Abstract
Background
The intricate etiology of autoimmune liver disease (AILD) involves genetic, environmental, and other factors that yet to be completely elucidated. This study comprehensively assessed the causal association between genetically predicted modifiable risk factors and AILD by employing Mendelian randomization.
Methods
Genetic variants associated with 29 exposure factors were obtained from genome-wide association studies (GWAS). Genetic association data with autoimmune hepatitis (AIH), primary biliary cholangitis (PBC) and primary sclerosing cholangitis (PSC) were also obtained from publicly available GWAS. Univariate and multivariate Mendelian randomization analyses were performed to identify potential risk factors for AILD.
Results
Genetically predicted rheumatoid arthritis (RA) (OR = 1.620, 95%CI 1.423–1.843, P = 2.506 × 10− 13) was significantly associated with an increased risk of AIH. Genetically predicted smoking initiation (OR = 1.637, 95%CI 1.055–2.540, P = 0.028), lower coffee intake (OR = 0.359, 95%CI 0.131–0.985, P = 0.047), cholelithiasis (OR = 1.134, 95%CI 1.023–1.257, P = 0.017) and higher C-reactive protein (CRP) (OR = 1.397, 95%CI 1.094–1.784, P = 0.007) were suggestively associated with an increased risk of AIH. Genetically predicted inflammatory bowel disease (IBD) (OR = 1.212, 95%CI 1.127–1.303, P = 2.015 × 10− 7) and RA (OR = 1.417, 95%CI 1.193–1.683, P = 7.193 × 10− 5) were significantly associated with increased risk of PBC. Genetically predicted smoking initiation (OR = 1.167, 95%CI 1.005–1.355, P = 0.043), systemic lupus erythematosus (SLE) (OR = 1.086, 95%CI 1.017–1.160, P = 0.014) and higher CRP (OR = 1.199, 95%CI 1.019–1.410, P = 0.028) were suggestively associated with an increased risk of PBC. Higher vitamin D3 (OR = 0.741, 95%CI 0.560–0.980, P = 0.036) and calcium (OR = 0.834, 95%CI 0.699–0.995, P = 0.044) levels were suggestive protective factors for PBC. Genetically predicted smoking initiation (OR = 0.630, 95%CI 0.462–0.860, P = 0.004) was suggestively associated with a decreased risk of PSC. Genetically predicted IBD (OR = 1.252, 95%CI 1.164–1.346, P = 1.394 × 10− 9), RA (OR = 1.543, 95%CI 1.279–1.861, P = 5.728 × 10− 6) and lower glycosylated hemoglobin (HbA1c) (OR = 0.268, 95%CI 0.141–0.510, P = 6.172 × 10− 5) were positively associated with an increased risk of PSC.
Conclusions
Evidence on the causal relationship between 29 genetically predicted modifiable risk factors and the risk of AIH, PBC, and PSC is provided by this study. These findings provide fresh perspectives on the management and prevention strategies for AILD.
Similar content being viewed by others
Introduction
Autoimmune liver disease (AILD) is a collection of liver pathologies resulting from autoimmune dysregulation, distinguished by liver lymphocyte infiltration, heightened levels of circulating immunoglobulins, elevated liver enzyme activity, and the generation of autoantibodies [1]. The group of diseases under consideration can be categorized into three main types based on clinical presentation, biochemistry, imaging, and histopathology [1,2,3]. Autoimmune hepatitis (AIH) is defined by injury to the parenchymal cells of the liver, specifically interfacial hepatitis [4, 5]. Primary biliary cholangitis (PBC) is characterized by non-suppurative, destructive injury and cholestasis of the interlobular bile ducts [3, 6, 7]. Lastly, primary sclerosing cholangitis (PSC) is identified by the presence of multilayered onion-skin-like fibrosis in the intermediate-sized intra- and extra-hepatic bile ducts, along with multifocal bile duct obstruction [8, 9]. Contemporary incidence rates of disease per 100,000 range from 0.84 to 2.75 for PBC, 0.1 to 4.39 for PSC and 0.4 to 2.39 for AIH [1, 10, 11].
AILD is known to progress slowly with a malignant tendency, potentially culminating in conditions such as liver fibrosis, cirrhosis, and even hepatocellular carcinoma [11,12,13]. Unfortunately, there is currently no curative treatment for AILD, with corticosteroids in combination with azathioprine being the primary first-line medication for AIH [14]. Additionally, drugs such as ursodeoxycholic acid (UDCA), hormonal medications, and immunosuppressive agents show some therapeutic efficacy for patients with PSC and PBC, although a subset of patients may exhibit poor response. For those in end-stage AILD, the quality of life is severely compromised, often necessitating liver transplantation as a final recourse [3, 14,15,16,17,18].
