Abstract
The dynamic changes of secondary metabolites and tyrosinase activity of Malus pumila flowers were investigated during blooming for full use of M. pumila flowers. Phlorizin, astragalin and afzelin were selected as the indicators to determine the optimum extraction conditions of M. pumila flowers by HPLC analysis and three-factor and three-level orthogonal design experiments. When the smashing mesh number was 40 mesh, the ultrasonic time was 30 min, the solid–liquid ratio was 1:120, and the centrifugal speed was 10,000 r/min, the total content of the three compounds was the highest. The total content of phlorizin, astragalin and afzelin increased firstly and then decreased, and the highest was on the third day (176.74 mg/g). At the same time, the tyrosinase activity of M. pumila flowers showed that it had an activation effect on tyrosinase during early blooming period, however it exhibited inhibitory effect during late blooming period.
Similar content being viewed by others
Introduction
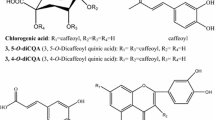
Malus pumila Mill has a long history of cultivation and is widely cultivated in temperate regions of the world [1]. The chemical constituents of M. pumila include flavonoids, terpenoids and organic acids [2,3,4], Which have been reported to excrete several pharmacological activities such as being antioxidant, anti-aging [5], anti-cancer [6], anti-bacterial [7], hypoglycemic [8], liver protective. M. pumila flowers can be used as tea [9]. Its main chemical components are dihydrochalcone such as phlorizin, phloretin, and other flavonoids such as quercetin, kaempferol and rutin [10]. In our previous study, nine compounds were isolated from M. pumila flowers, which exhibited activation or inhibition of tyrosinase [11]. In addition, we summarized the chemical compositions of different parts from M. pumila [12,13,14] as shown in Table 1.
Zhao et al. [15] reported that the content change of phlorizin was different in branches and leaves of M. pumila, phloretin was exclusively detected in the leaves. Tang [16] found that the main polyphenols in mature apples included catechins, proanthocyanidins and chlorogenic acids, while immature apples had mainly dihydrochalcone and flavonols. Renard et al. [17] reported that the content of proanthocyanidins increased continuously during the whole growth period of apples. Polyphenols began to synthesize 40 days after flowering. The content of flavonoids decreased significantly in the range of 35–100 days after flowering.
At present, the dynamic change of M. pumila is mainly focused on fruits, leaves and branches. However, there are fewer investigations on the dynamic change of M. pumila flowers, except for the dynamic changes of amino acids and protein [18]. In order to make full use of M. pumila resources, the dynamic changes of secondary metabolites and tyrosinase activity were investigated during M. pumila flowers blooming.
Methods
Chemicals and materials
Methanol was purchased from Tianjin DaMao Chemical Reagent Factory (Tianjin, China). Acetic acid was obtained from Tianjin FuChen Chemical Reagent Factory (Tianjin, China). Astragalin with purity greater than 98% was purchased from Chengdu Pufei De Biotech Co., Ltd. (Chengdu, China). Phlorizin and afzelin with purity greater than 98% were isolated in our previous research.
The LC-20AT high performance liquid chromatography system (Shimadzu, Kyoto, Japan) equipped with a degasser, a quaternary gradient low pressure pump, the CTO-20A column oven, a SPD-M20AUV-detector, an SIL-20A auto sampler was used. Chromatographic separation was performed on an Agilent ZORBAX SB-C18 column (4.6 mm × 5 mm, 5 μm) and extraction was carried out with KQ-500DB ultrasonic cleaner (Jiangsu Kunshan Ultrasonic Instrument Co., Ltd. Jiangsu, China). TGL-16 type high speed centrifuge was obtained from Jiangsu Jintan Zhongda instrument factory (Jiangsu, China). AB135-S 1/10 million electronic balance was purchased from Mettler Toledo Instruments Co., Ltd (Shanghai, China).
Plant materials
The M. pumila flowers were collected during the period from March 26th 2018 to April 3rd 2018 in the campus of Henan University (Kaifeng, Henan, China) and identified by Professor Changqin Li of National R & D Center for Edible Fungus Processing Technology. They were collected once a day on nine batches. Specimens (2018-0326-0403) were deposited in the National R & D Center for Edible Fungus Processing Technology.
