Abstract
Background
The relationship between diabetes and oxidative stress has been previously reported. Exercise represents a useful non-pharmacological strategy for the treatment in type 2 diabetic (T2DM) patients, but high intensity exercise can induce a transient inflammatory state and increase oxidative stress. Nutritional strategies that may contribute to the reduction of oxidative stress induced by acute exercise are necessary. The aim of this study was to examine if n-3 PUFA supplementation intervention can attenuate the inflammatory response and oxidative stress associated with high intensity exercise in this population. As a primary outcome, lipoperoxidation measurements (TBARS and F2-isoprostanes) were selected.
Methods
Thirty T2DM patients, without chronic complications, were randomly allocated into two groups: placebo (gelatin capsules) or n-3 PUFA (capsules containing 180 mg of eicosapentaenoic acid and 120 mg of docosahexaenoic acid). Blood samples were collected fasting before and after 8 weeks supplementation. In the beginning and at the end of protocol, an acute exercise was performed (treadmill), and new blood samples were collected before and immediately after the exercise for measurements of oxidative stress and high-sensitivity C-reactive protein (hs-CRP).
Results
After the supplementation period, a decrease in triglycerides levels was observed only in n-3 PUFA supplementation group (mean difference and 95% CI of 0.002 (0.000–0.004), p = 0.005). Supplementation also significantly reduced TRAP levels after exercise (mean difference and 95% CI to 9641 (− 20,068–39,351) for − 33,884 (− 56,976 - -10,793), p = 0.004, Cohen’s d effect size = 1.12), but no significant difference was observed in n-3 PUFA supplementation group in lipoperoxidation parameters as TBARS (mean difference and 95% CI to − 3.8 (− 10–2.4) for − 2.9 (− 1.6–7.4) or F2-isoprostanes (mean difference and 95% CI -0.05 (− 0.19–0.10) for − 0.02 (− 0.19–0.16), p > 0.05 for both.
Conclusion
PUFA n-3 supplementation reduced triglycerides as well as TRAP levels after exercise, without a significant effect on inflammatory and oxidative stress markers.
This study is registered at ClinicalTrials.gov with the registration number of NCT03182712.
Similar content being viewed by others
Background
Hyperglycemia is the hallmark metabolic abnormality associated with the complications of type 2 diabetes mellitus (T2DM). High rates of glucose utilization are associated with incremental free radical formation and protein modification (by glucose auto-oxidation, protein glycation, and polyol pathway activation) [1]. The redox imbalance resulting from the increase of reactive oxygen and nitrogen species, and the concomitant lowering of antioxidant defenses have been associated to micro- and macrovascular diabetic complications [2, 3]. Nevertheless, the underlying mechanisms related to this imbalance are not entirely clear.
The relationship between hyperglycemia and increased lipid peroxidation has been previously reported [2, 4, 5]. Elevated levels of thiobarbituric acid reactive substances (TBARS) have been found in the blood of both type 1 [6] and type 2 [4] diabetic patients. The gold standard lipid peroxidation marker, F2-isoprostane, a toxic product generated by non-enzymatic, free radical-catalyzed peroxidation of arachidonic acid, has also been shown to be elevated in plasma and urine of T2DM [6, 7]. Disrupted redox signalling or elevated oxidative stress (from prolonged periods of hyperglycemia and/or elevated pro-inflammatory cytokines) is thought to underlie the vascular dysfunction observed in individuals with glucose intolerance and diabetes [3]. It has also been shown that individuals with T2DM have more pronounced systemic inflammation and oxidative stress than those with normal glucose tolerance [8]. In addition, inflammatory markers, such as high-sensitivity C-reactive protein (hs-CRP), are associated with higher risk for cardiovascular diseases and are also important markers for the control and prevention of diabetes complications [9].
Regular physical exercise and diet represents a useful non-pharmacological strategy for the prevention and treatment of T2DM [3]. The beneficial effects of chronic exercise are associated with improved mitochondrial viability and antioxidant defenses, reducing oxidative stress and provides acute and chronic benefits [8, 10, 11], however, high intensity exercise can induce a transient inflammatory state and increase oxidative stress [10, 11]. Thus, nutritional strategies that may contribute to the reduction of oxidative stress induced by acute exercise may represent additional advantages to T2DM people.
