Abstract
Background
The aim of this study was to assess if FDG-PET could guide dose prescription heterogeneity and decrease arbitrary location of hotspots in SBRT.
Methods
For three patients with stage I lung cancer, a CT-simulation and a FDG-PET were registered to define respectively the PTVCT and the biological target volume (BTV). Two plans involving volumetric modulated arc therapy (VMAT) and simultaneous integrated boost (SIB) were calculated. The first plan delivered 4 × 12 Gy within the PTVCT and the second plan, with SIB, 4 × 12 Gy and 13.8 Gy (115% of the prescribed dose) within the PTVCT and the BTV respectively. The Dmax-PTVCT had to be inferior to 60 Gy (125% of the prescribed dose). Plans were evaluated through the D95%, D99% and Dmax-PTVCT, the D2 cm, the R50% and R100% and the dice similarity coefficient (DSC) between the isodose 115% and BTV. DSC allows verifying the location of the 115% isodose (ideal value = 1).
Results
The mean PTVCT and BTV were 36.7 (±12.5) and 6.5 (±2.2) cm3 respectively. Both plans led to similar target coverage, same doses to the OARs and equivalent fall-off of the dose outside the PTVCT. On the other hand, the location of hotspots, evaluated through the DSC, was improved for the SIB plans with a mean DSC of 0.31 and 0.45 for the first and the second plans respectively.
Conclusions
Use of PET to decrease arbitrary location of hotspots is feasible with VMAT and SIB for lung cancer.
Similar content being viewed by others
Introduction
Stereotactic body radiation therapy (SBRT) is considered as a standard treatment for patients with inoperable early stage lung cancer and leads to local control rates over 90% [1]-[4]. Historically, SBRT is delivered using a large number of non-overlapping treatment beams or arcs to create a sharper dose gradient between the target volume and the surrounding normal tissues. These delivery techniques result in large dose heterogeneity with a frequent dose prescription specified at the 80% (or lower) isodose and hotspots within the planning target volume (PTV). The recent ROSEL guidelines about SBRT recommend the maximum PTV dose to be between 110% and 140% of the prescription dose [5]. However, the location of these hotspots within the PTV is uncertain and uncontrolled by conventional SBRT delivery techniques.
Recent developments including volumetric modulated arc therapy (VMAT) with one or multiple arcs and varying openings of the multi-leaf collimator (MLC), dose rates and gantry speed, have resulted in more homogeneous dose distributions in SBRT [6]. However, clinical results of SBRT have historically been obtained with overdoses ranging from 120 to 140% inside the PTV, which possibly contribute the success of SBRT. In addition to delivering more homogeneous treatments, if used with simultaneous integrated boost (SIB), VMAT can also create a dose heterogeneity and controlled hotspots inside the PTV.
For non-small cell lung cancers (NSCLC), positron emission tomography (PET) with [18F]-Fluorodeoxyglucose (FDG) is frequently used to optimize radiotherapy for advanced stages with a better distinction between tumour and atelectasis, and a better detection of involved nodes. PET is not used for SBRT planning because the definition of the gross tumor volume (GTV) on computed tomography (CT) imaging in NSCLC stage I cases is not difficult. However, several studies have shown that FDG-PET could detect the sites at risk of failure inside GTV [7],[8]. These observations led us to use FDG-PET to guide hotspots and dose prescription heterogeneity for NSCLC SBRT with VMAT.
For this purpose, a feasibility study was conducted by analyzing the dosimetries of three patients. For each patient, two VMAT plans were performed and compared: the first plan delivered SBRT using only CT data, whereas the second plan delivered SBRT using CT and FDG-PET data to guide dose prescription heterogeneity.
Material and methods
Patient group
Three patients, enrolled in a clinical trial assessing the impact of 4D-FDG-PET in radiotherapy for lung cancer, were selected for this dosimetric study. The study was approved by the institutional review board of the universitary hospital and the French health authority and patients gave written informed consent to participate. All patients were male with a median age of 70 years [62–72] and peripheral T1N0M0 NSCLC (two adenocarcinomas and one squamous cell carcinoma). The tumour was located in the left inferior lobe with attachment to the chest wall for patient 1, in the middle lobe for patient 2, and in the left upper lobe for patient 3.