Experimental methods
Preparation of the standard solution
The standard concentrations of phlorizin, astragalin and afzelin were prepared at concentration of 0.2040, 0.1960 and 0.1990 mg/mL with methanol.
Preparation of test sample solution
Malus pumila flowers powder (20.00 mg) was dissolved with appropriate solvent. The sample was extracted by ultrasound for 30 min and centrifugation for 3 min at 8000 r/min. The supernatant was filtered by 0.22 μm microporous membrane and the subsequent filtrate was taken as the test solution.
The solvent type, extraction concentration, particle size, sample-solvent ratio, ultrasonic time and centrifugal speed were investigated in turn. Every experiment was carried out in parallel three times.
Chromatographic conditions
Tyrosinase activity assay
The absorbance was measured at 492 nm with a microplate reader, and the results were evaluated through the following formula [19]:
Results and discussion
Linear relationship
The peak area (X) is the vertical axis, and the sample quality (Y, μg) the abscissa, respectively. In Table 3, phlorizin, astragalin and afzelin exhibited good linearity in the ranges of 0.4080–14.28 (μg/mL), 0.3920–13.72 (μg/mL) and 0.3980–13.93 (μg/mL), respectively.
Optimization extraction process of flavonoids in M. pumila flowers
Single factor test
Types of extract solvent
Five solvents, 70% ethanol, 95% ethanol, methanol, acetonitrile and water were selected. Samples were prepared according to the conditions in “Preparation of test sample solution” section above and injected into the HPLC analysis by above chromatographic conditions (Table 2). The results showed that acetonitrile could only extract two kinds of flavonoids from M. pumila flowers (Fig. 2), whereas methanol was the best solvent.
Influence of different extract solvents (n = 3) (Additional file 1: Table S4)
Selection of mesh number
In Fig. 3, the extract rate of target analytes was the highest when the number of smashing mesh was 40 meshes, but the extract rate was lower when the number of smashing mesh was larger. So, 40 meshes were selected.
Effect of mesh numbers on extract yield (n = 3) (Additional file 1: Table S5)
Effect of ultrasonic time
The ultrasonic time of 10, 20, 30, 40, 50 and 60 min were chosen respectively. According to the above experimental conditions, results were showed in Fig. 4. When the ultrasonic time was 20 min, the extract rate of the target analytes reached the maximum. With the increase of time, the target analytes extraction rate showed a downward trend, which might be the decomposition of effective components due to ultrasonic overheating [20, 21]. Hence, 20 min was selected.
Effect of ultrasonic times on extract yield (n = 3) (Additional file 1: Table S6)
Selection of centrifugal speed
Under the optimal conditions, five different centrifugal speeds (2000, 4000, 6000, 8000 and 10,000 r/min) were chosen to evaluate the effect of centrifugal speed on the extract yield. In Fig. 5, the extract rate reached the maximum at 8000 r/min. Thus, in the experiments, 8000 r/min was chosen as the center point of the orthogonal factors.
Effect of centrifugal speeds on extract yield (n = 3) (Additional file 1: Table S7)
Sample-solvent ratio
The solid–liquid ratios were 1:20, 1:40, 1:60, 1:80, 1:100 and 1:120, which were used to evaluate the extract rate of the target analytes. In Fig. 6, when the solid–liquid ratio was 1:100, the maximum extract rate was reached. Continuing to increase the ratio, the extract rate was decreased. It indicated that the increase of solvent amount can increase the contact area between the active ingredients and the solvent, and the dissolution probability of the active ingredients will also increase accordingly in a certain range and the yield will be improved continuously. However, when the ratio of solid–liquid reaches a certain value, the degree of assistant effect of ultrasonic cavitation on plant cell rupture decreases, and the dissolution of effective components is close to saturation, the extract rate decreases [22, 23].
Effect of solid–liquid ratios on extract yield (n = 3) (Additional file 1: Table S8)
Orthogonal test (Additional file 1: Table S9)
A 3-factor 3-level orthogonal experiment was designed and statistically analyzed by SPSS 19.0 [24]. The investigated levels of each factor were selected depending on the above experiment results of the single-factor. Independent variables with three variation levels were shown in Table 4.
