Abstract
In recent decades, bacteria’s therapeutic role has aroused attention in medicinal and pharmaceutical research. While bacteria are considered among the primary agents for causing cancer, recent research has shown intriguing results suggesting that bacteria can be effective agents for cancer treatment – they are the perfect vessels for targeted cancer therapy. Several bacterial strains/species have been discovered to possess inherent oncolytic potentials to invade and colonize solid tumors in vivo. The therapeutic strategy of using bacteria for treating cancer is considered to be effective; however, the severe side effects encountered during the treatment resulted in the abandonment of the therapy. State-of-the-art genetic engineering has been recently applied to bacteria therapy and resulted in a greater efficacy with minimum side effects. In addition, the anti-cancer potential of tumor-targeting bacteria through oral administration circumvents the use of the intravenous route and the associated adverse effects. This review aims to provide a comprehensive summary of the latest literature on the role of bacteria in cancer treatment.
Similar content being viewed by others
Background
Despite the advancement in cancer treatment and detection, cancer has remained a major health problem and one of the leading causes of deaths worldwide. About 600,000 cancer deaths were projected to occur in the United States in 2016, out of 1.6 million newly reported cancer cases [1]. Similarly, about 3million cancer deaths were estimated to occur in China in 2015, out of 4 million reported cancer cases [2].
The conventional chemotherapeutic agents used for the treatment of cancer possess non-specific toxicity toward normal body cells. Also the body cells that are exposed to chemotherapy often become resistant to drugs because of enhanced capability to repair DNA defects in cellular machinery which intervenes apoptosis. The exposed cells increase the production of enzymes that cause detoxification of drug and drug delivery services [3]. These inherent complications of chemotherapy, which include drug resistive mechanism of cells caused by chemotherapy, have caused scientists to focus on examining the potential of using bacteria and their compounds for anti-cancer therapy [3].
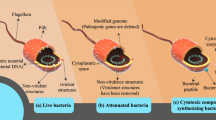
Bacteria are carcinogens and tumor promoters [4]. Bacteria produce toxins that disrupt the cellular signal thus perturbing the regulation of cell growth. Also, they are potential tumor promoters through inducing inflammation. Some bacteria strains notable for causing cancer are presented in Fig. 1. The strains include Helicobacter pylori, which is associated with gastric cancer [5], Salmonella typhi which is associated with hepatobiliary carcinoma [6], Campylobacter Jejuni which is associated with small intestinal lymphomas [7], Chlamydia psittaci which is associated with ocular lymphomas [8], Mycobacte-rium tuberculosis which is associated with lung cancer [9], and Citrobacter rodentium, which is associated with human colorectal cancer [10]. In addition, the enzymes produceed by bacteria are potential carcinogens, such as peptidyl arginine deaminase (PAD) enzymes that are found in oral bacteria and associated with pancreatic cancer [11, 12]. Also, quorum sensing peptides, such as PhrG from Bacillus subtilis, competence stimulating peptide (CSP) from Streptococcus mitis and extracellular death factors (EDF) from Escherichia coli together with their tripeptide analogue, are reported to promote tumor cell invasion and angiogenesis through type I collagen extracellular matrix, which influences tumor metastasis [13]. The quorum sensing peptides down-regulate microRNA-222 and initiate angiogenesis which promotes neovascularization and results in tumor metastasis [14].
Conversely, bacteria have shown great potential for cancer therapy. Bacteria of many species demonstrate the surprising ability to invade and colonize solid tumors, which often results in neoplasm growth retardation, and in some instances, complete tumor clearance [15]. Different strains of Clostridia, Bifidobacteria and Salmonella are capable of colonizing the hypoxic area of the tumor and destroy the tumor cells. Therefore, they are potential strains for selective tumor targeting therapy [16,17,18,19,20,21] (Fig. 1).
Bacteria create anti-tumor effects through the depletion of nutrients required for cancer cell metabolism [22]. The tumor tissues that are deoxygenated nurture the accumulation of obligate anaerobic bacteria – which only survive in the anoxic region [23]. Observation has shown that the systemic administration of Salmonella bacteria flushed into the solid tumor through severe haemorrhaging area, the area which leads to necrotic regions in which bacteria proliferate [24], colonized the tumor and decreased the proliferation of the tumor. The necrotic regions are formed because of the reduction of oxygen and nutrient supply, which leads to the breaking down of blood vessels in the hemorrhagic area. This causes the tumor cells in the center of the tumor to die from starvation and suffocation [24]. The tumor microenvironment may be conducive to bacterial survival and growth, as it may provide protection from the host immune system and nutrients [25].
