Abstract
Background
Causative variants in BRCA1 and BRCA2 are well-established risk factors for breast and ovarian cancer. In Poland, the causative founder variants in the BRCA1 are responsible for a significant proportion of ovarian cancer cases, however, regional differences in the frequencies of various mutations may exist. The spectrum and frequency of BRCA1/2 mutations between ovarian cancer patients have not yet been studied in the region of South-East Poland.
Methods
We examined 158 consecutive unselected cases of ovarian cancer patients from the region of Podkarpacie. We studied 13 Polish causative founder variants in BRCA1 (c.5266dupC, c.4035delA, c.5251C > T, c.181 T > G, c.676delT, c.68_69delAG, c.3700_3704delGTAAA, c.1687C > T, c.3756_3759delGTCT) and in BRCA2 (c.658_659delGT, c.7910_7914delCCTTT, c.3847_3848delGT, c.5946delT).
Results
A BRCA1 causative founder variants were detected in 10 of the 158 (6.3%) ovarian cancer cases. BRCA2 causative founder variants were not observed. The c.5266dupC mutation was detected in 6 patients, c.181 T > G mutation in 3 patients and the c.676delT mutation in 1 patient. The median age of diagnosis of the 10 hereditary ovarian cancers was 55.5 years of age.
Conclusions
The frequency of 13 causative founder variants in Podkarpacie was lower than in other regions of Poland. Testing of three BRCA1 mutations (c.5266dupC, c.181 T > G, c.676delT) should be considered a sensitive test panel.
Similar content being viewed by others
Background
There are approximately 3600 new ovarian cancer cases and over 2000 deaths as a result of this cancer in Poland annually [1]. It has been shown that ovarian cancer patients from Poland are characterized by a high proportion of a limited number of recurrent mutations in BRCA1 [2,3,4,5,6,7]. Three BRCA1 causative founder variants (c.5266dupC, c.181 T > G, and c.4035delA) account for approximately 80% of all detectable BRCA1 and BRCA2 mutations in breast-ovarian cancer families in Poland [2, 3]. A high proportion of BRCA-carriers with a limited number of recurrent mutations have an impact on the test costs reduction, it enhances the availability and testing effectiveness. In 2003, Menkiszak et al. observed that 13.5% of ovarian cancer patients in the Szczecin region carry one of these three common causative founder variants in BRCA1 [8]. Since 2003, several other less frequent recurrent BRCA1 and BRCA2 mutations have been reported as well as some regional differences in mutation frequency and spectrum have been observed [2,3,4,5,6,7,8]. Currently, five BRCA1 causative founder variants (c.5266dupC, c.181 T > G, and c.4035delA, c.68_69delAG, c.3700_3704delGTAAA) are recommended in national screening program.
The aim of this study was to evaluate the prevalence and spectrum of 13 BRCA1 and BRCA2 causative founder variants in unselected patients diagnosed with ovarian cancer from Region of Podkarpacie. These observations may be important to optimize the genetic testing strategy for ovarian cancer patients in the region.
Methods
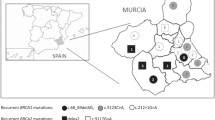
The ovarian cancer cases were identified among clinical base patients of the Department of Obstetrics and Gynecology of Fryderyk Chopin University Hospital No 1 in Rzeszów, Poland between January 2013 and January 2017. All patients were inhabitants of the South-East region of Poland. The study group consisted of 158 consecutive, newly diagnosed cases of ovarian cancer after surgical treatment, unselected based on age or family history. The mean age of diagnosis was 58.5 years (range 22–84 years). The reference pathologist reviewed a representative slide from each cancer to confirm the diagnosis. 20% of patients were diagnosed in I and II clinical stage and 80% in III and IV according to FIGO. 14.8% of ovarian cancers showed pathological grading G1, 11.1% - G2, and 74.1% – G3. A cancer family history was obtained during an appointment with clinical geneticist.