Through the orthogonal test of ultrasonic time (min), solid–liquid ratio (times) and centrifugal speed (r/min), the variance analysis was carried out by SPSS 19.0 software. The results were presented in Tables 5 and 6. The ultrasonic time had the greatest influence, followed by the solid–liquid ratio. The primary and secondary sequence of experimental factors were: (B) ultrasonic time > (A) solid–liquid ratio > (C) centrifugal speed. A1B3C3 was the optimal extraction process, namely: the solid–liquid ratio was 1:120, the ultrasonic time was 30 min, and the centrifugal speed was 10,000 r/min.
Determination of content in different periods
In Fig. 7, the total content of phlorizin, astragalin and afzelin reached the highest level on the third day (176.74 mg/g) during blooming of M. pumila flowers. The contents of astragalin and afzelin decreased after 1st April, while the contents of phlorizin increased after 2nd April. It indicated that the phlorizin was transferred and enriched in fruits, resulting in the fruit containing a large amount of phlorizin [25]. Moreover, light intensity, soil acidity and alkalinity and external temperature also affect the formation of secondary metabolites [26].
Changes of three secondary metabolites in M. pumila flowers (Additional file 1: Table S10)
Method validation
Repeatability (Additional file 1: Table S11)
Six M. pumila flowers samples were prepared by “Preparation of test sample solution” method, and the contents of phlorizin, astragalin and afzelin were determined under “Chromatographic conditions” section. The results showed that RSDs of phlorizin, astragalin and afzelin were 0.92%, 1.88% and 2.17% respectively, which indicated that the method had good repeatability.
Precision (Additional file 1: Table S12)
The mixed standard solution of phlorizin, astragalin and afzelin was prepared under “Preparation of the standard solution” conditions. According to “Chromatographic conditions” section, mixed standard solution was injected continuously for 6 times. The results showed that RSDs of phlorizin, astragalin and afzelin were 0.8%, 1.02% and 0.75% respectively, indicating that the instrument had good precision.
Stability (Additional file 1: Table S13)
Six M. pumila flowers samples were prepared by “Preparation of test sample solution” conditions. According to “Chromatographic conditions” section, samples were injected at 0, 4, 8, 12, 16, 20 and 24 h, respectively. The results showed that RSDs of phlorizin, astragalin and afzelin were 0.35%, 0.95% and 0.84% respectively, which indicated that phlorizin, astragalin and afzelin in the samples were basically stable within 24 h.
Recovery (Additional file 1: Table S14)
Six samples of M. pumila flowers were prepared according to 3.2 conditions. The contents of phlorizin, astragalin and afzelin were determined. Then the standard solution equivalent to 80% of the three target components in the sample was added. The average recoveries of phlorizin, astragalin and afzelin were 98.20%, 98.96% and 101.03% respectively, and their RSDs values were 0.20%, 0.24% and 0.13%, respectively.
Tyrosinase activity assay
The response of tyrosinase activity was determined by tyrosinase and dopa rate oxidation trace method in vitro. In Table 7 and Fig. 8, M. pumila flowers could stimulate the activity of tyrosinase in the early stage of blooming. Meanwhile, inhibition in the activity of tyrosinase occured in the late stage of blooming, which was gradually decreased. The reason may be that in the late stage of blooming, the content of secondary metabolites which inhibit the activity of tyrosinase increased, so the total extracts of M. pumila flowers showed inhibition activity of tyrosinase.
Tyrosinase is a key enzyme in melanin synthesis, and its activity is positively correlated with the amount of melanin [27, 28]. It is well known that melanin protects the skin from UV damage but its excessive production causes freckles, melasma, skin cancer, and age spots [29, 30]. Xie et al. [31] studied the inhibition kinetics of flavonoids on mushroom tyrosinase and suggested that flavonoids could induce reversible inhibition of enzyme activity through copper ions in the active center of chelating enzyme. Beside the effect of flavonoids, there might be other tyrosinase inhibitors, which could bind to free enzymes as well. It can bind with enzyme–substrate complex, resulting in reversible inhibition of enzyme activity. Several studies have shown that many flower extracts had good tyrosinase activity, such as orchid extract [32], lavender essential oil [33], areca nut boiling water extracts [34], and can be used as effective tyrosinase inhibitors. At present, there were few reports about the effect of M. pumila flowers on tyrosinase activity. The obtained results indicated that the content of three flavonoids reached the highest on 28th March during blooming of M. pumila flowers. The methanol extracts of the flowers picked on 30th March had a higher inhibitory effect on tyrosinase, which may advocated that tyrosinase activity not only related to flavonoids, but also related to the types of secondary metabolites extracted as well as the mechanism of action.