Bacteria mediated tumor therapy (BMTT) has been demonstrated for centuries. However, the associated adverse side effects hinder its development. Adequate balance between the control of infection and the therapeutic benefit of bacteria is an essential requirement for a successful BMTT, but this can only be achieved via the heat-inactivation method [26]. Recently, state-of-art genetic engineering has increased the ability to alter bacterial strains [27]. Such alteration reduces bacteria side effects while increasing their therapeutic benefits. For example, the well-established bacillus Calmette–Guerin (BCG) vaccine for the treatment of human bladder cancer is arguably superior to intravesical chemotherapy for superficial disease. The therapy is commonly used as the first-line adjuvant treatment [28]. Similarly, tumor-detecting bacteria provide a sensitive and minimally invasive method to detect tumor recurrence, monitor treatment efficacy, and identify the onset of metastatic disease [29]. For example, probiotic Escherichia coli Nissle 1917 has been used to develop the orally administered diagnosis that can noninvasively indicate the presence of liver metastasis by producing easily detectable signals in urine [30]. Such genetic engineering approaches have paved the way for further development of promising bacteria-based cancer therapy.
Bacteria as anti-cancer agents through enhancing human immunity
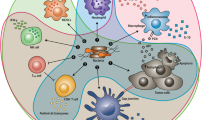
An important factor that applies to the spontaneous regression of cancer is the duality of the immune system [31]. Bacteria interact with the host as either pathogen or normal flora. The pathogenic interaction of bacteria enhances the immune system of the host in different ways.
Activating inflammasome pathways
The ∆ppGpp Salmonella typhimurium strain activates inflammasome pathways by damaging the signals released from cancer cells. This phenomenon significantly increases the amount of inflammatory cytokine IL-1β, TNF-α and Il-18 in tumors, which results in drastic tumor growth suppression [32]. IL-1β is the proinflammatory cytokine that plays a pivotal role in immunity against pathogens [33]. The IL-1β is secreted by LPS (lipopolysaccharides) during the activation of toll like receptor (TLR4) and inflammasomes, which then causes the damage to cancer cells [32]. The cancer cells are also damaged when bacteria activate inflammasomes in BMDM, which is involved in phagocytosis of damaged cancer cells by macrophages [32]. Therefore, the ∆ppGpp Salmonella typhimurium shows therapeutic efficacy for cancer through involving the inflammasome pathway [32].
CD4, CD25 and CD8 anti-tumor effectors T cell responses
Anaerobic bacteria such as E. Coli, which are capable of engulfing the solid tumors, are indirectly involved in clearance of some tumor cells (e.g. CT26) through infectious-defense mechanism. Once these bacteria invade the host, they stimulate the initiation of the defense mechanism of the host, which results in the production of lymphocytes T cells. The produced lymphocytes T cells are significantly involved in anti-tumor activity. During the induction phase of bacterial infection, CD8+ T cells are the only effectors responsible for tumor clearance; whereas, in the memory phase the clearance also involves CD8+ and CD4+T cells [34]. CD8+ T is reported to take part in the clearance of the original tumor after bacterial infection [34]. Similarly, the anti-tumor effectors T cells (CD4+ and CD8+) have the potential to block the formation of a new set of tumors. The CD8+ T cell is said to have the additional ability to eradicate even the already established tumors [34]. Furthermore, the lymphocytes (CD4 and CD25) and cytokines are reported to be the novel therapies for colon cancer in humans [35]. Their role in host immune response on carcinogenesis is invaluable [34, 35]. The regulatory cells (like CD4 and CD25) are capable of reducing the severity of inflammatory bowels and lower the risk of colon cancer [35]. In addition, the introduction of regulatory cells into chronically infected mice with established cancer showed the reduction of the severity of colitis, epithelial dysplasia, and cancer [35].