DNA was isolated from 5 to 10 ml of blood. All women were tested for the presence of 13 causative founder variants in BRCA1 (nine mutations) and BRCA2 (four mutations). The c.4035delA and c.5266dupC mutations were detected using allele-specific oligonucleotide polymerase chain reaction (PCR) [7]. The other mutations of BRCA1 (c.5251C > T, c.181 T > G, c.676delT, c.68_69delAG, c.3700_3704delGTAAA, c.1687C > T, c.3756_3759delGTCT) and in BRCA2 (c.658_659delGT, c.7910_7914delCCTTT, c.3847_3848delGT, c.5946delT) were genotyped using TaqMan assays (Applied Biosystems/Life Technologies, Carlsbad, CA) on Roche LightCycler 480. All mutations were confirmed by Sanger direct sequencing. Sequencing reactions were performed using a BigDye Terminator v3.1 Cycle Sequencing Kit (Life Technologies) according to the manufacturer’s protocol. Sequencing products were analyzed on the ABI Prism 3100 Genetic Analyzer (Life Technologies).
Results
A BRCA1 causative founder variants were detected in 10 of the 158 (6.3%) unselected ovarian cancer cases. The c.5266dupC mutation was the most common, having been diagnosed in six patients, followed by the c.181 T > G mutation observed in three patients and the c.676delT mutation in one patient (Table 1). The median age of diagnosis of the 10 hereditary ovarian cancers patients was 55.5 years (range 41–82 years), compared with a median age of diagnosis of 58.75 years (range 22–84 years) for the 148 cases without a mutation. A mutation was found in 9.7% (3/31) of women diagnosed with ovarian cancer at or under the age of 50 compared to 5.5% (7/127) of women diagnosed at a later age. Among the 10 women with ovarian cancer and a BRCA1 mutation, six reported a first- or second-degree relative with breast or ovarian cancer (60%). A mutation was present in 15.4% (6/39) of ovarian cancer patients with a positive family history and in 3.4% (4/119) of women with a negative family history. A significant family history, defined as a presence of first- or second-degree relative with breast or ovarian cancer, was observed in 24.7% (39/158) of patients with ovarian cancer (Table 2).
Discussion
The Podkarpacie region is located in the South-Eastern part of Poland, bordered by Ukraine and Slovakia. We found that 6.3% of unselected cases of ovarian cancer in this region carried one of 13 causative founder variants in the BRCA1 or BRCA2. This is a lower rate than in other regions of Poland, where the frequency of BRCA1 causative founder variants was observed in about 10–13.5% of ovarian cancer patients [2,3,4,5,6,7,8]. The frequency is, however, high enough to support genetic testing for all ovarian cancer patients in the region.
Since Podkarpacie is on a boundary region of Poland next to Ukraine, it is possible that the population slightly differs from other regions of Poland in terms of genetic makeup. In the nineteenth century, the region was part of Habsburg empire with significant minorities of Ukrainians and Jews, as well as a small proportion of Germans, Austrians, Hungarians and Armenians. Recently, Gorodetska et al. observed a slightly lower frequency of c.5266dupC and c.181 T > G BRCA1 mutations in a series of ovarian cancer patients from Ukraine as compared to other Slavic populations [9,10,11,12,13,14,15,16]. Genetic testing of BRCA1 mutations has been in place in the region since 2000. Several hundred mutation carriers have been diagnosed and a significant number of prophylactic oophorectomies have been performed. It may also, to some extent, affect the lower frequency of detected mutations than previously observed in other regions.
Our study was limited to screening for only 13 causative founder variants in BRCA1/2 and possibly other mutations will be detected with whole BRCA1/2 genes sequencing. In this study, the spectrum of detected BRCA1 mutations was similar to the rest of Poland and to other Slavic countries [9,10,11,12,13,14,15,16]. We observed the BRCA1 c.5266dupC mutation in 60% of carriers and the BRCA1 c.181 T > G mutation in 30% of carriers. In one case, the BRCA1 c.676delT mutation was detected. This mutation is rare in Polish population but should be included in the panel of recurrent mutations in the screening tests. Among the familial breast and ovarian cancers from this region, we also observed a higher frequency of the c.68_69delAG BRCA1 mutation (data not shown), but this mutation was not observed in this series of consecutive ovarian cancers.