Among many dietary components and supplements, studies have demonstrated beneficial effects of Polyunsaturated Fatty Acids Omega-3 (n-3 PUFA) on different conditions, including diabetes [12,13,14]. These effects include alterations in lipoprotein metabolism, platelet and endothelial function, and eicosanoid production. N-3 PUFA play an important role on cell membranes, are considered essential fatty acids for human beings, and should be provided by food intake [3]. Although fatty acid unsaturation favors free radical activity (increasing lipoperoxidation), the available literature is controversial when it relates to oxidative stress following n-3 PUFA supplementation [4, 15, 16].
Therefore, the aim of this study was to examine if n-3 PUFA supplementation intervention can attenuate the inflammatory response/oxidative stress associated with high intensity exercise in this population. As a primary outcome, lipoperoxidation measurements (TBARS and F2-isoprostanes) were selected. The antioxidant variables (superoxide dismutase, TRAP and uric acid) were considered secondary outcomes, and complement the oxidative stress responses. Additionally, biochemical changes in the lipid and glycemic profile before and after n-3 supplementation were investigated. We hypothesized that n-3 PUFA supplementation may attenuate oxidative stress parameters after exercise.
Methods
Subjects
Participants (adult male and female subjects with T2DM) were recruited via advertising on local newspapers and workplaces. The exclusion criteria were: in use of aspirin or insulin, HbA1c above 9.0%, dyslipidemia or having a diagnosis of any complication of diabetes mellitus. The study protocol was in accordance with the Helsinki declaration and was approved by the Clinics Hospital of Porto Alegre (HCPA) Ethics and Research Committee (06–222). All subjects gave written, informed consent before participation.
Study design
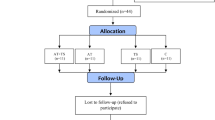
This study has a randomized, double blind, parallel group design. Initially, physical evaluations were carried out, including interview with an expert physician and aerobic power test with electrocardiogram. After that, participants attended the outpatient clinic on four occasions: 1) Anthropometric evaluation and blood and urine collections in fasting; 2) First sub-maximal exercise test and beginning of supplementation; 3) Blood and urine sampling (fasting) after 8 weeks of supplementation, and 4) Second sub-maximal exercise test. Figure 1 illustrates the flowchart of experimental sessions.
Anthropometric evaluation
During their first visit, the following anthropometric parameters were evaluated: body mass, height, circumferences and skinfolds. Body mass (kg) and height (m) was determined by a digital scale coupled to a stadiometer Welmy® (W 110 H, Santa Bárbara d’Oeste, SP, Brasil), with accuracy of 0.1 kg and 0.01 cm, respectively. Body mass index (BMI, kg/m2) and waist-to-hip ratio was calculated according to World Health Organization [17]. Body fat percent was determined by the Jackson & Pollock formula [18] and later converted to fat percentage, according to formula proposed by Siri [19]. All measurements of skinfolds were made on the right side of the body by using a compass (Cescorf®) with accuracy of 0.1 mm. After the supplementation period, body weight was evaluated.
Blood and urine collection
Blood and urine were collected before and after supplementation period, after 12 h fasting, for analysis of total cholesterol and fractions, triglycerides, HbA1c and glycemia and albuminury (urine), 24 h before each exercise session. In the day of submaximal exercise, subjects were in the fed state, and blood collect occurred immediately before and after exercise (See Fig. 1).
Exercise protocols
Peak oxygen consumption (VO2) and carbon dioxide production (VCO2) were measured breath by breath during the exercise via an automated ergospirometer metabolic cart (CPX-D, Medical Graphics Corporation, USA). The equipment was calibrated before each exercise test according to the manufacturer’s instructions. O2 and CO2 analyzers were calibrated using gas standards of known concentrations before each exercise test.
