Abstract
Background
The presence of autoantibodies is considered an important characteristic of rheumatoid arthritis (RA); therefore, both anticitrullinated protein antibodies (ACPA) and rheumatoid factor (RF) are included in the 2010 classification criteria for rheumatoid arthritis (RA). However, a considerable number of RA patients lack both these autoantibodies. Recently, several novel autoantibodies have been identified but their value for the classification of RA patients is unclear. Therefore, we studied the value of novel autoantibodies using the presence of anticarbamylated protein (anti-CarP) antibodies as an example for predicting RA development in patients with undifferentiated arthritis (UA).
Methods
There were 1352 UA patients included in the Leiden Early Arthritis Clinic (EAC) cohort according to the 1987 criteria. When the 2010 criteria were used, there were 838 UA patients. Of these, we evaluated whether they fulfilled the 1987 or 2010 criteria after 1 year, respectively. Logistic regression analyses were performed with RA as outcome and ACPA, RF, and anti-CarP antibodies as predictors. Analyses were repeated after stratification for ACPA and RF.
Results
Thirty-three percent of the 1987-UA patients and 6% of the 2010-UA patients progressed to RA during the first year of follow-up. For the 1987-UA patients, anti-CarP antibodies were associated with progression to RA, an association which remained when a correction was made for the presence of ACPA and RF (odds ratio (OR) 1.7, 95% confidence interval (CI) 1.2–2.4). After stratification for ACPA and RF, anti-CarP antibodies were associated with progression to RA only for ACPA- and RF-negative patients (OR 2.1, 95% CI 1.3–3.7). For the 2010-UA patients, anti-CarP antibodies were associated with progression to RA; however, they were not when a correction was made for the presence of ACPA and RF (OR 0.8, 95% CI 0.3–2.1).
Conclusions
Our finding that anti-CarP antibodies have no additional value when RA is defined according to the 2010 criteria might be inherent to the composition of the 2010 criteria and therefore might also apply to other novel autoantibodies. Potentially it would be interesting to evaluate other, non-autoantibody biomarkers.
Similar content being viewed by others
Background
Rheumatoid arthritis (RA) is characterized by the presence of autoantibodies, the most characteristic among which are anticitrullinated protein antibodies (ACPA) and rheumatoid factor (RF). These are used as diagnostic tools and are included in the classification criteria for RA [1]. Nonetheless, in approximately one-third of early RA patients these autoantibodies are lacking [2]. It is important to better characterize these patients since early intervention in seronegative RA is also important. Therefore, research has focused on identifying novel autoantibodies and several have been identified [3,4,5,6,7]. Based on this research, two issues have been raised. First, stratified analyses are pivotal to prove an additive value of a test. A novel autoantibody should predict an outcome in patients negative for both ACPA and RF, or in patient groups with a similar presence of ACPA and/or RF (e.g., ACPA+RF+novel autoantibody+ vsACPA+RF+novel autoantibody- patients). Thus far, studies that have evaluated the predictive value of novel autoantibodies are often stratified for ACPA but not for RF, leaving the question unanswered if the findings attributed to the novel autoantibody were actually driven by the concomitant presence of RF [5, 8]. A second issue is that, although different disease stages of RA have been studied, the value of novel autoantibodies in identifying the patients that will develop RA among patients presenting with undifferentiated arthritis (UA) is undetermined. Only one study evaluated the role of novel autoantibodies (UH-RA.1, UH-RA.21) in UA patients as an early marker of RA development [4]. The ultimate aim of this study was to increase our understanding on the value of recently identified autoantibodies to predict RA development using accurate stratification for ACPA and RF. An interesting novel family of autoantibodies are the anticarbamylated protein (anti-CarP) antibodies which target proteins modified by carbamylation. These antibodies are present in RA patients and are associated with the severity of radiographic progression [7, 9]. In this study, we investigated the value of the novel anti-CarP antibodies in predicting RA development in patients with UA, independent of ACPA and RF [7].
