Abstract
Objective
Evaluate the feasibility and potential impacts on hand function using a wearable stimulation device (the VTS Glove) which provides mechanical, vibratory input to the affected limb of chronic stroke survivors.
Methods
A double-blind, randomized, controlled feasibility study including sixteen chronic stroke survivors (mean age: 54; 1-13 years post-stroke) with diminished movement and tactile perception in their affected hand. Participants were given a wearable device to take home and asked to wear it for three hours daily over eight weeks. The device intervention was either (1) the VTS Glove, which provided vibrotactile stimulation to the hand, or (2) an identical glove with vibration disabled. Participants were randomly assigned to each condition. Hand and arm function were measured weekly at home and in local physical therapy clinics.
Results
Participants using the VTS Glove showed significantly improved Semmes-Weinstein monofilament exam results, reduction in Modified Ashworth measures in the fingers, and some increased voluntary finger flexion, elbow and shoulder range of motion.
Conclusions
Vibrotactile stimulation applied to the disabled limb may impact tactile perception, tone and spasticity, and voluntary range of motion. Wearable devices allow extended application and study of stimulation methods outside of a clinical setting.
Similar content being viewed by others
Background
Over 15 million people have a stroke each year, making it one of the leading causes of disability in the United States and worldwide [1,2,3]. Upper limb disability occurs in about 50% of cases [4, 5] and diminished tactile perception in about 35-55% [6, 7]. Current methods of therapy for upper limb dysfunction after stroke focus on activities which use the limb; however, these forms of rehabilitation are not accessible to survivors with very limited function.
Somatosensory stimulation may be an effective and accessible modality for rehabilitation. Most fundamentally, somatosensory input is known to drive cortical organization and skill acquisition [8,9,10]. Somatosensory input has also been associated with sensorimotor recovery after CNS injury in animal [11, 12] as well as human studies [13]. Afferent input is also integral to limb use. Tactile perception and proprioception are factors in motor performance and are thought to co-activate with motorcortical circuits [14,15,16].
Afferent electrical stimulation has been studied as a means for providing sensory input to the disabled extremity of stroke survivors [17,18,19,20,21], and preliminary evidence shows changes in tactile perception, motor function and brain activity. Mechanical, vibratory stimulation can be applied without the placement, electrodes or gel of electrical stimulation. Afferent electrical stimulation most often targets cutaneous sensory receptors in the skin; while vibrotactile stimulation can activate both muscle afferent fibers and cutaneous sensory receptors without inducing movement. Vibrotactile stimulation has been coupled with other methods such as robotic manipulation or music practice exercises for rehabilitation [22, 23], and applied to the arm for in-situ dexterity improvement [24]. Improved spasticity and significant neuromuscular changes have been found in laboratory studies of whole-body vibration (WBV) [25,26,27,28] and focal muscle/tendon vibration [29,30,31,32,33,34,35].
Despite encouraging data, vibrotactile stimulation is not widely used outside the clinic because there are no mobile devices that can deliver and study this form of mechanical stimulation for prolonged periods of time. Here we designed a lightweight, wireless, wearable device to apply vibrotactile stimulation to the hand. Wearable devices are closely coupled with the body, and thus allow stimulation for extended periods of time and in the background of daily life. The intervention is mobile and simple to apply without access to a clinic. Users simply wear the device, requiring little exertion and time, which may facilitate adherence. The device was deployed in a controlled feasibility trial of chronic stroke survivors with upper limb sensorimotor deficits. If wearable stimulation proves to be effective it could directly impact healthcare delivery, because it may provide a mobile, affordable rehabilitation option for patients who otherwise would not have access to high intensity stroke rehabilitation.
Methods
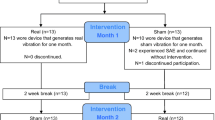
The study was a double-blind, randomized controlled study performed in Atlanta, Georgia. Eligible participants were randomly assigned to the vibrotactile stimulation glove (VTS) or sham control glove (control) condition. All were asked to wear the device on their affected hand for three hours each day for eight weeks. As a feasibility study, the trial was not listed with clinicaltrials.gov but was approved and overseen by the Office of Research Integrity’s IRB board of Georgia Institute of Technology. All participants were screened using the Mini Mental State Exam (MMSE) and provided written consent before beginning the study.
Participants
The study included 16 chronic stroke survivors with upper extremity deficits (ages 28-68; 1-13 years post stroke (Mean=3.7, SD=3.3); 8 VTS condition/8 control condition). Participants were recruited through stroke support groups in the Atlanta metropolitan area. Figure 2 shows a breakdown of participant demographics. Individuals with various levels of arm function could participate. The protocol requires no exercises and thus is accessible to patients with very limited movement. Because this investigation is preliminary, no prior data are available for optimal sample size calculation.
Inclusion criteria
-
History of stroke > 1 year prior
-
Impaired touch sensation in the hand (Semmes-Weinstein monofilament exam score of \(\ge\) 0.2 grams on 3 of 20 measured locations on the hand)
-
Passive range of motion allows user to don a glove
-
English speaker, age 18+
Exclusion criteria
-
Intact sensation in the hand (determined by Semmes-Weinstein monofilament exam)
-
Active Range of Motion within normal limits for all joints of the fingers
-
Cognitive deficits, dementia or aphasia (MMSE score of <22) that prevent informed consent
-
Other neurological condition that may affect motor response (e.g. Parkinson’s, ALS, MS)
-
Pain in the limb that substantially interferes with ADLs or prior arm injury
-
Enrollment in a conflicting study, Botox treatment, or other upper extremity rehabilitation program during the study period
Study design
The study consisted of eight weeks using the stimulation or sham device during daily life. Participants wore the glove daily and met with blinded study administrators for weekly visits to measure sensorimotor function.