The TNF-α innate immune system in bacteria-based tumor necrosis
The tumor necrosis factor (TNF- α) has the potential to damnify the vascular endothelial cells. TNF-α plays the significant part in the formation of the large haemorrhaging area that appears within the tumor. Systematic administering of Salmonella enterica serovar Typhimurium to mice models indicated that haemorrhaging increases the flushing of bacteria into the solid tumor resulting in necrosis [24]. Moreover, the activeness of the innate immune system of the host, especially neutrophilic granulocytes, is proportional to the area of necrotic. Neutrophiles function to separate the bacteria-containing necrotic region from the bacteria that migrate into the tumor from viable tumor cells. The depletion of host neutrophils increases the number of bacteria in the tumor and increases the ability of bacteria to migrate into vital tumor tissue [36]. Thus, the complete eradication of the established tumors could be attained with the increasing size of necrosis. Similarly, the depletion of host neutrophils amplifies the bacteria-mediated tumor therapy.
Bacteria as anti-cancer agents through released substances
Some substances secreted by bacteria, such as enzymes, can inhibit the growth of tumors. Several experimental studies discovered the therapeutic potential of different substances released from bacteria for treating cancer cell lines.
Bacteriocins
Bacteriocins are cationic peptides that are synthesized by almost all groups of bacteria ribosomally. Bacteriocins are non-immunogenic, biodegradable and contain cancer cell-specific toxicities. The bacteriocins have the potential to serve as synergistic agents to conventional cancer drugs [37]. Cancer cell membranes predominantly carry negative charge; thus bacteriocins preferentially bind to cancer cell membranes than to the normal cell membranes, which are neutral in charge and selective for binding of bacteria [38].
Colicins, the bacteriocin secreted from Enterobacteriaceae such as Escherichia coli (E.coli), are known to have anti-cancer activities against a variety of human tumor cell lines in vitro, including breast cancer, colon cancer, bone cancer and uteri cell line HeLa (human cervical adenocarcinoma) [38]. Microcin E492, part of Colicins from Klebsiella pneumoniae, was found to induce apoptosis in some human malignant cell lines such as HeLa, Jurkat (T cell derived from acute T cell leukemia), RJ2.25 (a variant of Burkitt’s lymphoma), and colorectal carcinoma cells, with no effect on normal cells [17]. Pediocin isolated from Pediococcus acidilactici K2a2-3 was reported to have cytotoxic activities against HT29 (human colon adenocarcinoma) and HeLa cell lines [39]. Similarly, Nisin (the bacteriocins from Lactobacillus lactis) possesses cytotoxic effect on MCF-7 (human breast adenocarcinoma cell line) [40], HepG2 (liver hepatocellular carcinoma) [41], and HNSCC (head and neck squamous cell carcinoma) [42], both in vitro and in vivo. Conversely, Nisin is non-toxic and safe to humans, WHO has approved it for human consumption.
Furthermore, partially purified bacteriocins produced by certain bacteria such as Pseudomonas aeruginosa have shown anti-cancer activities [43]. Pyocin, the bacteriocin produced by more than 90% of Pseudomonas aeruginosa strains [44,45,46], showed lethal effect on the L6OT mice fibroblast cell line [47]. Likewise, purified and partially purified pyocin S2 showed the cytotoxicity effect on tumor cell line HepG2 and Im9 (Human immunoglobulin-secreting cell line derived from multiple myeloma) with no effect on normal cell line HFFF (Human fetal foreskin fibroblast) [48].
Phenazine 1,6-di-carboxylic acid (PDC)
Multiple phenazine metabolites such as phenazine 1-carboxylic acid (PCA) and Phenazine 1,6-di-carboxylic acid (PDC) are derived from bacteria strains (e.g. Pseudomonus aeruginosa). The PDC phenazine was first isolated from Streptomyces species; it was demonstrated to be the potential agent for controlling metabolism and biofilm formation in Candida albicans [49, 50]. Compared to other phenazine metabolites, PDC showed a substantially broader spectrum of cytotoxicity effect towards a number of cancer cells of different origins, including HT29, HeLa, and MCF7 cell lines, with less activity on DU145 (Human prostate cancer cell lines) [51].
Bacteria as anti-cancer agents through biofilms
Biofilm is a primitive form of multicellular life that provides bacteria with tolerance strength against antibiotics and host defense mechanisms [52]. Biofilms are common to opportunistic bacterial pathogens such as Salmonella tyhimurium, and they (the biofilms) are decisive in the pathogenesis of chronic infectious diseases [53]. Salmonella tyhimurium and some other pathogens are known to cause severe haemorrhage within the tumor. Once the haemorrhae is activated, it induces the production of T cells that are very significant in biofilm induction [53]. Notwithstanding the etiopathogenesis of biofilms and its protective role that allows bacteria to escape from the host defense system [53], recent discoveries have revealed the potential ability and efficacy of biofilms in cancer therapy.