The maximal exercise tests were carried out on a cycle ergometer (The Byke, Cybex USA). All tests were started at a 25 W load, with 25 W/min increments until exhaustion, followed by a 3-min recovery with a 25 W load. Cycling should be kept between 60 to 90 cpm, on the subject’s most comfortable cadence [20]. Volunteers were verbally encouraged to achieve their best performance during the maximal exercise test. The criteria used to verify maximal effort included a respiratory gas exchange ratio ≥ 1.15, a final peak heart rate at ≥95% of the age-predicted maximum (220-age) and/or a VO2 plateau through systematic increases in the workload [21]. Anaerobic threshold was determined by the second ventilatory threshold (VT2) [22].
All high intensity sub-maximal tests were performed in the morning, 2 h after the subject’s usual breakfast. Volunteers were asked not to have high-intensity physical activity, caffeine consumption or alcohol ingestion 24 h before the tests. The tests were performed at an intensity of VT2, monitored by VO2 during exercise. After a 3-min rest, the test started. During the first 5 min, the load was increased to approximately 55% of VT2. The load was then adjusted to reach 100% of VT2. The subjects continued the exercise for 30 min or until exhaustion [23].
Supplementation
Supplementation was given to the subjects in pharmacy manufactured capsules. The placebo capsules contained 500 mg of gelatin. The intervention group received n-3 PUFA-enriched capsules containing 180 mg of eicosapentaenoic acid (EPA), 120 mg of docosahexaenoic acid (DHA) and 2 mg of vitamin E (alpha-tocopherol) [Naturalis®, Brazil]. A low concentration of vitamin E was used in the formulation to prevent n-3 PUFA oxidation, as previously recommended [24]. Subjects in both groups were asked to consume three capsules a day (at breakfast, lunch and dinner), for 8 weeks.
Biochemical analyses
Venous blood samples were taken after fasting from the antecubital vein in EDTA coated (for plasma) or plain (for serum) tubes using standard aseptic techniques. Urine samples for measurement of albuminuria also were collected only before and at the end of the supplementation period. Total cholesterol, HDL-cholesterol, triglycerides and glucose were measured by enzymatic colorimetric assays; Friedewald’s formula was used to calculate for LDL-cholesterol fraction [25]. Glycated hemoglobin (HbA1C) was measured by an immunoassay; urinary albumin was measured by immunoturbidimetry.
Before and after each submaximal test, blood samples were immediately centrifuged at 4 °C, 4000 rpm, for 5 min, and plasma or serum were separated and stored at − 75 °C for further analysis. We analyzed hs-CRP after each testing using nephelometry. Lipid Peroxidation was assessed by two distinct techniques: 1) Plasma F2-isoprostanes analysis, using a commercial kit (Cayman®), and 2) TBARS analysis, based in the thiobarbituric acid reactivity with malondialdehyde, a lipid oxidation end-product [26]. Non-enzymatic plasma antioxidant properties were assessed by total reactive antioxidant potential (TRAP), determined by chemiluminescence [27]. Superoxide dismutase (SOD) activity was measured indirectly through the inhibition of adrenaline auto-oxidation to adrenochrome, as described by Bannister [28]. Plasma protein was determined as described by Lowry et al. [29]. Uric acid was determined by a commercial kit Uricostat enzymatic AA, Wiener Lab®, Rosario, Agentina).
Statistical analysis
Data were analyzed on SPSS 25.0 for Windows (IBM, USA). Normality was assessed by the Shapiro-Wilk test, and Levene’s test was used for homoscedasticity. For baseline value comparison between groups, Student’s t-test for independent samples or the Mann-Whitney test were performed. A generalized estimating equation (GEE) followed by Bonferroni’s post hoc test was used to verify supplementation and exercise effects adjusted for age, gender, duration of diabetes and use of drugs. The GEE model for each outcome was based on the goodness of fit. The normality of the residuals was verified by normal Q-Q plot. Some cardiometabolic variables were transformed due to the high variability among individuals, using a) logarithmic transformation for hs-CRP and F2-isoprostanes, b) square root transformation for albuminuria, uric acid and SOD, and c) inverse transformation for triglycerides and HbA1C. Cohen’s d was used to verify the effect size of the means. The accepted significance level was p < 0.05. Data were reported as mean ± standard deviation, and difference mean and 95% confidence interval (95% CI).