At the first visit, all participants received a device, cord and safety manual to take with them. Participants were instructed to wear the device, turned on, for three hours every day while awake. Users were notified that an onboard measurement unit would track usage time, and that 21 h of weekly use is required. All participants were advised to charge the glove each night using the cord provided, just as one might do with a cell phone. Then, participants wear the device on-the-go or at home during their normal routine. Wearing did not need to be continuous each day, but had to total three hours. The dosage was chosen to be intensive, while not requiring too much daily commitment for participants.
Apparatus
A wearable computing glove was designed to provide vibrotactile stimulation to participants throughout their daily life (Fig. 1). It can be taken home and used outside of the clinic environment. Additionally, the glove is worn while users conduct their daily life - making the rehabilitation low-effort and “passive.”
Wearable device
The wearable device (“VTS Glove”) is designed to be low-cost, lightweight, and mobile. The device is a fingerless glove with a vibration motor attached to each dorsal phalanx. This design allows a designated actuator for each finger, while stimulating a region where vibrations can reach the glabrous skin of the palm and the finger extensor tendons. The heart of the device is a circuit board and microcontroller, which activates these motors in a pre-programmed sequence when the switch is turned “on.” The onboard gyroscope logs movement data along with usage data onto a microSD card which is checked by proctors for protocol adherence each week. The glove is rechargeable and has a battery life that allows wireless stimulation for four hours between charges. Design and implementation of the device is reported in detail in a companion manuscript [36].
Stimulus design
For this experiment, stimulation characteristics were designed to target cutaneous mechanoreceptors – specifically the Pacinian corpuscles – which respond to direct vibration and vibration transmitted through the body at a frequency range of 10-400 Hz (preferentially responding around 250 Hz) [37]. Stimulation pattern and timing was designed to be intensive but not uncomfortable by using many vibration pulses with a changing location across the fingers.
Small, coin-shaped vibration motors from Precision Microdrives (ERM-type, Model #310-113) provide the stimulation for this experiment. These motors were driven at a voltage of 3.3V for an approximate amplitude of 1.5 g and 210 Hz vibration frequency (measured in a laboratory setting for validation at 1.3 g and 175 Hz when attached to the glove). Stimulation was the same for all participants and could be perceived by the investigators. Two stimulation sequences were used, each based on the finger pattern for a piano song. Song patterns provided a framework for pseudo-random stimulation and the option to later combine stimulation with music practice exercises for a lighthearted therapy routine. Each song pattern (Ode to Joy and Happy Birthday) was extended with a short sequence to balance stimulation evenly across all fingers. During each repetition the pattern played once quickly (250 ms vibrations, 100 ms pause between each stimulus) and once slowly (700 ms vibrations, 100 ms pauses). These songs were chosen for their recognizable, one-handed melodies with 5-7 notes which could be played on the keyboard with little-to-no hand shifting. The stimulation pattern was switched weekly, alternating between the two “songs.”
Conditions
Participants continued their standard of care, and none were enrolled in concurrent upper limb rehabilitation programs.
Intervention condition
Participants in the vibrotactile stimulation (VTS) condition received a glove with vibration enabled. The protocol includes no required exercises. Participants were asked to wear their glove, switched on (so the indicator light appears), for three hours daily while awake. Users should also charge the battery each night and as needed.
Control condition
Participants in the sham control condition receive a glove with vibration disabled. The appearance of the device was the same as the experimental condition. All indicator lights on the computer board activate in the same fashion. Instructions and language also matched those in the VTS condition: wear the glove on their affected hand, switched on, for three hours daily while awake, and charge the battery each night.
The control condition was assigned a sham device (rather than no intervention) to examine the tolerance of the wearable device with and without stimulation, evaluate if the vibrotactile stimulation itself may have an impact on measures, and provide some data on mechanisms underlying this technique by comparing the conditions.
Outcome measures
Baseline demographic information collected was sex, age, date of stroke, type of stroke, and side affected. Measurements are taken during weekly visits throughout the study. Visits occur at the patient’s home or a midway meeting spot. All measures were performed by trained proctors not involved in the intervention or data analysis. For all participants, key measurements were taken by a blinded occupational therapist. Those measures were taken at the beginning (day 0), middle (4 weeks), and end (8 weeks) of the study. The therapist and study proctor for each participant was consistent to minimize inter-rater variability.
The intent of this study was to examine the initial feasibility in this device and technique. Thus, data on engineering, design, comfort and usability was collected through weekly surveys and observations. Engineering, comfort, and usability data are presented in another manuscript along with subsequent design work [36]. Here we provide data on measures of arm function.
Adherence for users in both conditions was measured each week using self-reported usage times matched with data from the glove’s inertial measurement unit. If usage time had not been within three hours of the required weekly time (21 hours) for two consecutive weeks, the participant would have been released from the study. No such occurrences happened during the trial.