Methods
Patients
Between 1993 and 2015, 1352 UA patients (according to the 1987 criteria; 1987-UA) were included in the Leiden Early Arthritis Clinic (EAC) cohort. This became 838 UA patients when the 2010 criteria were used (2010-UA). The EAC is an inception cohort that was started in 1993 and includes patients with clinically confirmed arthritis with symptom duration < 2 years at presentation to the rheumatologist [10]. Baseline questionnaires, joint counts, and blood samples were collected, and radiographs were taken. Two weeks after inclusion, when the results of laboratory investigations and radiography were known, patients received their diagnosis. Classification criteria were only applied to patients with a clinical diagnosis or suspicion of RA, and patients who were not classified according to RA classification criteria were documented as having UA.
Anti-CCP2, RF, and anti-CarP measurements
Baseline serum samples were tested for ACPA, RF, and anti-CarP antibodies. Immunoglobulin (Ig)G antibodies to cyclic citrullinated peptide (CCP) were measured by second generation anti-CCP2 enzyme-linked immunosorbent assay (ELISA; Immunoscan RA Mark 2, Eurodiagnostica, Arnhem; cut-off 25 U/ml), and anti-CCP2 ELISA (EliA CCP, Phadia, Nieuwegein, the Netherlands; cut-off 7 U/ml). IgM RF was determined by an in-house ELISA. IgG anti-CarP antibodies were determined as described previously in the Leiden EAC [7]. As no commercial kit is available for anti-CarP antibodies, we used our own in-house anti-CarP assay based on carbamylated fetal calf serum and, as a control, nonmodified fetal calf serum as the coating antigens in the ELISA. Cut-off for positivity was based on the mean + 2 standard deviations (SDs) from a set of healthy controls.
Analyses
Analyses were first performed when RA was classified using the 1987 criteria; thereafter, analyses were repeated using the 2010 criteria since autoantibodies are more prominent in the 2010 criteria. Fulfilment of the 1987 criteria and 2010 criteria was evaluated after 1 year of follow-up for the 1987-UA and 2010-UA patients, respectively. Logistic regression analyses were performed with ACPA, RF, and anti-CarP antibodies as independent variables and RA as outcome, both in the total group of UA patients and after stratification for ACPA and RF status.
Results
Baseline characteristics of the 1352 1987-UA and 838 2010-UA patients are shown in Table 1. Of these UA patients, 33% (441/1352) and 6% (53/838) progressed to RA during the first year according to the 1987 and 2010 criteria, respectively. Of the 1352 1987-UA patients, 257 (19%) were anti-CarP positive and of the 838 2010-UA patients, 77 (9%) were anti-CarP positive.
The value of anti-CarP antibodies was first studied in the 1987-UA patients. The presence of anti-CarP antibodies at baseline was associated with progression to RA (odds ratio (OR) 4.2, 95% confidence interval (CI) 3.2–5.6), an association which remained when a correction was made for the presence of ACPA and RF (OR 1.7, 95% CI 1.2–2.4). There was no additional predictive value of anti-CarP antibody levels in anti-CarP-positive patients. The association of anti-CarP antibodies with progression to RA was then determined within the strata of patients with a similar ACPA and RF status. The majority of the UA patients (69%) were ACPA- and RF-negative; 7% (65/929) of these ACPA- and RF-negative patients had anti-CarP antibodies (Table 2). Within this subgroup, the presence of anti-CarP antibodies was statistically significantly associated with progression to RA (OR 2.1, 95% CI 1.3–3.7; Table 2). When absolute risks were examined, the pre-test risk for RA development in the ACPA- and RF-negative subgroup was 21%, and this increased to 35% when anti-CarP antibodies were present (Table 2). When exploring the negative predictive value (NPV), the pre-test risk of not developing RA was 79% which was similar to the NPV of 80%.