Conclusion
Under optimum extraction conditions (smashing mesh number: 40 meshes, ultrasonic time: 30 min, solid–liquid ratio: 1:120, centrifugal speed: 10,000 r/min), the total contents of phlorizin, astragalin and afzelin from M. pumila flowers reached the maximum (176.74 mg/g) on the third day of blooming, which could be the best time for harvest. Meanwhile, the tyrosinase activity of M. pumila flowers showed that it had an activation effect on tyrosinase during early blooming period, however it expressed inhibitory effect during late blooming period.
Availability of data and materials
The datasets and samples of the compounds are available from the authors.
Abbreviations
- M. pumila flowers:
-
Malus pumila flowers
- HPLC:
-
high-performance liquid chromatography
- Fig:
-
figure
- RSD:
-
relative standard deviation
- Mar:
-
March
- Apr:
-
April
References
Flora of China (1974) 36:381. http://frps.eflora.cn/frps?id=MaluspumilaMill
Li J, Shi Y (2014) Research status of apple polyphenols. Deciduous Fruits 46(06):18–20. https://doi.org/10.13855/j.cnki.lygs.2014.06.008
Tan S, Qin XX, Su Q, Zhou ZQ (2013) Current status of the study and utilization of the bioactive compounds of apple. Food Ind Technol 34(01):358–361+367. https://doi.org/10.13386/j.issn1002-0306.2013.01.084
Wei XC, Zhao YM, Song GM, Song XK, Liu DL (2016) Chemical constituents isolated from branches of apple. Chin Tradit Drug 47(11):1845–1849
Wang J, Li HY, Liu DL, Song XB, Yu HJ (2011) Research progress of apple nutrition components and health function. Food Res Dev 32(01):164–168
Yang KC, Tsai CY, Wang YJ et al (2009) Apple polyphenol phloretin potentiates the anticancer actions of paclitaxel through induction of apoptosis in human hep G2 cells. Mol Carcinog 48(5):420–431
Sun HN, Sun AD, Su YJ, Gao XJ et al (2010) Study on antibacterial effect of apple polyphenols. J Beijing Forestry Univ 32(04):280. https://doi.org/10.13332/j.1000-1522.2010.04.002
Li ZH (2014) Study on the extraction, isolation and antidiabetic constituents of apple polyphenols. China Univ Mining Technol. http://kns.cnki.net/KCMS/detail/detail.aspx?dbcode=CMFD&dbname=CMFD201501&filename=1014074365.nh&v=MjM5NTZQSVI4ZVgxTHV4WVM3RGgxVDNxVHJXTTFGckNVUkxLZVplUnZGQ25tVmIvUFZGMjZHck8vR3RMS3FwRWI=
Yang S (2013) Pharmacological effects of polysaccharides and flavonoids from apple on liver injury. Shaanxi Normal Univ. http://kns.cnki.net/KCMS/detail/detail.aspx?dbcode=CMFD&dbname=CMFD201401&filename=1014107600.nh&v=MTUxMDBGckNVUkxLZVplUnZGQ25tV3IvTVZGMjZHcks0R2RmTXI1RWJQSVI4ZVgxTHV4WVM3RGgxVDNxVHJXTTE=
Yin YZ, Li YW, Han QH (2016) HPLC fingerprint of Pumila Flos. J Cent South Pharm 14:684–687
Li WJ, Shi MJ, Wang PY et al (2018) Efficient determination of three flavonoids in Malus pumila flowers by ionic liquid-HPLC. J Mol Liq 8(263):139–146. https://doi.org/10.1016/j.molliq.2018.04.135
Xing XF, Ye Q (2018) Determination of active ingredients in apple extract by HPLC. Mod Food 22:116–123. https://doi.org/10.16736/j.cnki.cn41-1434/ts.2018.22.033
Jiang P (2018) Effects of different treatments on the antioxidant activities and components of apple. Southwest Univ Sci Technol. http://kns.cnki.net/KCMS/detail/detail.aspx?dbcode=CMFD&dbname=CMFD201802&filename=1018199840.nh&uid=WEEvREcwSlJHSldRa1FhdkJkVG1CcDc3d1ZNWGpkeDNPNCtLM3RWYmNtUT0=$9A4hF_YAuvQ5obgVAqNKPCYcEjKensW4IQMovwHtwkF4VYPoHbKxJw!!&v = MjIyMjdTN0RoMVQzcVRyV00xRnJDVVJMT2Z