Primary outcome measures
The Semmes–Weinstein Monofilament Exam (SWME) [38] is used to assess cutaneous tactile perception in the affected hand. A 5-piece monofilament hand kit was used. Locations on the dorsal and volar side of the hand are assessed: each fingertip, each dorsal proximal phalanx, index and pinkey volar proximal phalanx, six points on the palm, and two points on the dorsal hand. The SWME is a standardized measure with moderate reliability, greater than the static two-point discrimination test in some conditions [39, 40]. This assessment was done weekly using the same brand of filaments, same evaluator, and filaments were replaced if damaged or bent.
Secondary outcome measures
The Modified Ashworth Scale (MAS) [41] is used to assess resistance to passive motion from involuntary muscle tone and spasticity . In this study, MAS was measured for flexion and extension of the PIP/MCP finger joints, thumb, wrist, elbow and shoulder of the participant’s affected upper limb. MAS ratings are reported here on a scale of 0-5. Confounding factors for this measure were controlled whenever possible including: time of day, time after medication dosage, arm position, and rater.
Voluntary angular range of motion (Active Range of Motion or AROM) is used to assess motor impairment. This measure can capture changes in function when participant dexterity is too low to perform tests like the Jebsen-Taylor Hand Function Test [42]. Here, these measures were made for flexion and extension of the fingers, wrist, elbow and shoulder of the participant’s affected upper limb. Measures are taken in the neutral gravity plane whenever possible. Compensation from other muscles and synergy with spasticity are not included as voluntary range. Finger and elbow extension is measured from a flexed position, not from neutral, so as to report voluntary extension that may be used for activities such as releasing objects from grasp.
A trained occupational therapist performed all movement and spasticity measures in a clinical setting at the beginning, middle and end of the study. Each week, participants are also given a worksheet to report what they did while wearing the device, observations, and comments about the device.
Semmes–Weinstein Monofilament Exam results by group at baseline and eight weeks. This graph shows the group’s average sum of perceived forces across 20 locations on the hand. Smaller perceived forces equate to greater tactile perception. Logarithmic scale used to render all force levels. Error bars indicate standard deviation plotted on a linear scale where each tick mark indicates 500 grams
Trajectory of Semmes–Weinstein Monofilament Exam results over eight weeks for both conditions. This graph shows the group’s average sum of perceived forces across 20 locations on the hand. Smaller perceived force values equate to greater tactile perception. Logarithmic scale used to render all force levels. Shaded regions indicate the standard deviation over time (linear scale)
Data analysis
Using an intention-to-treat analysis, we processed data for all participants including two who had to withdraw prematurely due to unrelated circumstances. The last measured values were used for the determination of any missing values in the case of dropouts or a missed visit, conservatively assuming that no changes occurred since the last measure. Paired observations were compared using the Wilcoxon signed-ranks test, and measures between groups were compared using the Mann-Whitney U test. Repeated measures were compared using a Friedman test and the Conover post-hoc test. A p-value < 0.05 was considered statistically significant.
The Semmes-Weinstein monofilament exam was taken at 20 points on the hand, yielding one minimum perceivable force value per location. The minimum perceivable force level at each location was summed across all locations tested. A minimum sum of 1.4 grams (20 points \(\times\) 0.07 grams per point) corresponds to “normal” sensation at all points and a maximum sum of 6000 g (20 points x 300 grams per point) corresponds to only “residual deep pressure sensation” at all points. Smaller perceived forces equate to better tactile perception.
Angular range of motion is reported, for clarity, at four body areas: the shoulder, elbow, wrist, and fingers. The reported AROM value for each of these areas is the sum of the joint’s movement (i.e., voluntary angular motion for the shoulder is the sum of shoulder flexion, extension, and abduction). Finger AROM is measured at the MCP and PIP joints, and those values are summed. Thus, “Finger Flex.” and “Finger Ext.” include change in both the average MCP and PIP ranges. Healthy ranges for these measures would be as follows: shoulder \(= 320^{\circ }\), elbow (flexion + extension from flexed position) \(= 300^{\circ }\), wrist (flexion + extension + R/U deviation) \(= 250^{\circ }\), finger flex. (PIP + MCP flexion ROM per finger) \(= 210^{\circ }\), finger ext. (PIP + MCP extension ROM from flexed position, per finger) \(= 210^{\circ }\).
Results
Semmes–Weinstein monofilament exam (SWME)
Starting means (M=832.4 grams, SD=1206 for VTS; M=501.6 grams, SD=949.7 for control) were compared between groups using Mann-Whitney U test (U=18; z=−1.10; p=0.271). Starting ranges were 2705–1.79 for VTS, and 2448-1.66 for control. One participant in the VTS condition is not included in this range and these calculations because their starting measures prevent representation on the graphs. This user initially presented as insensate at all points but could accurately report deep pressure sensation at three points later in the study.
Baseline measures of the VTS experimental group were compared to measures at eight weeks (M=9.701 grams, SD=14.25) and results suggest that there is a significant difference (t-test: t(6)=−3.50; p=0.006; signed-ranks: Z=−1.89; p=0.039). As Fig. 3 shows, the VTS condition is able to sense smaller forces than the control condition at eight weeks (M=91.15 grams, SD=224.1). The sham control condition also showed a change in SWME measures, but this change was not statistically significant (t-test: t(7)=1.190; p=0.254; signed-ranks: Z=−1.40; p=0.098). We also performed a Friedman’s test that found a significant difference in the repeated measures for the VTS group (X2F(8)=24.04, p=0.002), and no significant difference between measures for the control group (X2F(8)=17.29, p=0.27). A signed-ranks test suggests that the difference from baseline in the VTS group becomes significant at week 4. The Conover post-hoc test adjusted by the Benjaminyi-Hochberg FDR method suggests a significant difference from baseline begins at week 5. Figure 4 shows the trends in these values throughout the entire study.