Next, the predictive value of anti-CarP antibodies was studied within the 2010-UA patients. Here, anti-CarP antibodies at baseline were associated with progression to 2010 RA within 1 year (OR 2.9, 95% CI 1.4–5.8). However, when adjustment was made for the presence of ACPA and RF, there was no additive predictive value of anti-CarP antibodies (OR 0.8, 95% CI 0.3–2.1). When analyzing groups of patients stratified according to the absence of ACPA and RF, the majority of 2010-UA patients were ACPA- and RF-negative (90%) and only 6% (49/755) of these patients had anti-CarP antibodies. Within this subgroup, no predictive value of anti-CarP antibodies was observed (Table 2). Evaluation of absolute risks in the ACPA- and RF-negative subgroup revealed that the pre-test risk of developing RA was 4% and the positive predictive value (PPV) was 2% when anti-CarP antibodies were present. Likewise, the pre-test risk of not developing RA in this subgroup was similar to the post-test risk (NPV) when patients tested negative for anti-CarP antibodies (both 96%, Table 2).
Discussion
This study was performed to increase our understanding of the value of recently identified autoantibodies to predict RA development using accurate stratification for ACPA and RF. Anti-CarP antibodies were studied as an example. We observed that the presence of anti-CarP antibodies was statistically significantly associated with the development of RA within ACPA- and RF-negative 1987-UA patients. In this group, the risk of developing RA increased from 21% to 35% when anti-CarP antibodies were present. However, when RA was defined according to the 2010 criteria, anti-CarP antibodies were not associated with RA development and the presence of these autoantibodies did not increase the risk of RA development compared to the pre-test risks.
Although they used different study designs and entire early arthritis populations, two previous studies found 2.2% and 0.4% improved classification when adding anti-CarP to ACPA and RF, thus showing little additive benefit [8, 11]. These findings are in line with our data.
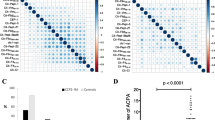
Presumably this finding is explained by the fact that ACPA and RF are heavily weighted in the 2010 criteria. Consequently, the majority of UA patients are ACPA- and RF-negative and these patients can only fulfill the 2010 criteria if they develop > 10 involved joints but they can fulfill the 1987 criteria over time with less extensive disease progression; hence the definition of the outcome matters. Additionally, autoantibodies frequently occur together (Fig. 1), as has been shown for several novel autoantibodies [3, 5]. These two explanations might also apply to other novel autoantibodies. Although novel autoantibodies other than anti-CarP antibodies were not evaluated here, we anticipate that similar findings will be obtained. Importantly, our findings relate to the earlier identification of patients with RA; novel autoantibodies can still be useful for other outcomes, such as radiographic progression [7].
Concomitant presence of anticitrullinated protein antibodies (ACPA), rheumatoid factor (RF), and anticarbamylated protein (anti-CarP) antibodies in patients with 1987-undifferentiated arthritis (UA) and 2010-UA. Depicted are the percentages of the 1352 1987-UA (a) and the 838 2010-UA (b) patients positive for ACPA, RF, and/or anti-CarP antibodies
Conclusions
More research is needed to identify early RA patients among (2010 criteria-negative) UA patients, but based on the composition of the current classification criteria it will be interesting to evaluate other, non-autoantibody biomarkers.
Abbreviations
- ACPA:
-
Anticitrullinated protein antibodies
- anti-CarP:
-
Anticarbamylated protein
- CCP:
-
Cyclic citrullinated peptide
- CI:
-
Confidence interval
- EAC:
-
Early Arthritis Clinic
- ELISA:
-
Enzyme-linked immunosorbent assay
- Ig:
-
Immunoglobulin
- NPV:
-
Negative predictive value
- OR:
-
Odds ratio
- RA:
-
Rheumatoid arthritis
- RF:
-
Rheumatoid factor
- SD:
-
Standard deviation
- UA:
-
Undifferentiated arthritis
References
Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2010;69:1580–8.