The mean age of diagnosis of the 10 cases with BRCA1 mutations was 55.5 years, which is slightly higher than the mean age observed in other regions of Poland [6,7,8]. Possibly there are lifestyle/environmental factors which may influence later age of diagnosis, however, for non-carriers, the mean age of diagnosis was similar in Podkarpacie and in the rest of Poland (58.75 vs. 56.2–62.3 years) [6,7,8].
A strong breast or ovarian cancer family history was reported by 24.7% of ovarian cancer patients. It is significantly more frequent than observed by other authors [6, 8]. We think that this phenomenon may be caused by a larger number of family members than in other regions of Poland. Urbanization in this region used to be lower and fertility rate higher. We observed strong cancer family history in 6 out of 10 mutation carriers, which is more frequent than observed in other regions [6,7,8]. It can be explained by a relatively larger number of family members. However, between familial cases as well as between all patients with ovarian cancer we observed the lower frequency of mutation carriers, therefore we think that we could have missed some mutations while testing only causative founder variants.
There are several limitations to our study. The number of cases is relatively small and the results are based on 10 mutation-positive ovarian cancer cases. The increase of the study group was not possible because the registry of unselected, consecutive ovarian cancer patients was organized in 2013, and other hospitals did not create such a registry. Due to the high mortality of patients with ovarian cancer, a retrospective study based on a group diagnosed before 2013 may be biased. We screened only for the limited number of recurrent mutations and it is possible that other rare mutations were missed. Since the frequency of BRCA-carriers was significantly lower, including cases with family history of breast or ovarian cancer, than in other regions, the study based on sequencing of whole BRCA1 and BRCA2 should be performed.
Conclusions
Approximately 6% of unselected ovarian cancer patients in the region of Podkarpacie Poland carry a BRCA1 causative founder variants. These causative variants frequency was significantly lower than in other regions of Poland but it is sufficiently high to support the recommendation that all ovarian cancer patients undergo genetic testing regardless of the age of diagnosis or family history.
Abbreviations
- PCR:
-
Polymerase chain reaction
References
Wojciechowska U, Didkowska J, Zatoński W. Cancer. Poland: National Cancer Registy; 2008. http://onkologia.org.pl/wp-content/uploads/Nowotwory2008.pdf
Górski B, Byrski T, Huzarski T, Jakubowska A, Menkiszak J, Gronwald J, et al. Founder mutations in the BRCA1 gene in polish families with breast–ovarian cancer. Am J Hum Genet. 2000;66(6):1963–8.
Górski B, Jakubowska A, Huzarski T, Byrski T, Gronwald J, Grzybowska E, et al. A high proportion of founder BRCA1 mutations in polish breast and ovarian cancer families. Int J Cancer. 2004;110:683–6.
Wojcik P, Jasiowka M, Strycharz E, Sobol M, Hodorowicz-Zaniewska D, Skotnicki P, et al. Recurrent mutations of BRCA1, BRCA2 and PALB2 in the population of breast and ovarian cancer patients in southern Poland. Hered Cancer Clin Pract. 2016;14:5.
Gaj P, Kluska A, Nowakowska D, Bałabas A, Piątkowska M, Dabrowska M, et al. High frequency of BRCA1 founder mutations in polish women with nonfamilial breast cancer. Familial Cancer. 2012;11(4):623–8.
Brozek I, Ochman K, Debniak J, Morzuch L, Ratajska M, Stepnowska M, et al. High frequency of BRCA1/2 germline mutations in consecutive ovarian cancer patients in Poland. Gynecol Oncol. 2008;108(2):433–7.