Modified Ashworth Scale (MAS)
Modified Ashworth Scale (MAS) was measured in a clinical setting for flexion and extension of MCP/PIP finger joints, thumb, wrist, elbow, and shoulder. Here, results are reported for the fingers (average of PIP and MCP joints) which showed the most change in values. Starting means (M=3.11, SD=1.08 for VTS; M=2.25, SD=0.78 for control) were compared using a Mann-Whitney U test (U=16; z=1.63; p=0.10) and no significant difference was found. Each participant’s mean MAS can be found in Fig. 5. Differences in experimental group MAS were found to be significant using a Wilcoxon signed-ranks test comparing starting measures to measures at 8 weeks (Z=-2.31; p=0.01). MAS difference at 8 weeks for the VTS condition was an average of -1.44 points on the Ashworth scale for each of the two measured joints on the affected limb (MCP and PIP). Differences in control group MAS at 8 weeks were also compared using the Wilcoxon signed-ranks test (Z=1.06; p=0.15) but the average difference (M=−0.27) was not considered significant. Change from baseline was compared between conditions and found to be significantly different (Mann-Whitney: U=7.5; z=−2.48; p=0.007 ). Participant 7 had severe spasticity before the study, which led to a Baclofen pump and wrist fusion surgery. These interventions were failing to stop the progression of tone and spasticity in their hand; however, their tone was reduced after participation in the study. Two users (5 and 7) agreed to follow-up six months post-study. There was no significant relapse in values at follow-up vs. study end.
Increase in angular degrees of voluntary movement for four upper body locations between baseline and study end. Shoulder, elbow and wrist values include both flexion and extension (from flexed) ranges. Finger flexion and extension is shown separately to provide greater detail, and these values include both MCP and PIP ranges. Zero values most often occurred when the participant had no voluntary movement in the joint at baseline and 8 weeks
Active range of motion (AROM)
Starting means for arm motion and finger flexion had a significant difference between conditions. The control group included fewer members with low to moderate starting function. Baseline function may be a factor in the AROM results for the control group, but further study is needed to examine its influence. The control group showed no significant difference in shoulder (Starting Mean\(=202.9^{\circ }\), \(\hbox {SD}=135.0^{\circ }\), Avg. Change at Week \(\hbox {Eight}=2.7^{\circ }\)), elbow (Starting \(\hbox {Mean}=113.8^{\circ }\), SD=115.7\(^{\circ }\), \(\hbox {Avg.Change}=-2.9^{\circ }\)), wrist (Starting \(\hbox {Mean}=48.6^{\circ }\), SD=38.9\(^{\circ }\), \(\hbox {Avg.Change}=3.0^{\circ }\)), finger flexion (Starting \(\hbox {Mean}=117.8^{\circ }\), SD=71.9\(^{\circ }\), Avg. \(\hbox {Change}=10.6^{\circ }\)) or finger extension range (\(\text {Starting Mean}=46.3^{\circ }\), \(\hbox {SD}=52.0^{\circ }\), \(\text {Avg.Change}=20.7^{\circ }\)).
The experimental VTS condition showed improvements in sum of shoulder (Starting Mean=63.5\(^{\circ }\), SD=66.7\(^{\circ }\), Avg. Change at Week Eight=44.6\(^{\circ }\)), elbow (Starting Mean=54.1\(^{\circ }\), SD=52.5\(^{\circ }\), Avg.Change =69.5\(^{\circ }\)), and finger flexion (Starting Mean=25.0\(^{\circ }\), SD=31.3\(^{\circ }\), Avg. Change=50.9\(^{\circ }\)) range of motion. A Wilcoxon signed-ranks test found these changes to be significant (Z=-2.03, -2.17, -2.10, p=0.02, 0.01, 0.02), and this finding was consistent with a paired t-test (t(7)=2.59, 2.98, 2.16, p=0.02, 0.01, 0.03). Change in range of motion for the wrist (Starting Mean=9.5\(^{\circ }\), SD=12.5\(^{\circ }\), Avg. Change=16.5\(^{\circ }\)) and finger extension (Starting Mean=10.9\(^{\circ }\), SD=18.1\(^{\circ }\), Avg.Change=57.2\(^{\circ }\)) was not found to be statistically significant. Changes in voluntary ROM are shown in Fig. 6.
Discussion
Participants who received vibrotactile stimulation showed significant change in measures whereas those in the control group did not. The wearable devices successfully delivered mobile stimulation throughout the duration of the study, and all participants were able to adhere to the daily wearing protocol.
Changes in SWME measures suggest that participants showed improved tactile perception. Figure 4 suggests that the trend in improvement was gradual. One participant reported the return of protective sensation in cases of joint hyper-extension, and one reported being able to feel the vibrations when they could not initially.