Imaging
For CT simulation, patients were in supine position with the arms above the head using an armrest. Data were acquired with a 3-mm slice in free breathing. No four dimensional (4D) acquisition was performed because 4D CT scan was not implemented in our department at the date of this study. For FDG-PET/CT, a 4D acquisition was performed in radiotherapy treatment position, 50 min after intravenously injection of 3.7 MBq/Kg of [18F]-FDG, on a PET/CT integrated system (Discovery RX, General Electric Medical System®, Milwaukee, WI) using the Real-time Position Management (RPM) device (Varian®) and the “Motion Free” software (General Electric Medical System®). The respiratory cycle was rebinned in six phases.
Target volumes definition
For the target volume definition, CT simulation and PET/CT were registered using a rigid algorithm. The GTVCT was delineated manually on CT simulation using a parenchymal window (−1000 and +200 Hounsfield Units). Patients 1 and 3 presented a non-mobile lesion (located in the upper lobe or attached to the chest), therefore a 5 mm isotropic margin was added to GTV to create PTVCT. For patient 2, a 8 mm isotropic margin was added.
The Biological Target Volume (BTV) was defined on 4D-PET and corresponded to the sum of the six BTV delineated on the six respiratory-gated PET images. BTV was delineated on each PET image using the fuzzy locally adaptive Bayesian (FLAB) method (9), which is an automatic segmentation method. This statistic algorithm combined with a fuzzy measure is particularly adapted to noisy and blurry PET images, and has been validated on both simulated and clinical datasets for accuracy and robustness. Also, BTV included internal target volume (ITV) in its definition.
The lung-PTV, spinal cord, oesophagus, heart, trachea, brachial plexus and chest wall were outlined as organs at risk (OAR) on CT simulation.
Dose prescription and treatment planning
For each patient, two RapidArc plans (Varian Medical System®) were calculated by the same radiophysicist using the Eclipse treatment planning software (Helios, Varian Medical System®) with the Analytic Anisotropic Algorithm v.10. For the first plan, the objective was to deliver 48 Gy in PTVCT in 4 fractions of 12 Gy. For the second plan, the objective was to deliver 48 Gy in PTVCT and to guide hotspots in BTV with SIB. Both plans adopted the following ROSEL recommendations: 95% of the PTVCT had to receive at least 100% of the prescribed dose and 99% of the PTVCT at least 90% of the prescribed dose. The maximum dose within the PTVCT (Dmax PTVCT) had to be lower than 125% of the prescribed dose. Additional constraints included the D2cm (maximal dose at 2 cm of the PTVCT), the R50% and R100% (ratios between PTVCT and the 50% and 100% isodoses, respectively) allowing to verify the rapid fall-off of the dose outside the PTVCT. The OARs dose constraints were in accordance with the RTOG 0915 trial. The Dmax of the spinal cord, the brachial plexus, the oesophagus, the heart and the trachea were inferior to 26, 27.2, 30, 34 and 34.8 Gy, respectively. For the chest wall, the dose to one cm3 (D1cc) was kept below 32Gy.
RapidArc plans were performed with three 180° coplanar arcs using 6MV photons at a maximum dose rate of 600 monitor units (MUs) per minute. Collimator angles of 15, 30 and 330° were used to minimize the tongue-and-groove effect. For the first plan, 12 Gy per fraction were prescribed at the barycentre of the PTVCT. For the second plan, 12 Gy and 13.8 Gy (115% of the prescribed dose) were prescribed with SIB in PTVCT and BTV, respectively.
Dosimetric analysis
In order to assess hotspots, we calculated the volume of the 115% isodose (Viso115%) and the Dice Similarity Coefficient (DSC). DSC allows to verify the location of the 115% isodose, and was defined as follows: DSC = (Viso115%∩VBTV)/(Viso115%∪VBTV). Ideally, DSC is equal to 1.
Results
The mean PTVCT and BTV were 36.7 (±12.5) and 6.5 (±2.2) cm3, respectively. For both plans, ROSEL recommendations were respected. The SIB technique (plan 2) led to similar target coverage than that obtained with conventional prescription on PTVCT (plan 1): the mean D95%, D99% and the Dmax of the PTVCT were 103.7% ± 1.3, 101.6% ± 2.1 and 123.5% ± 1.9 without SIB, versus 103.7% ± 2.4, 101.3% ± 2.1 and 124.4% ± 0.3 with SIB. The rapid fall-off of the dose outside the PTVCT was equivalent for both plans: the mean D2cm, R50% and R100% were 62.4% ± 3.9, 4.6% ± 0.7 and 1.1% ± 0.08 without SIB, versus 66.2% ± 3.9, 4.7% ± 0.9 and 1.09% ± 0.1 with SIB.
