cabezas F, Conget P, Ezquer M. Proregenerative microenvironment triggered by donor mesenchymal stem cells preserves renal function and structure in mice with severe diabetes mellitus. Biomed Res Int. 2015;2015:164703.
Ezquer F, Ezquer M, Simon V, Pardo F, Yanez A, Carpio D, et al. Endovenous administration of bone-marrow-derived multipotent mesenchymal stromal cells prevents renal failure in diabetic mice. Biol Blood Marrow Transplant. 2009;15:1354–65.
Calligaris SD, Conget P. Intravenous administration of bone marrow-derived multipotent mesenchymal stromal cells has a neutral effect on obesity-induced diabetic cardiomyopathy. Biol Res. 2013;46:251–5.
de Mayo T, Conget P, Becerra-Bayona S, Sossa CL, Galvis V, Arango-Rodriguez ML. The role of bone marrow mesenchymal stromal cell derivatives in skin wound healing in diabetic mice. 2017;12:e0177533.
Ezquer F, Ezquer M, Contador D, Ricca M, Simon V, Conget P. The antidiabetic effect of mesenchymal stem cells is unrelated to their transdifferentiation potential but to their capability to restore Th1/Th2 balance and to modify the pancreatic microenvironment. Stem Cells. 2012;30:1664–74.
Paz AH, Salton GD, Ayala-Lugo A, Gomes C, Terraciano P, Scalco R, et al. Betacellulin overexpression in mesenchymal stem cells induces insulin secretion in vitro and ameliorates streptozotocin-induced hyperglycemia in rats. Stem Cells Dev. 2011;20:223–32.
Yaochite JNU, de Lima KWA, Caliari-Oliveira C, Palma PVB, Couri CEB, Simões BP, et al. Multipotent mesenchymal stromal cells from patients with newly diagnosed type 1 diabetes mellitus exhibit preserved in vitro and in vivo immunomodulatory properties. Stem Cell Res Ther. 2016;7:14.
Guimaraes ET, Cruz Gda S, Almeida TF, Souza BS, Kaneto CM, Vasconcelos JF, et al. Transplantation of stem cells obtained from murine dental pulp improves pancreatic damage, renal function, and painful diabetic neuropathy in diabetic type 1 mouse model. Cell Transplant. 2013;22:2345–54.
Couri CB, Oliveira MB, Stracieri AL, et al. C-peptide levels and insulin independence following autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA. 2009;301:1573–9.
Malmegrim KC, de Azevedo JT, Arruda LC, Abreu JR, Couri CE, de Oliveira GL, et al. Immunological balance is associated with clinical outcome after autologous hematopoietic stem cell transplantation in type 1 diabetes. Front Immunol. 2017;8:167.
Cantu-Rodriguez OG, Lavalle-Gonzalez F, Herrera-Rojas MA, Jaime-Perez JC, Hawing-Zarate JA, Gutierrez-Aguirre CH, et al. Long-term insulin independence in type 1 diabetes mellitus using a simplified autologous stem cell transplant. J Clin Endocrinol Metab. 2016;101:2141–8.
Martinez-Gamboa M, Cruz-Vega DE, Moreno-Cuevas J, Gonzalez-Garza MT. Induction of nestin early expression as a hallmark for mesenchymal stem cells expression of PDX-1 as a pre-disposing factor for their conversion into insulin producing cells. Int J Stem Cells. 2017;10:76–82.
Benitez-Arvizu G, Palma-Lara I, Vazquez-Campos R, Sesma-Villalpando RA, Parra-Barrera A, Gutierrez-Iglesias G. Autologous mesenchymal stem cells and cutaneus autograft as a treatment for chronic ulcer secondary to diabetes mellitus 2. Cir Cir. 2015;83:532–6.
Estrada EJ, Valacchi F, Nicora E, Brieva S, Esteve C, Echevarria L, et al. Combined treatment of intrapancreatic autologous bone marrow stem cells and hyperbaric oxygen in type 2 diabetes mellitus. Cell Transplant. 2008;17:1295–304.
Suarez-Rodriguez R, Belkind-Gerson J. Cultured nestin-positive cells from postnatal mouse small bowel differentiate ex vivo into neurons, glia, and smooth muscle. Stem Cells. 2004;22:1373–85.
Yaochite JN, Caliari-Oliveira C, de Souza LE, Neto LS, Palma PV, Covas DT, et al. Therapeutic efficacy and biodistribution of allogeneic mesenchymal stem cells delivered by intrasplenic and intrapancreatic routes in streptozotocin-induced diabetic mice. Stem Cell Res Ther. 2015;6:31.
Voltarelli JC, Couri CE, Stracieri AB, Oliveira MC, Moraes DA, Pieroni F, et al. Autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA. 2007;297:1568–76.
Mesples A, Majeed N, Zhang Y, Hu X. Early immunotherapy using autologous adult stem cells reversed the effect of anti-pancreatic islets in recently diagnosed type 1 diabetes mellitus: preliminary results. Med Sci Monit. 2013;19:852–7.
International Diabetes Federation. IDF diabetes atlas, 8th edition. http://www.diabetesatlas.org/resources/2017-atlas.html. Accessed 4 Jan 2018.
Global Burden of Disease Collaborative Network. Global burden of disease study 2016 (GBD 2016) results. Seattle, United States: Institute for Health Metrics and Evaluation (IHME), 2017. http://ghdx.healthdata.org/gbd-results-tool. Accessed 4 Jan 2018.
NCD Risk Factor Collaboration (NCD-RisC). http://www.ncdrisc.org/data-downloads.html. Accessed 4 Jan 2018.
Authors’ contributions
MAS designed the manuscript. MAS, IMV, and RC wrote and revised the manuscript. LLH revised the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
All data generated or analyzed during this study are included in this manuscript.
Funding
This work was supported by Gorgas Memorial Institute for Health Studies Research Grant and Secretaria Nacional de Ciencia, Tecnología, e Innovación research grant 09-2018-ITE17-R1-001 from Panama. IMV is supported by the Sistema Nacional de Investigación (SNI, SENACYT). The funding sources had no involvement in the manuscript design, writing, or in the decision to submit the article for publication.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Solis, M.A., Moreno Velásquez, I., Correa, R. et al. Stem cells as a potential therapy for diabetes mellitus: a call-to-action in Latin America. Diabetol Metab Syndr 11, 20 (2019). https://doi.org/10.1186/s13098-019-0415-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13098-019-0415-0