Some participants in the VTS condition provided observations that the affected hand was more open and flexible. These observations were consistent with changes in Modified Ashworth Scale measures. Figure 5 shows each person’s starting and ending measures. All but one person in the experimental condition showed a reduction in MAS values. Participants in both conditions must frequently stretch open their affected hand to don the device, and stretching may be associated with changes in MAS. However, participants in the control group (who also stretched to don the device daily) did not show a significant change in MAS values, which suggests that stimulation rather than stretching is associated with these changes. Tone and spasticity lack effective or lasting treatment options, yet 40-50% of stroke survivors with upper extremity disability may be affected [43, 44] . More study is needed, but this promising preliminary evidence along with that in prior work suggests that afferent stimulation may be used to address spasticity and tone. The VTS Glove allows extended stimulation and further study of this technique. Future work can adjust stimulation characteristics to target different sensory receptors and examine optimal settings. Some participants with flexed fingers struggled to don the glove device, so the design was subsequently revised for accessibility.
Changes in voluntary range of motion may be due in part to reduction in involuntary tone. Some participants showed large increases in range, with near-normal finger extension and flexion at week eight. Other participants showed no change in voluntary range of motion. Further study can provide details on what markers, such as initial motor ability, predict outcomes using this device. Improved range of motion in proximal joints of the arm, such as the elbow, may be attributable to increased limb use and attention or to afferent input that reaches these proximal regions. Vibration can be widely conducted throughout the human body via bones and other tissues [45, 46].
Future work should examine if these results are maintained, but the informal follow-ups that were accepted by the two participants suggest that improvements may be lasting. Some participants had their stroke many years ago, and demonstrated change in measures. Participants used the device for over 140 h each, an intensity enabled by the wearable form factor and the passive stimulation method. In line with this result, a body of research has previously associated rehabilitation intensity (practice time) with improved outcomes [47,48,49]. Prior studies applying WBV and focal muscle vibration also found reductions in spasticity and disabled limb function [25,26,27, 31, 50]; however, most prior work is limited to stimulation for periods of 5-30 minutes in a laboratory setting because existing apparatus are large and immobile.
Participants in the experimental condition reported new capabilities on the weekly worksheet that included helping to cook, cleaning their hobby equipment, donning winter gloves and holding their partner’s hand. They also reported new tactile perception from the hand including sensing the vibrations, hyperextension during stretching, and the spray of water. Three participants reported a greater sense of embodiment or ownership of the limb. Participants took advantage of the mobile nature of the device: reporting wearing the device to events such as church, lunch, and the movies.
Study limitations
This investigation intends to establish the feasibility of wearable vibrotactile stimulation to improve diminished limb function. Participants include various levels of disability, which provides initial data on who may be suited for this stimulation. The Modified Ashworth Scale is a standard measure of tone and spasticity, but there are confounding factors for this measure. These factors were controlled whenever possible, including arm position, time of day, and rater.
Effects of the control condition
Some change in measures may be expected when using the sham device. The sham device provided cutaneous sensory stimulation via the fabric of the glove; while the VTS experimental device provided additional cutaneous, and proprioceptive, stimulation via vibration. Furthermore, both conditions encouraged attention and engagement with the limb. However, in contrast to the experimental group, the control group did not show significant changes.
Possible mechanisms behind changes in limb function
A wearable device can facilitate engagement with the disabled limb, which may help discourage maladaptive plastic changes from sensory deprivation and learned non-use. Learned non-use [51,52,53] is thought to be one of the reasons behind limited functional improvement of limbs after stroke: survivors learn to compensate and do not force themselves to re-learn the use of their limb. In addition, participants stretched open their affected hand to don and doff the device several times per day. This stretching was expected to impact Modified Ashworth measures. Lesion location was not recorded in the study, but this information would provide interesting additional data if recorded in future work.
The control condition allowed us to examine the impacts of these mechanisms. All participants interacted with a wearable device, but the experimental VTS group showed significantly different clinical measures after eight weeks. This difference suggests that engagement and stretching may not be the only mechanisms to influence the participants.
Changes in tactile perception may be due central mechanisms. Afferent input, transmitted by intact peripheral nervous pathways, may activate central nervous system regions. This sensory input could impact central organization as is found in constraint-induced movement therapy after brain injury, or during normal sensorimotor skill acquisition [52, 54, 55].
Vibration may help regulate electrophysiology associated with spasticity via afferent feedback. Reduced threshold of the stretch reflex has been implicated as one of the mechanisms behind symptoms of spasticity [56, 57]. Supraspinal control usually regulates this reflex, but can be disrupted in events such as spinal cord injury or stroke [56]. These reflexes are also mediated by afferent feedback produced during limb movement [58, 59]. Vibration provides similar feedback – like many small muscle stretches – activating cutaneous mechanoreceptors and proprioceptive afferents [60, 61]. Afferent feedback then may induce reflex suppression and involuntary muscle contraction – which may impact spasticity and are found during whole body vibration (WBV) and focal muscle/tendon vibration [25,26,27, 31, 50]. Presynaptic inhibition from afferent discharge is cited as a possible mechanism underlying reflex suppression during vibration [33]. Continuous passive motion is another treatment for spasticity, but removal of proprioceptive afferents was shown to prevent normalization [58, 62] suggesting that sensory feedback may underlie this method. Investigation of these factors is beyond the scope of this work, but the promising results warrant further study.
Improved voluntary range of motion may be unlocked when spasticity and tone decreases. Another hypothesis for changes in voluntary motion is that such sensory stimulation provides excitatory feedback and co-activation of motor systems, and helps restore somatosensation useful in motor function [22, 63,64,65]. This hypothesis is supported by work in sensory stimulation for motor learning and performance [66], and motor rehabilitation [67,68,69,70].