Anti-cancer drugs cause the induction of biofilm formation during cancer treatment, which results in metastasis distraction [54, 55]. Similarly, the formation of bacteria biofilm on cancer cells during the SOS response results in metastasis disruption. Thus bacteria biofilm shows potential usefulness in cancer treatment [56]. Bacteria biofilm can affect colon cancer development and progression through modifying cancer metabolome to produce a regulator of cellular proliferation [56]. Also, the bacterial macromolecules necessary for biofilm formation such as proteins and DNA coat cancer cells to block metastasis [57]. For example, polysaccharides released by Streptococcus agalactiae inhibit adhesion of cancer cells to endothelial cells, an essential step in cancer metastasis [58]. Furthermore, [59] demonstrates the potential application of iron oxide nanowires from a biofilm waste produced by bacteria (Mariprofundus ferroxydans) as a new multifunctional drug carrier for cancer therapy and cancer hyperthmia.
While the above hypotheses avow for the potential of bacterial biofilm in cancer therapy, the evidence is relatively insufficient to build-up the case. However, the efficacy of bacteria biofilm for metastasis distraction calls for the further examination and investigation of the anti-cancer ability of bacteria biofilm.
Bacteria as a carrier for cancer therapeutic agents
Apart from their direct anti-cancer effect, tumor-targeting bacteria can also be used as carriers for cancer therapeutic agents in cancer treatments. Recent studies have revealed that bacteria are capable of targeting both primary tumors and metastasis [60,61,62,63].
Bacteria-mediated anti-angiogenesis therapy
The growth and metastasis of solid tumors depend on the formation of new blood vessels (angiogenesis). Thus blocking tumor angiogenesis can be a reasonable approach to treat solid tumors. Jia et al. [64] applied the combination therapy of a low dose of Salmonella (attenuated, auxotrophic) and rhEndostatin in a murine model of malignant melanoma which resulted in the reduction of the tumor growth. The therapy is argued to be safer and effective, but also economically desirable – it decreases possible side effects and lowers the therapeutic expense. Moreover, Li et al. [65] used Bifidobacterium adolescentis (non-pathogenic) as a vector for the expression of endostatin within tumors. Their findings showed that Bifidobacterium adolescentis strongly inhibit the angiogenesis and significantly inhibit local tumor growth. Bifidobacterium longum efficiently delivered the anti-angiogenic protein (endostatin) to murine liver tumors and induced anti-tumor activity [66, 67]. In addition, the oral anti-angiogenic bacterial vaccines directed against vesicular endothelial growth factor receptor 2 (VEGFR-2) were proven efficacious in animal models of malignant melanoma, colorectal carcinoma and lung cancer [68].
The combined therapy of bacteriolytic and anti-angiogenic using tumor-targeting bacteria has also shown promising results. Bacteria are capable of invading the poorly perfuse tumor areas (which are not accessible by systemically administered agents) using their unique metabolic features. They cause the inhibition of angiogenisis to kill residual tumor cells, and hence significantly increase the chance for tumor eradication [69]. In addition, the anti-tumor effect can be enhanced through co-administration of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and endostatin [70].
The combined treatment of bacteria and viruses
Oncolytic viruses (OVs) have shown positive outcomes for cancer treatment through their tumor-selective replication and multi-modality attack against cancers [71]. The viral-mediated oncolysis cancer therapy approach is potentially more effective and less toxic than current treatment regimes. While bacteria are arguably better at targeting the tumor, viruses are argued to possess unprecedented abilities to kill cancer cells. Apart from the generic effective killing mechanisms, certain virus mutants have the ability to selectively kill cancer cells [72]. Bacteria have the ability to disseminate the virus inside the tumor and induce a strong immune response against tumor antigens [73]. In their experiment, Cronin et al. [74] found that non-pathogenic bacteria (Escherichia coli) expressing B18R enhanced the oncolytic potential of the vesicular stomatitis virus (VSVΔ51) to reduce tumor growth, and thus prolonged the survival of an aggressive tumor model.