For both plans, constraints of doses to the OARs were respected, except for the dose to the chest wall for patient 1, who presented a tumour attached to the rib.
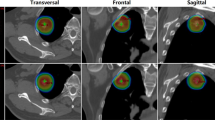
To assess the ability of the SIB to guide hotspots, we evaluated the volume of the 115% isodose and the location of this isodose through the analysis of the DSC (Table 1). The Viso115% was more important in plan 2, with a 33% and 16% increase for patients 1 and 3, respectively. On the contrary, for patient 2 the Viso115% was bigger in the plan 1 with a difference of 85%. Analysis on the location of the 115% isodose revealed that SIB technique improves DSC with a mean of 0.31 for plan 1 and 0.45 for plan 2.. SIB technique’s ability to guide hotspots in the BTV and to decrease arbitrary hotspots is illustrated in Figure 1.
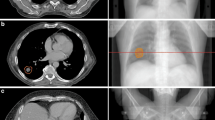
Comparison of both plans for the three patients: plans 1 in (A) and (B) and plans 2 with SIB in (C) and (D). PTVCT is shown in blue and BTV in yellow. With both techniques, the target coverage of the PTVCT with the 100% isodose is adequate but the 115% isodose is more focused on the BTV with the SIB technique.
Discussion
One limitation of this study is the lack of 4D-CT simulation, which impedes a good estimation of the ITV. An isotropic margin was also added automatically around the GTVCT, probably leading to an overestimation of the PTVCT. The present investigation, however, does not focus on target volume definition, but rather on the use of PET and VMAT to control and guide hotspots in SBRT. In this respect, the study demonstrates that it is feasible to guide dose prescription heterogeneity on FDG-PET data with VMAT and SIB. Indeed, plans 1 and 2 respected conventional dosimetric criteria of SBRT equally (D95% PTVCT, 99% PTVCT, Dmax PTVCT, D2cm, R50% and R100%) and imparted equivalent doses to the OARs. In contrast, better DSC was computed with plan 2. In this study, we chose to perform a SIB with a dose of 115% of the prescribed dose, because the BTV defined on 4D-FDG-PET is relatively large considering the ITV. FDG-PET was used because its ability to detect sites of relapse after radiotherapy is recognized. After conventional fractionated radiotherapy treatments, relapses are often located inside the area of highest uptake on baseline FDG-PET scan [7],[8]. That’s why some authors try to increase the radiation dose inside this BTV. Our purpose was based on this idea and was to integrate data of FDG-PET in SBRT focusing hotspots on BTV. Other PET tracers like hypoxia or proliferation tracers could be of interest to individualize more specific BTVs. These BTVs issued from more specific tracers are often smaller than those defined with FDG. The choice of isodose (115, 120% or higher) to focus hotspots could also vary depending on the tracer and volume of BTV. Whatever the choice of the tracer, an important difficulty stays the choice of the method of PET images segmentation. Many automatic segmentation methods are described in the literature and in the absence of a confrontation with gold standards or surgical specimens, it’s impossible to promote one method. More complex segmentation methods as gradient based or stochastic methods seem more robust than thresholding methods based on SUV [9],[10].
VMAT is particularly interesting to plan and deliver SBRT. The number of studies concerned with SBRT and VMAT has incredibly increased these last years. Several dosimetric comparisons confirmed an important reduction in treatment time compared to other delivery techniques like three-dimensional conformal radiotherapy, Cyberknife or Tomotherapy [6],[11]-[14] as well as a reduction of patient movements caused by discomfort and a reduction of the consecutive intra-fraction motion. VMAT can achieve better conformity, sharper dose fall-off outside the PTV and lower dose to normal lung [12]-[15].
However, concerns about use of modulation therapy for SBRT for tumors subject to respiratory motion have been previously highlighted with the “interplay effect” [16]. This effect describes the interplay between MLC motion and tumour motion with risk of hotspots or cold spots inside the tumor. Recent series have investigated the impact of tumour motion on 4D dose calculation for lung SBRT and found a negligible interplay effect, with differences between 3D and 4D calculations around 1% [17],[18]. Several reasons may explain this negligible effect. First, since high doses per fraction are delivered during SBRT, most VMAT segments receive a large number of MUs with a long segment delivery time (on the order of the breathing period). Moreover, modulation for lung SBRT is low with few OARs. Also, most segments contain an aperture shape conformed to the PTV and few segments have leaves blocking a part of the tumor. In the same way, the gantry speed is in general low to be able to deliver a large number of MUs at each segment. Few clinical studies about modulation intensity for lung SBRT are published but results of the Mayo clinic with IMRT [19], of Yamashita et al. [20] with VMAT or Navarria et al. [15] with VMAT unflattened beams (FFF) are good with excellent local control.