Conclusions
A controlled, randomized trial of 16 participants evaluated the feasibility of a wearable vibrotactile stimulation method to reduce upper limb disability in chronic stroke. All users were assigned to wear a computerized glove on their affected hand for three hours per day. Users in the sham control group received no stimulation and those in the experimental condition received vibrotactile stimulation from the glove.
The wireless, wearable device was used during daily life, not in a clinical setting. Participants who received vibrotactile stimulation demonstrated a significant change in measures of tactile perception, voluntary motion, and spasticity after eight weeks. Some participants reported increase in protective sensation, sense of embodiment, and return to activities of daily living such as cleaning, cooking and writing using their disabled hand.
Availability of supporting data
Not applicable.
References
Bonita R, Mendis S, Truelsen T, Bogousslavsky J, Toole J, Yatsu F. The global stroke initiative. Lancet Neurol. 2004;3(7):391–3.
Prevalence of stroke–united states, 2006-2010. Centers for Disease Control and Prevention (CDC and others). Morbidity and Mortality Weekly Report 61(20), 379 (2012)
Adamson J, Beswick A, Ebrahim S. Is stroke the most common cause of disability? J Stroke Cerebrovasc Dis. 2004;13(4):171–7.
Wade D, Langton-Hewer R, Wood V, Skilbeck C, Ismail H. The hemiplegic arm after stroke: measurement and recovery. J Neurol Neurosurg Psychiatry. 1983;46(6):521–4.
Parker V, Wade D, Hewer RL. Loss of arm function after stroke: measurement, frequency, and recovery. Int Rehab Med. 1986;8(2):69–73.
Connell LA, Lincoln N, Radford K. Somatosensory impairment after stroke: frequency of different deficits and their recovery. Clin Rehab. 2008;22(8):758–67.
Tyson SF, Hanley M, Chillala J, Selley AB, Tallis RC. Sensory loss in hospital-admitted people with stroke: characteristics, associated factors, and relationship with function. Neurorehab Neural Rep. 2008;22(2):166–72.
Feldman DE, Brecht M. Map plasticity in somatosensory cortex. Science. 2005;310(5749):810–5.
Van der Loos H, Woolsey TA. Somatosensory cortex: structural alterations following early injury to sense organs. Science. 1973;179(4071):395–8.
Buonomano DV, Merzenich MM. Cortical plasticity: from synapses to maps. Ann Rev Neurosci. 1998;21(1):149–86.
Xerri C, Merzenich MM, Peterson BE, Jenkins W. Plasticity of primary somatosensory cortex paralleling sensorimotor skill recovery from stroke in adult monkeys. J Neurophysiol. 1998;79(4):2119–488.
Jablonka J, Burnat K, Witte O, Kossut M. Remapping of the somatosensory cortex after a photothrombotic stroke: dynamics of the compensatory reorganization. Neuroscience. 2010;165(1):90–100.
Bird T, Choi S, Goodman L, Schmalbrock P, Nichols-Larsen DS. Sensorimotor training induced neural reorganization after stroke: a case series. J Neurol Phys Ther. 2013;37(1):27.
Pleger B, Schwenkreis P, Dinse HR, Ragert P, Höffken O, Malin J-P, Tegenthoff M. Pharmacological suppression of plastic changes in human primary somatosensory cortex after motor learning. Exp Brain Res. 2003;148(4):525–32.
Porter LL. Patterns of projections from area 2 of the sensory cortex to area 3a and to the motor cortex in cats. Exp Brain Res. 1992;91(1):85–93.
Mattay VS, Callicott JH, Bertolino A, Santha AK, Van Horn JD, Tallent KA, Frank JA, Weinberger DR. Hemispheric control of motor function: a whole brain echo planar fmri study. Psychiatry Res. 1998;83(1):7–22.
Dewald JA, Given J, Rymer WZ. Long-lasting reductions of spasticity induced by skin electrical stimulation. IEEE Trans Rehab Eng. 1996;4(4):231–42.
Schabrun SM, Hillier S. Evidence for the retraining of sensation after stroke: a systematic review. Clin Rehab. 2009;23(1):27–39.
Dimitrijevic MM, Stokié DS, Wawro AW, Wun C-CC. Modification of motor control of wrist extension by mesh-glove electrical afferent stimulation in stroke patients. Arch Phys Med Rehab. 1996;77(3):252–8.
Smith PS, Dinse HR, Kalisch T, Johnson M, Walker-Batson D. Effects of repetitive electrical stimulation to treat sensory loss in persons poststroke. Arch Phys Med Rehab. 2009;90(12):2108–11.
Tu-Chan AP, Natraj N, Godlove J, Abrams G, Ganguly K. Effects of somatosensory electrical stimulation on motor function and cortical oscillations. J Neuroeng Rehab. 2017;14(1):113.
Cordo P, Lutsep H, Cordo L, Wright WG, Cacciatore T, Skoss R. Assisted movement with enhanced sensation (ames): coupling motor and sensory to remediate motor deficits in chronic stroke patients. Neurorehab Neural Rep. 2009;23(1):67–77.
Estes, L.T., Backus, D., Starner, T.: A wearable vibration glove for improving hand sensation in persons with spinal cord injury using passive haptic rehabilitation. In: 2015 9th International Conference on Pervasive Computing Technologies for Healthcare (PervasiveHealth), pp. 37–44 (2015). IEEE
Enders LR, Hur P, Johnson MJ, Seo NJ. Remote vibrotactile noise improves light touch sensation in stroke survivors’ fingertips via stochastic resonance. J Neuroeng Rehab. 2013;10(1):105.