Bacteria-based microrobot (Bacteriobot)
The bacteriorobot technique is a new innovative theranostic methodology of bacteria-based fabrication for tumor therapy. The technique uses bacteria as microactuators and microsensors to deliver microstructures for targeting and treating solid tumors [75]. Various types of biomedical microrobots have been invented through the convergence of technologies from micro electromechanical system (MEMS) with nano- and bio-technologies [76, 77]. In an attempt to develop microrobot therapy, Park et al. [75] encapsulated therapeutic bacteria (Salmonella typhimurium) in biocompatible/biodegradable alginate microbeads and attached flagellated bacteria (Salmonella typhimurium) on the microbead to fabricate a bacteria-based microrobot in the targeted tumor region. The bacteria-encapsulated delivery system protects the bacteria from being attacked by the immune system, which is safer than the direct inoculation of bacteria [78].
Conclusion
Bacteria are the double-edged sword in cancer therapy. Using bacteria for cancer therapy is feasible and its potential to treat solid tumors has been known for decades. However, the clinical application of this therapy never became routine because of the adverse uncontrollable side effects. In an effort to overcome these side effects, some attenuated species of bacteria capable of treating cancer have been recently identified and studied. These species of bacteria are considered safe for cancer therapeutic application with little or no side effects. While bacteria alone may not demonstrate fully therapeutic potential, their modifications as anti-tumor agents, anti-oncogenes or immunogenic antigens, and their combination with other therapeutic processes will improve their potential for cancer therapy. The arena of using bacteria as an anti-cancer agent is still new; further studies are imperative to scrutinize the clinical significance of bacteria-based cancer therapy. The findings presented in this review suggest that this promising cancer therapy needs to be optimized and developed further.
Abbreviations
- BCG:
-
Bacillus Calmette–Guerin
- BMDM:
-
Bone marrow derived macrophages
- BMTT:
-
Bacteria mediated tumor therapy
- CT26:
-
Murine colon carcinoma
- DU145:
-
Human prostate cancer cell lines
- HeLa:
-
Human cervical adenocarcinoma
- HepG2:
-
Liver hepatocellular carcinoma
- HFFF:
-
Human fetal foreskin fibroblast
- HNSCC:
-
Head and neck squamous cell carcinoma
- HT29:
-
Human colon adenocarcinoma
- Jurkat:
-
T cell derived from acute T cell leukemia
- L6OT:
-
Mice fibroblast cell line
- LPS:
-
lipopolysaccharides
- MCF7 cells:
-
Human breast adenocarcinoma cell line
- MEMS:
-
Micro Electro Mechanical System
- NSCLC:
-
Non Small Cell Lung Cancer
- OVs:
-
Oncolytic viruses
- PAD:
-
Peptidyl arginine deaminase
- PAP:
-
Pseudomonas aeruginosa preparation
- PCA:
-
Phenazine 1-carboxylic acid
- PDC:
-
Phenazine 1,6-di-carboxylic acid
- RJ2.25:
-
Variant of Burkitt’s lymphoma
- TLR4:
-
Toll like receptor
- TNF- α:
-
Tumor necrosis factor
- TRAIL:
-
Tumor necrosis factor-related apoptosis-inducing ligand
- VEGFR-2:
-
Vesicular endothelial growth factor receptor 2
- VSVΔ51:
-
Vesicular Stomatitis Virus
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30.
Chen W, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–32.
Raguz S, Yague E. Resistance to chemotherapy: new treatments and novel insights into an old problem. Br J Cancer. 2008;99(3):387–91.
Lax AJ. Opinion: bacterial toxins and cancer--a case to answer? Nat Rev Microbiol. 2005;3(4):343–9.
Correa P, et al. Helicobacter pylori and gastric carcinoma. Serum antibody prevalence in populations with contrasting cancer risks. Cancer. 1990;66(12):2569–74.
Caygill CP, et al. The association between typhoid carriage, typhoid infection and subsequent cancer at a number of sites. Eur J Cancer Prev. 1995;4(2):187–93.
Mesnard B, et al. Immunoproliferative small intestinal disease associated with campylobacter jejuni. Dig Liver Dis. 2012;44(9):799–800.
Ferreri AJ, et al. Evidence for an association between chlamydia psittaci and ocular adnexal lymphomas. J Natl Cancer Inst. 2004;96(8):586–94.
Gupta PK, et al. Mycobacterium tuberculosis H37Rv infected THP-1 cells induce epithelial mesenchymal transition (EMT) in lung adenocarcinoma epithelial cell line (A549). Cell Immunol. 2016;300:33–40.