Nishimura K, Sugiyama D, Kogata Y, Tsuji G, Nakazawa T, Kawano S, et al. Meta-analysis: diagnostic accuracy of anti-cyclic citrullinated peptide antibody and rheumatoid factor for rheumatoid arthritis. Ann Intern Med. 2007;146:797–808.
Raza K, Schwenzer A, Juarez M, Venables P, Filer A, Buckley CD, et al. Detection of antibodies to citrullinated tenascin-C in patients with early synovitis is associated with the development of rheumatoid arthritis. RMD Open. 2016;2:e000318.
De Winter LM, Hansen WLJ, van Steenbergen HW, Geusens P, Lenaerts J, Somers K, et al. Autoantibodies to two novel peptides in seronegative and early rheumatoid arthritis. Rheumatol Oxf Engl. 2016;55:1431–6.
Juarez M, Bang H, Hammar F, Reimer U, Dyke B, Sahbudin I, et al. Identification of novel antiacetylated vimentin antibodies in patients with early inflammatory arthritis. Ann Rheum Dis. 2016;75:1099–107.
Nell VPK, Machold KP, Stamm TA, Eberl G, Heinzl H, Uffmann M, et al. Autoantibody profiling as early diagnostic and prognostic tool for rheumatoid arthritis. Ann Rheum Dis. 2005;64:1731–6.
Shi J, Knevel R, Suwannalai P, van der Linden MP, Janssen GMC, van Veelen PA, et al. Autoantibodies recognizing carbamylated proteins are present in sera of patients with rheumatoid arthritis and predict joint damage. Proc Natl Acad Sci U S A. 2011;108:17372–7.
Truchetet M-E, Dublanc S, Barnetche T, Vittecoq O, Mariette X, Richez C, et al. Association of the presence of anti-carbamylated protein antibodies in early arthritis with a poorer clinical and radiologic outcome: data from the French ESPOIR Cohort. Arthritis Rheumatol. 2017;69:2292–302.
Ajeganova S, van Steenbergen HW, Verheul MK, Forslind K, Hafström I, Toes REM, et al. The association between anti-carbamylated protein (anti-CarP) antibodies and radiographic progression in early rheumatoid arthritis: a study exploring replication and the added value to ACPA and rheumatoid factor. Ann Rheum Dis. 2017;76:112–8.
de Rooy DPC, van der Linden MPM, Knevel R, Huizinga TWJ, Mil AHM van der H. Predicting arthritis outcomes—what can be learned from the Leiden Early Arthritis Clinic? Rheumatology. 2011;50:93–100.
Regueiro C, Nuño L, Ortiz AM, Peiteado D, Villalba A, Pascual-Salcedo D, et al. Value of measuring anti-carbamylated protein antibodies for classification on early arthritis patients. Sci Rep. 2017;7:12023.
Funding
This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (Starting grant, agreement no. 714312) and from the Netherlands Organisation for Health Research and Development (Vidi grant).
Availability of data and materials
Data can be requested from the corresponding author.
Author information
Authors and Affiliations
Contributions
DMB, LAT, AHMvdHvM and HWvS contributed to the conception and study design. DMB and HWvS analyzed the data. DMB, LAT, AHMvdHvM, and HWvS contributed to the interpretation of the data. DMB, LAT, AHMvdHvM, and HWvS contributed to the acquisition of the data. DMB, AHMvdHvM, and HWvS wrote the first version of the manuscript and all authors revised it critically. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved under number P10.108 by the medical ethics committee of the Leiden University Medical Center, which is named Commissie Medische Ethiek. All patients signed informed consent.
Competing interests
LAT is listed as an inventor on a patent on the detection of anti-CarP antibodies. The remaining authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Boeters, D.M., Trouw, L.A., van der Helm-van Mil, A.H.M. et al. Does information on novel identified autoantibodies contribute to predicting the progression from undifferentiated arthritis to rheumatoid arthritis: a study on anti-CarP antibodies as an example. Arthritis Res Ther 20, 94 (2018). https://doi.org/10.1186/s13075-018-1591-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13075-018-1591-2