Ratajska M, Krygier M, Stukan M, Kuźniacka A, Koczkowska M, Dudziak M, et al. Mutational analysis of BRCA1/2 in a group of 134 consecutive ovarian cancer patients. Novel and recurrent BRCA1/2 alterations detected by next generation sequencing. J Appl Genet. 2015;56(2):193–8.
Menkiszak J, Gronwald J, Górski B, Jakubowska A, Huzarski T, Byrski T, et al. Hereditary ovarian cancer in Poland. Int J Cancer. 2003;106(6):942–5.
Gorodetska I, Serga S, Lahuta T, Ostapchenko L, Demydov S, Khranovska N, Skachkova O, Inomistova M, Kolesnik O, Svintsitsky V, Tsip N, Peresunko A, Kmit’ N, Manzhura O, Rossokha Z, Popova O, Salomakhina H, Kyriachenko S, Kozeretska I. Prevalence of two BRCA1 mutations, 5382insC and 300T > G, in ovarian cancer patients from Ukraine. Familial Cancer. 2017;11 https://doi.org/10.1007/s10689-017-9978-9. [Epub ahead of print]
Oszurek O, Gorski B, Gronwald J, Prosolow Z, Uglanica K, Murinow A, et al. Founder mutations in the BRCA1 gene in west Belarusian breast–ovarian cancer families. Clin Genet. 2001;60:470–1.
Uglanitsa N, Oszurek O, Uglanitsa K, Savonievich E, Lubiński J, Cybulski C, et al. The contribution of founder mutations in BRCA1 to breast cancer in Belarus. Clin Genet. 2010;78(4):377–80.
Bogdanova N, Antonenkova NN, Rogov YI, Karstens JH, Hillemanns P, Dörk T. High frequency and allele-specific differences of BRCA1 founder mutations in breast cancer and ovarian cancer patients from Belarus. Clin Genet. 2010;78(4):364–72.
Sokolenko AP, Mitiushkina NV, Buslov KG, Bit-Sava EM, Iyevleva AG, Chekmariova EV, et al. High frequency of BRCA1 5382insC mutation in Russian breast cancer patients. Eur J Cancer. 2006;42:1380–4.
Pohlreich P, Zikan M, Stribrna J, Kleibl Z, Janatova M, Kotlas J, et al. High proportion of recurrent germ line mutations in the BRCA1 gene in breast and ovarian cancer patients from the Prague area. Breast Cancer Res. 2005;7:R728–36.
Krajc M, Teugels E, Zgajnar J, Goelen G, Besic N, Novakovic S, et al. Five recurrent BRCA1/2 mutations are responsible for cancer predisposition in the majority of Slovenian breast cancer families. BMC Med Genet. 2008;9:83.
Konecny M, Milly M, Zavodna K, Weismanova E, Gregorova J, Mlkva I, et al. Comprehensive genetic characterization of hereditary breast/ovarian cancer families from Slovakia. Breast Cancer Res Treat. 2011;126(1):119–30.
Acknowledgements
Authors thank to Stanisława Pasternak, Małgorzata Drozd, Renata Gibaszek and Lidia Jabłońska for excellent assistance in material and data collection. Authors thank for support to Breast Cancer Campaign Estée Lauder Companies.
Funding
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
TK the study design, database management, sample collection and computing results, writing manuscript; AJ sample collection, the manuscript review and approval; EM data collection, the manuscript review and approval; RJ data collection, the manuscript review and approval; JL the study design, molecular analysis, the manuscript review and approval; SN the study design, the manuscript review and approval, JG: the study design, database management, sample collection and computing results, molecular analysis, writing manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All participants gave informed written consent prior blood donating. The study was approved by Ethics Committee of the Pomeranian Medical University in Szczecin, Poland (decision No. BN-001/174/05.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Kluz, T., Jasiewicz, A., Marczyk, E. et al. Frequency of BRCA1 and BRCA2 causative founder variants in ovarian cancer patients in South-East Poland. Hered Cancer Clin Pract 16, 6 (2018). https://doi.org/10.1186/s13053-018-0089-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13053-018-0089-x