Results
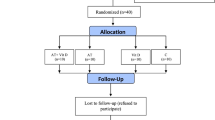
Figure 2 shows the flow diagram of patient recruitment and randomization. Of the 90 individuals who manifested interest in participating in the study, 60 were excluded based on the adopted criteria. In total, 30 individuals performed all baseline assessments, and all subjects completed the intervention. The characteristics of the participants are presented in Table 1. There were no differences in age, diabetes duration, weight, height, body mass index, percentage of body fat and peak oxygen consumption between the groups. Waist to hip ratio was higher in male subjects in the n3-PUFA group (p = 0.03).
After the supplementation period, individuals did not change your body weight (0.94 ± 0.36% in n-3 PUFA supplementation and 0.75 ± 0.25% in placebo group, respectively, p > 0.05 for both). Table 2 shows the biochemical parameters before and after supplementation period. n-3 PUFA supplementation significantly reduced triglycerides by 17% (P = 0.005), compared with baseline values, and by 26% when compared with the placebo group (P = 0.05). No other interaction condition vs time was observed in the experimental groups.
Regarding submaximal exercise tests, there were no differences in time duration in both groups before (14 ± 5.02 min and 15 ± 6.1 min) and after supplementation (15 ± 4.7 min and 13 ± 5.8 min) for n-3 PUFA supplementation and placebo groups, respectively (p > 0.05). Table 3 shows the results about oxidative stress and inflammation parameters after supplementation and submaximal exercises. No significant changes were observed for TBARS nor F2-Isoprostanes concentrations (primary outcome). In the n-3 PUFA supplementation group, a statistical significant interaction (condition vs time) was observed for TRAP count values, with decrease after supplementation and exercise. Cohen’s d effect size was consider high for this mean difference (d ≥ 0.8). Although no statistically significant interaction was found for SOD levels (p = 0.095), the n-3 PUFA supplementation group presented an increase after supplementation (p = 0.002, Cohen’s d effect size = 0.49), with a tendency in their concentrations after exercise. There was no significant difference for hs-CRP between the groups.
Discussion
The main finding of this study was that 8 weeks of n-3 PUFA supplementation reduces TRAP concentration after a high intensity exercise, with no other statistically significant changes in other oxidative stress parameters or inflammation. Additionally, as previously demonstrated, n-3 PUFA supplementation reduced triglycerides levels. Our hypothesis was partially rejected, due to the fact that our primary outcomes (TBARS and F2-isoprostanes) were not attenuated by n-3 PUFA supplementation.
As previously mentioned, physical exercise is considered a powerful tool to prevent and to treat diabetes and the associated comorbidities. Nowadays, due to the lack of time, many people are using high intensity exercise sessions (with a lower volume), as an alternative, to improve their health, metabolic function and body composition. However, high intensity exercise can induce a transient inflammatory state and increase oxidative stress [10, 11] that may promote, in diabetic population, an undesired effect. The increase in VO2 (load dependent) caused by exercise can lead to increased blood and tissue levels of reactive oxygen species [30]. Laaksonen et al. [31] and Atalay et al. [32] showed increased reactive oxygen species production after 40 min of sub-maximal exercise (intensity equivalent to 60% of VO2peak) in type 1 diabetic subjects. In our study, exercise performed with intensity equivalent to 70–80% of VO2peak was not able to increase the production of lipoperoxides as measured by TBARS and F2-Isoprostanes (primary outcomes). If exercise intensity was to be an issue, our data are corroborated by the findings of Davison et al. [33], that did not observe differences in the concentrations of malondialdehyde even after strenuous exercise. In addition, the lack of changes in TBARS and F2-isoprostanes may be attributed, at least in part, by the shorter period of supplementation of the present work in comparison with others.
We hypothesized that including n-3 PUFA to our subjects would induce benefits when they exercise at high intensity, however, with except of changes in TRAP, our intervention fail to change levels of lipoperoxidation, SOD and UA. Accinni and colleagues [34] also found an increase in TRAP in dyslipidemic volunteers supplemented with n-3 PUFA for 4 months, however, with a significant reduction in the levels of TBARS (P = 0,002). After treating healthy volunteers with 3.6 g/day of n-3 PUFA, Nalsen et al. [16] did not observe changes in the values of TRAP; however, they found a significant (P = 0.015) reduction in lipoperoxidation as measured by F2-isoprostanes concentration. The difference in results among studies may be related to different exercise protocols, duration of the tests, and different methods employed when measuring the oxidative stress markers.