Abercromby AF, Amonette WE, Layne CS, Mcfarlin BK, Hinman MR, Paloski WH. Variation in neuromuscular responses during acute whole-body vibration exercise. Med Sci Sports Exerc. 2007;39(9):1642–50.
Delecluse C, Roelants M, Verschueren S. Strength increase after whole-body vibration compared with resistance training. Med Sci Sports Exerc. 2003;35(6):1033–41.
Ness LL, Field-Fote EC. Effect of whole-body vibration on quadriceps spasticity in individuals with spastic hypertonia due to spinal cord injury. Restorat Neurol Neurosci. 2009;27(6):623–33.
Pang M, Lau R, Yip S. The effects of whole-body vibration therapy on bone turnover, muscle strength, motor function, and spasticity in chronic stroke: a randomized controlled trial. Eur J Phys Rehab Med. 2013;49(4):439–50.
Binder C, Kaya AE, Liepert J. Vibration prolongs the cortical silent period in an antagonistic muscle. Muscle Nerve. 2009;39(6):776–80.
Kossev A, Siggelkow S, Kapels H-H, Dengler R, Rollnik J. Crossed effects of muscle vibration on motor-evoked potentials. Clin Neurophysiol. 2001;112(3):453–6.
Marconi B, Filippi GM, Koch G, Giacobbe V, Pecchioli C, Versace V, Camerota F, Saraceni VM, Caltagirone C. Long-term effects on cortical excitability and motor recovery induced by repeated muscle vibration in chronic stroke patients. Neurorehab Neural Rep. 2011;25(1):48–60.
Noma T, Matsumoto S, Shimodozono M, Etoh S, Kawahira K. Anti-spastic effects of the direct application of vibratory stimuli to the spastic muscles of hemiplegic limbs in post-stroke patients: a proof-of-principle study. J Rehab Med. 2012;44(4):325–30.
Rittweger J. Vibration as an exercise modality: how it may work, and what its potential might be. Eur J Appl Physiol. 2010;108(5):877–904.
Siggelkow S, Kossev A, Schubert M, Kappels H-H, Wolf W, Dengler R. Modulation of motor evoked potentials by muscle vibration: the role of vibration frequency. Muscle Nerve. 1999;22(11):1544–8.
Steyvers M, Levin O, Verschueren S, Swinnen S. Frequency-dependent effects of muscle tendon vibration on corticospinal excitability: a TMS study. Exp Brain Res. 2003;151(1):9–14.
Seim, C.: Wearable vibrotactile stimulation: How passive stimulation can train and rehabilitate. PhD thesis, Georgia Institute of Technology (2019)
Johnson KO. The roles and functions of cutaneous mechanoreceptors. Curr Opin Neurobiol. 2001;11(4):455–61.
Weinstein, S.: Reminiscences on the history and development of the semmes-weinstein monofilaments. In: Proceedings of the Conference on Biomechanics of Deformity and Treatment of the Insensitive Hand, May 4-6, Gillis W. Long Hansen’s Disease Center, Carville, LA (1992)
Bulut T, Tahta M, Sener U, Sener M. Inter-and intra-tester reliability of sensibility testing in healthy individuals. J Plastic Surg Hand Surg. 2018;52(3):189–92.
Bulut T, Akgun U, Ozcan C, Unver B, Sener M. Inter-and intra-tester reliability of sensibility testing in digital nerve repair. J Hand Surg. 2016;41(6):621–3.
Bohannon RW, Smith MB. Interrater reliability of a modified ashworth scale of muscle spasticity. Phys Ther. 1987;67(4):522–5.
Jebsen R, Taylor N, Trieschmann R, Trotter M, Howard L. An objective and standardized test of hand function. Arch Phys Med Rehab. 1969;50(6):311.
Dajpratham P, Kuptniratsaikul V, Kovindha A, Kuptniratsaikul PS-a, Dejnuntarat K. Prevalence and management of poststroke spasticity in thai stroke patients: a multicenter study. Med J Med Assoc Thailand. 2009;92(10):1354.
Watkins C, Leathley M, Gregson J, Moore A, Smith T, Sharma A. Prevalence of spasticity post stroke. Clin Rehab. 2002;16(5):515–22.
Matsumoto Y, Griffin M. Dynamic response of the standing human body exposed to vertical vibration: influence of posture and vibration magnitude. J Sound Vibr. 1998;212(1):85–107.
Gurram R, Rakheja S, Gouw GJ. Vibration transmission characteristics of the human hand-arm and gloves. Int J Ind Ergon. 1994;13(3):217–34.
Teasell R, Bitensky J, Salter K, Bayona NA. The role of timing and intensity of rehabilitation therapies. Top Stroke Rehab. 2005;12(3):46–57.
Kwakkel G. Impact of intensity of practice after stroke: issues for consideration. Disability Rehab. 2006;28(13–14):823–30.
Jette DU, Warren RL, Wirtalla C. The relation between therapy intensity and outcomes of rehabilitation in skilled nursing facilities. Arch Phys Med Rehab. 2005;86(3):373–9.
Noma T, Matsumoto S, Etoh S, Shimodozono M, Kawahira K. Anti-spastic effects of the direct application of vibratory stimuli to the spastic muscles of hemiplegic limbs in post-stroke patients. Brain Injury. 2009;23(7–8):623–31.