Conclusion
Use of PET to guide dose prescription heterogeneity with VMAT and SIB for lung cancer is feasible and seems promising. These preliminary results have to be confirmed with more patients and confronted to a clinical study. Moreover, other PET tracers more specific than FDG could be evaluated for this dose painting.
References
Baumann P, Nyman J, Hoyer M, Wennberg B, Gagliardi G, Lax I, Drugge N, Ekberg L, Friesland S, Johansson K-A, Lund J-A, Morhed E, Nilsson K, Levin N, Paludan M, Sederholm C, Traberg A, Wittgren L, Lewensohn R: Outcome in a prospective phase II trial of medically inoperable stage I non-small-cell lung cancer patients treated with stereotactic body radiotherapy. J Clin Oncol Off J Am Soc Clin Oncol 2009, 27: 3290-3296. 10.1200/JCO.2008.21.5681
Brock J, Ashley S, Bedford J, Nioutsikou E, Partridge M, Brada M: Review of hypofractionated small volume radiotherapy for early-stage non-small cell lung cancer. Clin Oncol R Coll Radiol G B 2008, 20: 666-676. 10.1016/j.clon.2008.06.005
Fakiris AJ, McGarry RC, Yiannoutsos CT, Papiez L, Williams M, Henderson MA, Timmerman R: Stereotactic body radiation therapy for early-stage non-small-cell lung carcinoma: four-year results of a prospective phase II study. Int J Radiat Oncol Biol Phys 2009, 75: 677-682. 10.1016/j.ijrobp.2008.11.042
Lagerwaard FJ, Haasbeek CJA, Smit EF, Slotman BJ, Senan S: Outcomes of risk-adapted fractionated stereotactic radiotherapy for stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2008, 70: 685-692. 10.1016/j.ijrobp.2007.10.053
Hurkmans CW, Cuijpers JP, Lagerwaard FJ, Widder J, van der Heide UA, Schuring D, Senan S: Recommendations for implementing stereotactic radiotherapy in peripheral stage IA non-small cell lung cancer: report from the quality assurance working party of the randomised phase III ROSEL study. Radiat Oncol Lond Engl 2009, 4: 1. 10.1186/1748-717X-4-1
Verbakel WFAR, Senan S, Cuijpers JP, Slotman BJ, Lagerwaard FJ: Rapid delivery of stereotactic radiotherapy for peripheral lung tumors using volumetric intensity-modulated arcs. Radiother Oncol J Eur Soc Ther Radiol Oncol 2009, 93: 122-124. 10.1016/j.radonc.2009.05.020
Abramyuk A, Tokalov S, Zöphel K, Koch A, Szluha Lazanyi K, Gillham C, Herrmann T, Abolmaali N: Is pre-therapeutical FDG-PET/CT capable to detect high risk tumor subvolumes responsible for local failure in non-small cell lung cancer? Radiother Oncol J Eur Soc Ther Radiol Oncol 2009, 91: 399-404. 10.1016/j.radonc.2009.01.003
Aerts HJWL, van Baardwijk AAW, Petit SF, Offermann C, van Loon J, Houben R, Dingemans A-MC, Wanders R, Boersma L, Borger J, Bootsma G, Geraedts W, Pitz C, Simons J, Wouters BG, Oellers M, Lambin P, Bosmans G, Dekker ALAJ, De Ruysscher D: Identification of residual metabolic-active areas within individual NSCLC tumours using a pre-radiotherapy (18)Fluorodeoxyglucose-PET-CT scan. Radiother Oncol J Eur Soc Ther Radiol Oncol 2009, 91: 386-392. 10.1016/j.radonc.2009.03.006
Hatt M, Cheze le Rest C, Turzo A, Roux C, Visvikis D: A fuzzy locally adaptive Bayesian segmentation approach for volume determination in PET. IEEE Trans Med Imaging 2009, 28: 881-893. 10.1109/TMI.2008.2012036
Wanet M, Lee JA, Weynand B, De Bast M, Poncelet A, Lacroix V, Coche E, Grégoire V, Geets X: Gradient-based delineation of the primary GTV on FDG-PET in non-small cell lung cancer: a comparison with threshold-based approaches, CT and surgical specimens. Radiother Oncol J Eur Soc Ther Radiol Oncol 2011, 98: 117-125. 10.1016/j.radonc.2010.10.006