Regarding SOD and UA, our secondary outcomes, there were no significant changes after the exercise, similarly to the results described by Laaksonen et al. [31] and Atalay et al. [32]. If our subjects were more obese or dyslipidemic, the results could be different (49). There is still very limited information on the effects of exercise in acute oxidative stress in subjects with type 2 diabetes. The trend for increased activity of SOD after supplementation with n-3 PUFA was not reported in the work of Kesavulu et al. [4]. However, Smaoui et al. [35] showed a positive correlation between SOD activity and concentrations of n-3 PUFA in erythrocytes. Erdogan et al. [36] also found increased activity of SOD in rats after 0.4 / kg body weight fish oil supplementation for 30 days. This increase in SOD and TRAP could be explained by the formation of cyclopentenone prostaglandins. Supplementation with n-3 PUFA, particularly with EPA and DHA, has been documented to trigger a momentary lipoperoxidation process, thus leading to the formation of prostaglandins, particularly cyclopentenone prostaglandins. These are responsible for the dissociation of Keap1/Nrf2 (NF-E2-related factor). Nrf2 is the main transcription factor for the activation of the expression of antioxidant enzymes (e.g. SOD, catalase, glutathione reductase) as well as phase 2 enzymes (e.g. glutathione S-transferase, NADPH oxidase) and thus also increases TRAP [37]. The production of lipoperoxide is not considered enough to cause damage to the body, but important to stimulate the defense systems.
Subjects who develop T2DM have high levels of inflammatory markers such as hs-CRP when compared to healthy subjects [37, 38]. Supplementation with n-3 PUFA has been suggested to minimize the inflammatory processes [39]. However, our study failed to detect any significant change in the plasma levels of hs-CRP after supplementation with n-3 PUFA. Our findings corroborate the data published by Madsen et al. [40] who worked with healthy subjects supplemented with 2 or 6.6 g / day of n-3 PUFA, for a period of 12 weeks. Both groups showed no change in the values of hs-CRP. Mori et al. [15] and Fatemeh Azizi-Soleiman et al. [41] also found no change in plasma levels of CRP after supplementation with EPA or DHA, for 6 weeks and 8 weeks, respectively, in type 2 diabetic subjects. Other studies also have shown that either EPA or DHA reduce in vivo oxidant stress without changing markers of inflammation, in treated, hypertensive, type 2 diabetic subjects [15]. Finally, despite the lack of changes of hs-CRP, we cannot exclude the potential anti-inflammatory effects of our supplementation, since we did not look at other inflammatory markers such as TNF-α, IL-8, eHSP70 etc. This is a limitation of our study.
Plasma glucose and HbA1c did not change significantly after the period of supplementation. Some studies reported increased levels of glucose, HbA1c and low insulin activity, in type 2 diabetes, after the consumption of large amounts of fish oil (around 10 g/day) [42]. However, when of n-3 PUFA is given in smaller quantities (1 to 4 g/day), no changes in glycemic parameters are observed [43]. In our study, a significant reduction (17%) in the values of plasma triglycerides was observed after the period of supplementation with n-3 PUFA. These results can be explained by the reduction of fractions of VLDL - thus minimizing the hepatic production of triglycerides - and a possible reduction of free fatty acids [44]. As in other studies, there were no significant changes in other lipid parameters [43, 45,46,47]. This can be partially explained by the fact that the diabetic subjects in the present study were not dyslipidemic, and were relatively lean (overweight on average). This differs from many studies in the literature. In a meta-analysis covering studies between 1966 and 2006, Hartweg et al. [48], showed that n-3 PUFA supplementation is, indeed, efficient to reduce triglycerides and VLDL levels, without changing other lipid sub fractions. In contrast, Abe et al. [49], after 6 weeks of supplementation with 4 g/day n-3 PUFA, found an 11% reduction in total cholesterol and a 14% increase in HDL. These changes are not a consensus in the available literature. In addition, a 4-h infusion of intralipid (n-3 FA) failed to affect insulin sensitivity, insulin secretion, or markers of oxidative stress in subjects with T2DM [50]. It could explain the lack of changes in the glycemic profile.