Grant VM, Gibson A, Shields N. Somatosensory stimulation to improve hand and upper limb function after stroke-a systematic review with meta-analyses. Top Stroke Rehab. 2018;25(2):150–60.
Liepert J, Miltner W, Bauder H, Sommer M, Dettmers C, Taub E, Weiller C. Motor cortex plasticity during constraint-induced movement therapy in stroke patients. Neurosci Lett. 1998;250(1):5–8.
Wolf SL, Winstein CJ, Miller JP, Taub E, Uswatte G, Morris D, Giuliani C, Light KE, Nichols-Larsen D, Investigators E, et al. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the excite randomized clinical trial. Jama. 2006;296(17):2095–104.
Schaechter JD, Kraft E, Hilliard TS, Dijkhuizen RM, Benner T, Finklestein SP, Rosen BR, Cramer SC. Motor recovery and cortical reorganization after constraint-induced movement therapy in stroke patients: a preliminary study. Neurorehab Neural Rep. 2002;16(4):326–38.
Liepert J, Bauder H, Miltner WH, Taub E, Weiller C. Treatment-induced cortical reorganization after stroke in humans. Stroke. 2000;31(6):1210–6.
Dietz, V., Sinkjaer, T.: Spasticity. In: Handbook of Clinical Neurology. Elsevier, New York;2012, pp. 197–211.
Powers R, Marder-Meyer J, Rymer W. Quantitative relations between hypertonia and stretch reflex threshold in spastic hemiparesis. Ann Neurol. 1988;23(2):115–24.
Lynskey JV, Belanger A, Jung R. Activity-dependent plasticity in spinal cord injury. J Rehab Res Dev. 2008;45(2):229.
Sheean G. The pathophysiology of spasticity. Eur J Neurol. 2002;9:3–9.
Burke D, Hagbarth K-E, Löfstedt L, Wallin BG. The responses of human muscle spindle endings to vibration of non-contracting muscles. J Physiol. 1976;261(3):673–93.
Fallon JB, Macefield VG. Vibration sensitivity of human muscle spindles and golgi tendon organs. Muscle Nerve. 2007;36(1):21–9.
Ollivier-Lanvin K, Keeler BE, Siegfried R, Houlé JD, Lemay MA. Proprioceptive neuropathy affects normalization of the h-reflex by exercise after spinal cord injury. Exp Neurol. 2010;221(1):198–205.
Doyle S, Bennett S, Fasoli SE, McKenna KT. Interventions for sensory impairment in the upper limb after stroke. Cochrane Database Syst Rev. 2010;6:1.
Platz T. Impairment-oriented training (iot)-scientific concept and evidence-based treatment strategies. Restor Neurol Neurosci. 2004;22(3–5):301–15.
Raghavan P. The nature of hand motor impairment after stroke and its treatment. Curr Treatm Opt Cardiovasc Med. 2007;9(3):221–8.
Rosenkranz K, Rothwell JC. Differential effect of muscle vibration on intracortical inhibitory circuits in humans. J Physiol. 2003;551(2):649–60.
Cordo P, Wolf S, Lou J-S, Bogey R, Stevenson M, Hayes J, Roth E. Treatment of severe hand impairment following stroke by combining assisted movement, muscle vibration, and biofeedback. J Neurol Phys Ther. 2013;37(4):194–203.
Forner-Cordero A, Steyvers M, Levin O, Alaerts K, Swinnen SP. Changes in corticomotor excitability following prolonged muscle tendon vibration. Behav Brain Res. 2008;190(1):41–9.
Golaszewski SM, Bergmann J, Christova M, Kunz AB, Kronbichler M, Rafolt D, Gallasch E, Staffen W, Trinka E, Nardone R. Modulation of motor cortex excitability by different levels of whole-hand afferent electrical stimulation. Clin Neurophysiol. 2012;123(1):193–9.
Kaelin-Lang A, Luft AR, Sawaki L, Burstein AH, Sohn YH, Cohen LG. Modulation of human corticomotor excitability by somatosensory input. J Physiol. 2002;540(2):623–33.
Acknowledgements
We would like to acknowledge and thank the other advisors to the project: Edelle Field-Fote (Shepherd Center/Emory University/Georgia Tech), Sarah Callahan (Shepherd Center) and Samir Belagaje (Emory University/Grady Hospital Stroke and Neuroscience Center). Thanks also to the clinicians of PT Solutions Midtown Atlanta and Physio Clinic Midtown Atlanta for taking measurements in this study.
Funding
This research was supported, in part, by the National Science Foundation (NSF) Graduate Research Fellowship program, a grant from the Georgia Tech Graphics, Visualization, and Usability (GVU) consortium, and a Microsoft Research PhD Fellowship.
Author information
Authors and Affiliations
Contributions
All authors (CES, SLW, and TES) contributed to the study design and methodology. CES designed and fabricated the devices used in the research, recruited participants, and interfaced with clinicians. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved and overseen by the Office of Research Integrity’s IRB board for the Georgia Institute of Technology. All participants were screened using the Mini Mental State Exam (MMSE) and provided written consent before beginning the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Seim, C.E., Wolf, S.L. & Starner, T.E. Wearable vibrotactile stimulation for upper extremity rehabilitation in chronic stroke: clinical feasibility trial using the VTS Glove. J NeuroEngineering Rehabil 18, 14 (2021). https://doi.org/10.1186/s12984-021-00813-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12984-021-00813-7