Ong CL, Verbakel WFAR, Cuijpers JP, Slotman BJ, Lagerwaard FJ, Senan S: Stereotactic radiotherapy for peripheral lung tumors: a comparison of volumetric modulated arc therapy with 3 other delivery techniques. Radiother Oncol J Eur Soc Ther Radiol Oncol 2010, 97: 437-442. 10.1016/j.radonc.2010.09.027
Zhang GG, Ku L, Dilling TJ, Stevens CW, Zhang RR, Li W, Feygelman V: Volumetric modulated arc planning for lung stereotactic body radiotherapy using conventional and unflattened photon beams: a dosimetric comparison with 3D technique. Radiat Oncol Lond Engl 2011, 6: 152. 10.1186/1748-717X-6-152
Holt A, van Vliet-Vroegindeweij C, Mans A, Belderbos JS, Damen EMF: Volumetric-modulated arc therapy for stereotactic body radiotherapy of lung tumors: a comparison with intensity-modulated radiotherapy techniques. Int J Radiat Oncol Biol Phys 2011, 81: 1560-1567. 10.1016/j.ijrobp.2010.09.014
McGrath SD, Matuszak MM, Yan D, Kestin LL, Martinez AA, Grills IS: Volumetric modulated arc therapy for delivery of hypofractionated stereotactic lung radiotherapy: A dosimetric and treatment efficiency analysis. Radiother Oncol J Eur Soc Ther Radiol Oncol 2010, 95: 153-157. 10.1016/j.radonc.2009.12.039
Navarria P, Ascolese AM, Mancosu P, Alongi F, Clerici E, Tozzi A, Iftode C, Reggiori G, Tomatis S, Infante M, Alloisio M, Testori A, Fogliata A, Cozzi L, Morenghi E, Scorsetti M: Volumetric modulated arc therapy with flattening filter free (FFF) beams for stereotactic body radiation therapy (SBRT) in patients with medically inoperable early stage non small cell lung cancer (NSCLC). Radiother Oncol J Eur Soc Ther Radiol Oncol 2013, 107: 414-418. 10.1016/j.radonc.2013.04.016
Bortfeld T, Jiang SB, Rietzel E: Effects of motion on the total dose distribution. Semin Radiat Oncol 2004, 14: 41-51. 10.1053/j.semradonc.2003.10.011
Ong C, Verbakel WFAR, Cuijpers JP, Slotman BJ, Senan S: Dosimetric impact of interplay effect on RapidArc lung stereotactic treatment delivery. Int J Radiat Oncol Biol Phys 2011, 79: 305-311. 10.1016/j.ijrobp.2010.02.059
Rao M, Wu J, Cao D, Wong T, Mehta V, Shepard D, Ye J: Dosimetric impact of breathing motion in lung stereotactic body radiotherapy treatment using intensity modulated radiotherapy and volumetric modulated arc therapy [corrected]. Int J Radiat Oncol Biol Phys 2012, 83: e251-e256. 10.1016/j.ijrobp.2011.12.001
Videtic GMM, Stephans K, Reddy C, Gajdos S, Kolar M, Clouser E, Djemil T: Intensity-modulated radiotherapy-based stereotactic body radiotherapy for medically inoperable early-stage lung cancer: excellent local control. Int J Radiat Oncol Biol Phys 2010, 77: 344-349. 10.1016/j.ijrobp.2009.05.004
Yamashita H, Takahashi W, Haga A, Kida S, Saotome N, Nakagawa K: Stereotactic body radiotherapy for small lung tumors in the university of Tokyo hospital. BioMed Res Int 2014, 2014: 136513. 10.1155/2014/136513
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
BH and MA carried out the planification of the dosimetric study. MH and FL participated in the segmentation of PET images. RT, PL, AP and PF drafted the manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Henriques de Figueiredo, B., Antoine, M., Trouette, R. et al. Use of FDG-PET to guide dose prescription heterogeneity in stereotactic body radiation therapy for lung cancers with volumetric modulated arc therapy: a feasibility study. Radiat Oncol 9, 300 (2014). https://doi.org/10.1186/s13014-014-0300-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13014-014-0300-9