To the best of our knowledge, this is the first study to examine the interactions between n-3 PUFA supplementation and high-intensity exercise on oxidative stress and inflammation in subjects with T2DM. However, we recognize that the study has several limitations. First, the sample size was small, although the effect size on the variables with statistical significance was high. So, at this point, should be interpreted with caution before being extrapolated to the general population. Second, we have only studied acute effect of high intensity exercise; therefore, it is not possible to state that the effects would be similar after other intensities of exercise. Finally, we did not analyze the supplement offered to the participants, so we cannot be 100% sure if the product provides the amount of n-3 PUFA. We believe in the suitability of the company, but we suggest that future studies test the product before the beginning of the trial. Despite some limitations, the strength of this study is that we analyzed a large number of variables related to oxidative stress, including two measurements of lipoperoxidation (TBARS and F2-siprostanes) and measurements of enzymatic (SOD) and non-enzymatic antioxidants (uric acid and TRAP). Thus, this study contributes to narrow the gap in the literature on the effects of antioxidants nutrients in oxidative stress parameters after acute exercise. Further clarification is needed regarding the clinical relevance of the n-3 PUFA supplementation for T2DM in different intensities of exercise.
Conclusions
Eight weeks of supplementation with n-3 PUFA did not attenuate oxidative stress parameters after a high intensity exercise in T2DM subjects, with exception to TRAP values. On the other hand, n-3 PUFA cause reduction of triglyceride levels, at rest, without altering membrane oxidative damage or inflammation (as assessed by hs-CRP). Studies including other inflammation markers such as IL-8, MCP-1, eHSP70, or others, are required to better assess this response. Likewise, longer periods of observation and/or and other intensities of exercise may be necessary to induce significant increments in oxidative stress parameters in diabetic patients.
Abbreviations
- DHA:
-
Docosaexaenoic acid
- EPA:
-
Eicosapentaenoic acid
- HR:
-
Heart rate
- hs-CRP:
-
High sensitivity C-reactive protein
- n-3PUFA:
-
Polyunsaturated fatty acids omega-3
- SOD:
-
Superoxide dismutase
- T2DM:
-
Type 2 diabetes mellitus
- TBARS:
-
Thiobarbituric acid reactive substances
- TRAP:
-
Total reactive antioxidant potential
- VCO2 :
-
Carbon dioxide production
- VO2 :
-
Oxygen consumption
- VT2:
-
Second ventilator threshold
References
Shah MS, Brownlee M. Molecular and cellular mechanisms of cardiovascular disorders in diabetes. Circ Res. 2016;118(11):1808–29.
Vlassara H, Uribarri J. Advanced glycation end products (AGE) and diabetes: cause, effect, or both? Curr Diab Rep. 2014;14(1):453.
Newsholme P, et al. Exercise and possible molecular mechanisms of protection from vascular disease and diabetes: the central role of ROS and nitric oxide. Clin Sci (Lond). 2009, 118(5):341–9.
Kesavulu MM, et al. Effect of omega-3 fatty acids on lipid peroxidation and antioxidant enzyme status in type 2 diabetic patients. Diabetes Metab. 2002;28(1):20–6.
Peerapatdit T, et al. Antioxidant status and lipid peroxidation end products in patients of type 1 diabetes mellitus. J Med Assoc Thai. 2006;89(Suppl 5):S141–6.
Davi G, et al. Enhanced lipid peroxidation and platelet activation in the early phase of type 1 diabetes mellitus: role of interleukin-6 and disease duration. Circulation. 2003;107(25):3199–203.
Davi G, et al. In vivo formation of 8-iso-prostaglandin f2alpha and platelet activation in diabetes mellitus: effects of improved metabolic control and vitamin E supplementation. Circulation. 1999;99(2):224–9.
Krause M, et al. The effects of aerobic exercise training at two different intensities in obesity and type 2 diabetes: implications for oxidative stress, low-grade inflammation and nitric oxide production. Eur J Appl Physiol. 2013;114(2):251–60.