De Spiegeleer B, et al. The quorum sensing peptides PhrG, CSP and EDF promote angiogenesis and invasion of breast cancer cells in vitro. PLoS One. 2015;10(3):e0119471. https://doi.org/10.1371/journal.pone.0119471.
Ogrendik M. Oral bacteria in pancreatic cancer: mutagenesis of the p53 tumour suppressor gene. Int J Clin Exp Pathol. 2015;8(9):11835–6.
Jacob JA. Study links periodontal disease Bacteria to pancreatic Cancer risk. JAMA. 2016;315(24):2653–4.
De Spiegeleer B, et al. The quorum sensing peptides PhrG, CSP and EDF promote angiogenesis and invasion of breast Cancer cells in vitro. PLoS One. 2015;10(3):e0119471.
Wynendaele E, et al. Crosstalk between the microbiome and cancer cells by quorum sensing peptides. Peptides. 2015;64(Supplement C):40–8.
Leschner S, Weiss S. Salmonella-allies in the fight against cancer. J Mol Med (Berl). 2010;88(8):763–73.
Wei MQ, et al. Facultative or obligate anaerobic bacteria have the potential for multimodality therapy of solid tumours. Eur J Cancer. 2007;43(3):490–6.
Chang J, et al. Pseudomonas aeruginosa preparation plus chemotherapy for advanced non-small-cell lung cancer: a randomized, multicenter, double-blind phase III study. Med Oncol. 2015;32(5):139.
Hetz C, et al. Microcin E492, a channel-forming bacteriocin from Klebsiella pneumoniae, induces apoptosis in some human cell lines. Proc Natl Acad Sci U S A. 2002;99(5):2696–701.
Pawar V, et al. Murine solid tumours as a novel model to study bacterial biofilm formation in vivo. J Intern Med. 2014;276(2):130–9.
Ganai S, et al. In tumors Salmonella migrate away from vasculature toward the transition zone and induce apoptosis. Cancer Gene Ther. 2011;18(7):457–66.
Yan L, et al. Photodynamic treatment of tumor with Bacteria expressing KillerRed. PLoS One. 2015;10(7):e0131518.
Danino T, et al. Measuring growth and gene expression dynamics of tumor-targeted S. Typhimurium bacteria. J Vis Exp. 2013;77:e50540.
Forbes NS. Engineering the perfect (bacterial) cancer therapy. Nat Rev Cancer. 2010;10(11):785–94.
Leschner S, et al. Tumor invasion of Salmonella enterica serovar typhimurium is accompanied by strong hemorrhage promoted by TNF-alpha. PLoS One. 2009;4(8):e6692.
Nallar SC, Xu DQ, Kalvakolanu DV. Bacteria and genetically modified bacteria as cancer therapeutics: current advances and challenges. Cytokine. 2017;89:160–72.
Coley WB. The treatment of inoperable sarcoma by bacterial toxins (the mixed toxins of the Streptococcus erysipelas and the Bacillus prodigiosus). Proc R Soc Med. 1910;3(Surg Sect):1–48.
Felgner S, et al. Optimizing Salmonella enterica serovar typhimurium for bacteria-mediated tumor therapy. Gut Microbes. 2016;7(2):171–7.
Alexandroff AB, et al. Recent advances in bacillus Calmette-Guerin immunotherapy in bladder cancer. Immunotherapy. 2010;2(4):551–60.
Panteli JT, et al. Genetically modified bacteria as a tool to detect microscopic solid tumor masses with triggered release of a recombinant biomarker. Integr Biol (Camb). 2015;7(4):423–34.
Danino T, et al. Programmable probiotics for detection of cancer in urine. Sci Transl Med. 2015;7(289):289ra84. https://doi.org/10.1126/scitranslmed.aaa3519.
Kucerova P, Cervinkova M. Spontaneous regression of tumour and the role of microbial infection--possibilities for cancer treatment. Anti-Cancer Drugs. 2016;27(4):269–77.
Phan TX, et al. Activation of inflammasome by attenuated Salmonella typhimurium in bacteria-mediated cancer therapy. Microbiol Immunol. 2015;59(11):664–75.
Netea MG, et al. IL-1beta processing in host defense: beyond the inflammasomes. PLoS Pathog. 2010;6(2):e1000661.