Lu YZ, Wei Y (2016) Separation and purification of active components from apple leaves by elution-push countercurrent chromatography-semi-preparative high performance liquid chromatography. China Chem Soc, 1. http://kns.cnki.net/KCMS/detail/detail.aspx?dbcode=CPFD&dbname=CPFDLAST2017&filename=ZGHY201604004016&uid=WEEvREcwSlJHSldRa1FhdkJkVG1CcDc3d1ZNWGpkeDNPNCtLM3RWYmNtUT0=$9A4hF_YAuvQ5obgVAqNKPCYcEjKensW4IQMovwHtwkF4VYPoHbKxJw!!&v=MjQ4MDhlTU9VS3JpZlp1OXZGU3ZpVU
Zhao YM, Wang J, Song GM, Song XB, Liu DL, Xie WL (2013) Study on the dynamic changes of phlordzin and phloretin in leave and branches of apple tree. Food Res Dev 34(07):95–98
Tang CH, Peng ZY (2001) The development and application of apple polyphenol. China Food Additives (02):41–45. http://kns.cnki.net/KCMS/detail/detail.aspx?dbcode=CJFQ&dbname=CJFD2001&filename=ZSTJ200102010&v=MDc0MTkxRnJDVVJMS2VaZVJ2RkNublZyN01QejdmWkxHNEh0RE1yWTlFWklSOGVYMUx1eFlTN0RoMVQzcVRyV00=
Renard CMGC, Dupont N, Guillermin P (2007) Concentrations and characteristics of procyanidins and other phenolics in apples during fruit growth. Phytochemistry 68(08):1128–1138
Khanizadeh S, Buszard D, Zarkadas CG (1989) Seasonal variation of proteins and amino acids in apple flower buds (Malus pumila Mill, cv. McIntosh/M7). J Agric Food Chem 37(05):1246–1252
Wang JM, Peng LN, Shi MJ, Li CQ, Zhang Y, Kang WY (2017) Spectrum effect relationship and component knock-out in Angelica Dahurica Radix by high performance liquid chromatography-q exactive hybrid quadrupole-orbitrap mass spectrometer. Molecules 22:7. https://doi.org/10.3390/molecules22071231
Ren YM, Liu YQ (2018) Ultrasonic extraction of alcohol extract of Maca. Agric Prod Process (18):19–20 + 25. http://kns.cnki.net/KCMS/detail/detail.aspx?dbcode=CJFQ&dbname=CJFDLAST2018&filename=NCPJ201818008&uid=WEEvREcwSlJHSldRa1FhdkJkVWEyd2MxZ0xHYm92TXE3dENnTTcxN2EyRT0=$9A4hF_YAuvQ5obgVAqNKPCYcEjKensW4IQMovwHtwkF4VYPoHbKxJw!!&v=MTQzNTBuTnA0OUZiSVI4ZVgxTHV4WVM3R
Gao DD, Guo PH, Qi GZ (2015) Optimization of extraction technology of total flavonoids from Mentha haplocalyx by response surface methodology. Food Ind Sci Technol 36(2):299–303
Sun CB, Zhang J, Liu HQ (2018) Optimization of ultrasonic-assisted ethanol extraction for protein from Velvet Antler. Food Ind. (09):9–13. http://kns.cnki.net/KCMS/detail/detail.aspx?dbcode=CJFQ&dbname=CJFDLAST2018&filename=SPGY201809003&uid=WEEvREcwSlJHSldRa1FhdkJkVWEyd2MxZ0xHYm92TXE3dENnTTcxN2EyRT0=$9A4hF_YAuvQ5obgVAqNKPCYcEjKensW4IQMovwHtwkF4VYPoHbKxJw!!&v=MDMwMTc0UjhlWDFMdXhZUzdEaDFUM3FUc
Li JH (2005) Study on extraction, purification and function evaluation of active composition in pilose antler and function food development. China Agricultural University, Beijing
Shi MJ, Zhang Y, Song MM, Sun Y, Li CQ, Kang WY (2018) Screening the marker components in Psoralea corylifolia L. with the aids of spectrum-effect relationship and component knock-out by UPLC-MS2. Int J Mol Sci. https://doi.org/10.3390/ijms19113439
McCann MJ, Gill CIR, Brien G, Rao JR, McRoberts WC, Hughes P, McEntee R, Rowland IR (2007) Anti-cancer properties of phenolics from apple waste on colon carcinogenesis in vitro. Food Chem Toxicol 45:1224–1230. https://doi.org/10.1016/j.fct.2007.01.003
Wu H, Chen M, Fan Y, Elsebaei F, Zhu Y (2012) Determination of rutin and quercetin in Chinese herbal medicin by ionic liquid-based pressurized liquid extraction-liquid chromatography-chemiluminescence detection. Talanta 88:222–229. https://doi.org/10.1016/j.talanta.2011.10.036