Pickup JC. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care. 2004;27(3):813–23.
Colberg SR, et al. Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement executive summary. Diabetes Care. 2010;33(12):2692–6.
Fox CS, et al. Update on prevention of cardiovascular disease in adults with type 2 diabetes mellitus in light of recent evidence: a scientific statement from the American Heart Association and the American Diabetes Association. Diabetes Care. 2015;38(9):1777–803.
Capo X, et al. Effects of almond- and olive oil-based Docosahexaenoic- and vitamin E-enriched beverage dietary supplementation on inflammation associated to exercise and age. Nutrients. 2016;8(10).
Chen C, et al. Association between omega-3 fatty acids consumption and the risk of type 2 diabetes: a meta-analysis of cohort studies. J Diabetes Investig. 2017;8(4):480–8.
Taghizadeh M, et al. The effects of omega-3 fatty acids and vitamin E co-supplementation on clinical and metabolic status in patients with Parkinson's disease: a randomized, double-blind, placebo-controlled trial. Neurochem Int. 2017;108:183–9.
Mori TA, et al. Effect of eicosapentaenoic acid and docosahexaenoic acid on oxidative stress and inflammatory markers in treated-hypertensive type 2 diabetic subjects. Free Radic Biol Med. 2003;35(7):772–81.
Nalsen C, et al. Dietary (n-3) fatty acids reduce plasma F2-isoprostanes but not prostaglandin F2alpha in healthy humans. J Nutr. 2006;136(5):1222–8.
WHO, W.H.O.-. Obesity: preventing and managing the global epidemic. WHO, 2000.
Jackson AS, Pollock ML. Generalized equations for predicting body density of men. Br J Nutr. 1978;40(3):497–504.
Siri WE. Body composition from fluid spaces and density: analysis of methods. 1961. Nutrition. 1993;9(5):480–91. discussion 480, 492
Lucia A, et al. Heart rate and performance parameters in elite cyclists: a longitudinal study. Med Sci Sports Exerc. 2000;32(10):1777–82.
Midgley AW, et al. Criteria for determination of maximal oxygen uptake: a brief critique and recommendations for future research. Sports Med. 2007;37(12):1019–28.
Rodrigues-Krause J, et al. Oxygen consumption and heart rate responses to isolated ballet exercise sets. J Dance Med Sci. 2014;18(3):99–105.
Ribeiro JP, et al. Metabolic and ventilatory responses to steady state exercise relative to lactate thresholds. Eur J Appl Physiol Occup Physiol. 1986;55(2):215–21.
Valk EE, Hornstra G. Relationship between vitamin E requirement and polyunsaturated fatty acid intake in man: a review. Int J Vitam Nutr Res. 2000;70(2):31–42.
Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502.
Draper HH, Hadley M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1990;186:421–31.
Lissi E, et al. Evaluation of total antioxidant potential (TRAP) and total antioxidant reactivity from luminol-enhanced chemiluminescence measurements. Free Radic Biol Med. 1995;18(2):153–8.
Bannister JV, Calabrese L. Assays for superoxide dismutase. Methods Biochem Anal. 1987;32:279–312.
Lowry OH, et al. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193(1):265–75.
Ortenblad N, Madsen K, Djurhuus MS. Antioxidant status and lipid peroxidation after short-term maximal exercise in trained and untrained humans. Am J Phys. 1997;272(4 Pt 2):R1258–63.
Laaksonen DE, et al. Increased resting and exercise-induced oxidative stress in young IDDM men. Diabetes Care. 1996;19(6):569–74.
Atalay M, et al. Altered antioxidant enzyme defences in insulin-dependent diabetic men with increased resting and exercise-induced oxidative stress. Acta Physiol Scand. 1997;161(2):195–201.
Davison GW, et al. Exercise, free radicals, and lipid peroxidation in type 1 diabetes mellitus. Free Radic Biol Med. 2002;33(11):1543–51.
Accinni R, et al. Effects of combined dietary supplementation on oxidative and inflammatory status in dyslipidemic subjects. Nutr Metab Cardiovasc Dis. 2006;16(2):121–7.