Stern C, et al. Induction of CD4(+) and CD8(+) anti-tumor effector T cell responses by bacteria mediated tumor therapy. Int J Cancer. 2015;137(8):2019–28.
Erdman SE, et al. CD4(+)CD25(+) regulatory lymphocytes require interleukin 10 to interrupt colon carcinogenesis in mice. Cancer Res. 2003;63(18):6042–50.
Westphal K, et al. Containment of tumor-colonizing bacteria by host neutrophils. Cancer Res. 2008;68(8):2952–60.
Kaur S, Kaur S. Bacteriocins as potential anticancer agents. Front Pharmacol. 2015;6:272.
Chumchalova J, Smarda J. Human tumor cells are selectively inhibited by colicins. Folia Microbiol (Praha). 2003;48(1):111–5.
Villarante KI, et al. Purification, characterization and in vitro cytotoxicity of the bacteriocin from Pediococcus acidilactici K2a2-3 against human colon adenocarcinoma (HT29) and human cervical carcinoma (HeLa) cells. World J Microbiol Biotechnol. 2011;27(4):975–80.
Tagg JR, Dajani AS, Wannamaker LW. Bacteriocins of gram-positive bacteria. Bacteriol Rev. 1976;40(3):722–56.
Paiva AD, et al. Toxicity of bovicin HC5 against mammalian cell lines and the role of cholesterol in bacteriocin activity. Microbiology. 2012;158(Pt 11):2851–8.
Joo NE, et al. Nisin, an apoptogenic bacteriocin and food preservative, attenuates HNSCC tumorigenesis via CHAC1. Cancer Med. 2012;1(3):295–305.
Hill RP, Farkas-Himsley H. Further studies of the action of a partially purified bacteriocin against a murine fibrosarcoma. Cancer Res. 1991;51(5):1359–65.
Mackinnon EM. Chacterizing the pyocin activity of diverse Pseudomonas aeruginosa isolates. In: Department of Molecular Genetics. Toronto: University of Toronto; 2011. p. 65.
Ling H, et al. A predicted S-type pyocin shows a bactericidal activity against clinical Pseudomonas aeruginosa isolates through membrane damage. FEBS Lett. 2010;584(15):3354–8.
Michel-Briand Y, Baysse C. The pyocins of Pseudomonas aeruginosa. Biochimie. 2002;84(5-6):499–510.
Farkas-Himsley H, Cheung R. Bacterial proteinaceous products (bacteriocins) as cytotoxic agents of neoplasia. Cancer Res. 1976;36(10):3561–7.
Abdi-Ali A, et al. Cytotoxic effects of pyocin S2 produced by Pseudomonas aeruginosa on the growth of three human cell lines. Can J Microbiol. 2004;50(5):375–81.
Podojil M, Gerber NN. Biosynthesis of 1,6-phenazinediol 5,10-dioxide (iodinin). Incorporation of shikimic acid. Biochemistry. 1970;9(23):4616–8.
Morales DK, et al. Control of Candida albicans metabolism and biofilm formation by Pseudomonas aeruginosa phenazines. MBio. 2013;4(1):e00526–12.
Dasgupta D, et al. Isolation of phenazine 1,6-di-carboxylic acid from Pseudomonas aeruginosa strain HRW.1-S3 and its role in biofilm-mediated crude oil degradation and cytotoxicity against bacterial and cancer cells. Appl Microbiol Biotechnol. 2015;99(20):8653–65.
Kinnari TJ. The role of biofilm in chronic laryngitis and in head and neck cancer. Curr Opin Otolaryngol Head Neck Surg. 2015;23(6):448–53.
Komor U, et al. Biofilm formation by Pseudomonas aeruginosa in solid murine tumors - a novel model system. Microbes Infect. 2012;14(11):951–8.
Huang Q. Comment on “Bacteria form biofilms against cancer metastasis”; 2010. p. 203.
Wiemann B, Starnes CO. Coley's toxins, tumor necrosis factor and cancer research: a historical perspective. Pharmacol Ther. 1994;64(3):529–64.
Johnson CH, et al. Metabolism links bacterial biofilms and colon carcinogenesis. Cell Metab. 2015;21(6):891–7.
Weitao T. Bacteria form biofilms against cancer metastasis. Med Hypotheses. 2009;72(4):477–8.