Abu Tahir M, Pramod K, Ansari SH et al (2010) Current remedies for vitiligo. Autoimmun Rev 9(7):516. https://doi.org/10.1016/j.autrev.2010.02.013
Qing LI, Ai XU (2015) Modern research of traditional Chinese Medicine Treatment of Vitiligo. Med Recapitul 21:1
Nguyen H, Nguyen N, Nguyen M, Lee T, Do T, Hung T, Nguyen M (2016) Tyrosinase inhibitory activity of flavonoids from Artocarpus heterophyllous. Chem Cent J 10:1–6. https://doi.org/10.1186/s13065-016-0150-7
Zhang L, Zhao X, Tao G, Chen J, Zheng Z (2017) Investigating the inhibitory activity and mechanism differences between norartocarpetion and luteolin for tyrosinase. A combinatory kinetic study and computational simulation analysis. Food Chem 223:40–48. https://doi.org/10.1016/j.foodchem.2016.12.017
Xie LP, Chen QX, Huang H et al (2003) Inhibitory effects of some flavonoids on the activity of mushroom tyrosinase. Biochemistry 68(04):487–491. https://doi.org/10.1023/a:1023620501702
Bonte F, Cauchard JH, Archambault JC et al (2010) New orchid extract based technology for skin whitening. J Dis Control Prev 14(04):353–356
Jing HF, Liu BH, Liu XT et al (2010) Study on inhibition of lavender essential oil on the activity of tyrosinase. Chin Wild Plant Resour 29(05):29–31
Cheng FF, Hai H, Hyang YL et al (2011) Inhibitory effect of areca inflorescence boiling water extracts on tyrosinase-catalyzing reaction. Chin J Trop Crops 11:1932–1936. https://doi.org/10.1080/00949651003724790
Acknowledgements
Not applicable.
Funding
This work was supported by Key Project in Science and Technology Agency of Henan Province (182102110473), Innovation and Entrepreneurship Training Program, Henan University (201810475069).
Author information
Authors and Affiliations
Contributions
WK and ZL conceived the research idea. LC, XH, WL, YL, ZY, XL and YH collected the plant specimens, conducted the experiments, analyzed and interpreted the data as well as prepared the first draft. WK, ZL, and LC critically read and revised the paper. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1.
Table S1–S3. The raw data for Table 3 Linear regression equation of phlorizin, astragalin and afzelin in the manuscript. Table S4. The raw data for Fig. 2 Influence of different extract solvents in the manuscript. Table S5. The raw data for Fig. 3 Effect of mesh numbers on extract yield in the manuscript. Table S6. The raw data for Fig. 4 Effect of ultrasonic times on extract yield in the manuscript. Table S7. The raw data for Fig. 5 Effect of centrifugal speeds on extract yield in the manuscript. Table S8. The raw data for Fig. 6 Effect of solid-liquid ratios on extract yield in the manuscript. Table S9. The raw data for orthogonal test. Table S10. The raw data for Fig. 7 Changes of three secondary metabolites in M. pumila flowers in the manuscript. Table S11. The raw data for 4.5.1 Repeatability experiment in the manuscript. Table S12. The raw data for 4.5.2 Precision experiment in the manuscript. Table S13. The raw data for 4.5.3 Stability experiment in the manuscript. Table S14. The raw data for 4.5.4 Recovery experiment in the manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Cui, L., Hou, X., Li, W. et al. Dynamic changes of secondary metabolites and tyrosinase activity of Malus pumila flowers. BMC Chemistry 13, 81 (2019). https://doi.org/10.1186/s13065-019-0602-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13065-019-0602-y