Smaoui M, et al. Association between dietary fat and antioxidant status of Tunisian type 2 diabetic patients. Prostaglandins Leukot Essent Fatty Acids. 2006;74(5):323–9.
Erdogan H, et al. Effect of fish oil supplementation on plasma oxidant/antioxidant status in rats. Prostaglandins Leukot Essent Fatty Acids. 2004;71(3):149–52.
Laaksonen DE, et al. C-reactive protein and the development of the metabolic syndrome and diabetes in middle-aged men. Diabetologia. 2004;47(8):1403–10.
Pradhan AD, et al. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286(3):327–34.
Calder PC. Polyunsaturated fatty acids and inflammation. Prostaglandins Leukot Essent Fatty Acids. 2006;75(3):197–202.
Madsen T, et al. The effect of dietary n-3 fatty acids on serum concentrations of C-reactive protein: a dose-response study. Br J Nutr. 2003;89(4):517–22.
Azizi-Soleiman F, et al. Effects of pure eicosapentaenoic and docosahexaenoic acids on oxidative stress, inflammation and body fat mass in patients with type 2 diabetes. Int J Prev Med. 2013;4(8):922–8.
Hendra TJ, et al. Effects of fish oil supplements in NIDDM subjects. Controlled study. Diabetes Care. 1990;13(8):821–9.
Petersen M, et al. Effect of fish oil versus corn oil supplementation on LDL and HDL subclasses in type 2 diabetic patients. Diabetes Care. 2002;25(10):1704–8.
Rivellese AA, et al. Long-term effects of fish oil on insulin resistance and plasma lipoproteins in NIDDM patients with hypertriglyceridemia. Diabetes Care. 1996;19(11):1207–13.
Luo J, et al. Moderate intake of n-3 fatty acids for 2 months has no detrimental effect on glucose metabolism and could ameliorate the lipid profile in type 2 diabetic men. Results of a controlled study. Diabetes Care. 1998;21(5):717–24.
Mansoori A, et al. Effect of DHA-rich fish oil on PPARgamma target genes related to lipid metabolism in type 2 diabetes: a randomized, double-blind, placebo-controlled clinical trial. J Clin Lipidol. 2015;9(6):770–7.
Moore CS, et al. Oily fish reduces plasma triacylglycerols: a primary prevention study in overweight men and women. Nutrition. 2006;22(10):1012–24.
Hartweg J, et al. Meta-analysis of the effects of n-3 polyunsaturated fatty acids on lipoproteins and other emerging lipid cardiovascular risk markers in patients with type 2 diabetes. Diabetologia. 2007;50(8):1593–602.
Abe Y, et al. Soluble cell adhesion molecules in hypertriglyceridemia and potential significance on monocyte adhesion. Arterioscler Thromb Vasc Biol. 1998;18(5):723–31.
Mostad IL, et al. Addition of n-3 fatty acids to a 4-hour lipid infusion does not affect insulin sensitivity, insulin secretion, or markers of oxidative stress in subjects with type 2 diabetes mellitus. Metabolism. 2009;58(12):1753–61.
Acknowledgments
We thank Naturalis® Company for providing the capsules of omega-3.
Funding
This work was funded from CAPES (the Brazilian Ministry of Education’s fund) and by FIPE-HCPA (the Research Fund of Hospital de Clínicas de Porto Alegre).
Availability of data and materials
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
APTF, KBS, MK, PIHB Jr., RF and JCFM and ARO designed the study. KBS, GCS, RR, JSR and JRF carried out the data collection. APTF and KBS performed the statistical analysis. MK, RR, JSR and JRF performed the biochemical analyzes. All authors helped to draft the manuscript, read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the HCPA Ethics and Research Committee (06–222). The study was conducted in accordance with the requirements of the declarations of Helsinki. All participants were informed about the procedures and signed an informed consent form prior to enrollment in the study.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Fayh, A.P.T., Borges, K., Cunha, G.S. et al. Effects of n-3 fatty acids and exercise on oxidative stress parameters in type 2 diabetic: a randomized clinical trial. J Int Soc Sports Nutr 15, 18 (2018). https://doi.org/10.1186/s12970-018-0222-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12970-018-0222-2