Miyake K, Yamamoto S, Iijima S. Blocking adhesion of cancer cells to endothelial cell types by S. Agalactiae type-specific polysaccharides. Cytotechnology. 1996;22(1-3):205–9.
Kumeria T, et al. Naturally derived Iron oxide nanowires from Bacteria for magnetically triggered drug release and Cancer hyperthermia in 2D and 3D culture environments: Bacteria biofilm to potent Cancer therapeutic. Biomacromolecules. 2016;17(8):2726–36.
Min J-J, et al. Noninvasive real-time imaging of tumors and metastases using tumor-targeting light-emitting Escherichia coli. Mol Imaging Biol. 2008;10(1):54–61.
Weibel S, et al. Colonization of experimental murine breast tumours by Escherichia coli K-12 significantly alters the tumour microenvironment. Cell Microbiol. 2008;10(6):1235–48.
Min J-J, et al. Quantitative bioluminescence imaging of tumor-targeting bacteria in living animals. Nat Protoc. 2008;3(4):629–36.
Yu YA, et al. Visualization of tumors and metastases in live animals with bacteria and vaccinia virus encoding light-emitting proteins. Nat Biotechnol. 2004;22(3):313–20.
Jia LJ, et al. Enhanced therapeutic effect by combination of tumor-targeting Salmonella and endostatin in murine melanoma model. Cancer Biol Ther. 2005;4(8):840–5.
Li X, et al. Bifidobacterium adolescentis as a delivery system of endostatin for cancer gene therapy: selective inhibitor of angiogenesis and hypoxic tumor growth. Cancer Gene Ther. 2003;10(2):105–11.
Xu YF, et al. A new expression plasmid in Bifidobacterium longum as a delivery system of endostatin for cancer gene therapy. Cancer Gene Ther. 2007;14(2):151–7.
Fu GF, et al. Bifidobacterium longum as an oral delivery system of endostatin for gene therapy on solid liver cancer. Cancer Gene Ther. 2005;12(2):133–40.
Niethammer AG, et al. A DNA vaccine against VEGF receptor 2 prevents effective angiogenesis and inhibits tumor growth. Nat Med. 2002;8(12):1369–75.
Gardlik R, et al. Gene therapy for cancer: bacteria-mediated anti-angiogenesis therapy. Gene Ther. 2011;18(5):425–31.
Hu B, et al. Bifidobacterium longum as a delivery system of TRAIL and endostatin cooperates with chemotherapeutic drugs to inhibit hypoxic tumor growth. Cancer Gene Ther. 2009;16(8):655–63.
Russell SJ, Peng KW, Bell JC. Oncolytic virotherapy. Nat Biotechnol. 2012;30(7):658–70.
Krzykawski MP. Combined bacterial and viral treatment: a novel anticancer strategy. Cent Eur J Immunol. 2015;40(3):366–72.
Kawai K, et al. Bacillus Calmette-Guerin (BCG) immunotherapy for bladder cancer: current understanding and perspectives on engineered BCG vaccine. Cancer Sci. 2013;104(1):22–7.
Cronin M, et al. Bacterial-mediated knockdown of tumor resistance to an oncolytic virus enhances therapy. Mol Ther. 2014;22(6):1188–97.
Park SJ, et al. New paradigm for tumor theranostic methodology using bacteria-based microrobot. Sci Rep. 2013;3:3394.
Sharma NN, Mittal RK. Nanorobot movement: Challenges and biologically inspired solutions. Int J Smart Sensing Intell Syst. 2008;1(1):87–109.
Gong J, et al. Microparticles and their emerging role in cancer multidrug resistance. Cancer Treat Rev. 2012;38(3):226–34.
Park SJ, et al. Effect of chitosan coating on a bacteria-based alginate microrobot. Biotechnol Bioeng. 2015;112(4):769–76.
Acknowledgments
We would like to convey our sincere gratitude to Zakaria Ali Khamis of the University of Turku, Finland and Linda Giles for their critical reading of the manuscript and editorial assistance.
Funding
Not applicable
Availability of data and materials
Not applicable
Author information
Authors and Affiliations
Contributions
All authors contribute in writing and reading the manuscript in the order of authorship. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Song, S., Vuai, M.S. & Zhong, M. The role of bacteria in cancer therapy – enemies in the past, but allies at present. Infect Agents Cancer 13, 9 (2018). https://doi.org/10.1186/s13027-018-0180-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13027-018-0180-y