Several previous studies have initially explored the association between certain modifiable risk factors and AILD risk. About one-third of AILD patients are accompanied by extrahepatic autoimmune diseases, including rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), inflammatory bowel disease (IBD), and psoriasis [19,20,21,22]. Nonetheless, no comprehensive observational study has been conducted to elucidate the causal connection between the occurrence of these extrahepatic autoimmune disorders and AILD. In addition to being frequently associated with extrahepatic autoimmune diseases, osteoporosis is one of the most serious complications of PBC, leading to an increased risk of fracture [23,24,25,26,27]. Several factors affecting bone metabolism may be responsible for osteoporosis in patients with PBC, such as calcium dysregulation and vitamin D3 deficiency [25,26,27]. Previous studies have demonstrated that serum vitamin D3 levels in AIH and PBC patients are significantly lower than those in healthy controls, and are negatively correlated with liver fibrosis and cholestasis, respectively [4, 28,29,30]. Vitamin D3 deficiency may be a contributory factor in the development of AILD, but there are still few relevant studies [31,32,33,34]. AILD often presents a gender-dependent pattern. Peroxisome proliferator activated receptor alpha (PPAR-α) plays a crucial role in the innate immune defense [35]. Suppression of PPAR-α expression in female patients with PBC is accompanied by reduced testosterone levels, which should raise interest in the role of testosterone in the development of PBC [36,37,38]. In addition to the above factors, the association between many other modifiable risk factors and AILD has not been fully investigated, such as lifestyle, serum parameters, glucose metabolism, lipid metabolism, and obesity characteristics.
Mendelian randomization (MR) is a data analysis technique for assessing etiological inferences in epidemiological studies, based on the widely published genome-wide association study (GWAS) pooled datasets, which employs genetic variations as instrumental variables (IVs) to estimate the causal relationship between possible exposures and the outcome of interest [39, 40]. MR exploits the fact that genes are fixed and Mendel’s first and second laws of inheritance [41, 42]. It is a method comparable to randomized controlled trials without bias due to unobserved confounders, measurement errors and reverse causality [41, 43]. In the absence of randomized controlled studies, MR can be a time-saving and cost-effective way to assess and screen for potential causal associations, making it also known as " randomized controlled study built by nature”.
The unsatisfactory pharmacologic treatment of AILD underlines the imperative need to thoroughly investigate the etiology. Therefore, this study explores the causal effects of 29 genetically predicted modifiable risk factors on the risk of AIH, PBC, and PSC. This work aims to provide a comprehensive overview of the potential modifiable risk factors for AILD and offer new insights into the etiology of AILD.
Methods
MR design
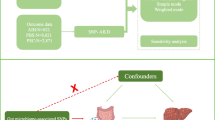
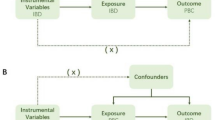
MR was used to explore causal associations between modifiable risk factors and different AILDs. MR is based on three key assumptions: (i) IVs are related to exposure; (ii) IVs are independent of confounders that may affect the exposure-outcome association; (iii) there is no way for IVs to affect the outcome other than by being related to exposure (Fig. 1). A total of 29 main risk factors were selected, which were divided into 6 categories: lifestyle behaviors, related diseases, serum parameters, lipid metabolism, glucose metabolism and obesity characteristics. Single nucleotide polymorphisms (SNPs) associated with these risk factors were extracted as IVs. The data sets used in this study were all sourced from public databases and received ethical approval prior to implementation. Therefore, no additional ethical approval was required for this study.
IVs selection
Rigorous fltering steps were performed to control the IVs quality before MR analysis. The significant and independent SNPs for all factors were chosen as IVs based on the following criteria: [1] genome-wide association significance threshold of p-value < 5 × 10− 8; [2] all SNPs are independent with the threshold of LD r2 < 0.01 in a 10 Mb window. SNPs were also ruled out if palindromic sequences with intermediate allele frequencies were present. Proxy SNPs were utilized when merging exposure and outcome data [3]. The PhenoScanner was used to remove SNPs associated with confounders (http://www.phenoscanner.medschl.cam.ac.uk/). [4] Steiger filtering was applied to test the direction of causality of each SNP on exposure and outcome. SNPs meeting the criterion of “FALSE and P < 0.05” were excluded [5]. The MR-pleiotropy residual sum and outlier (MR-PRESSO) test was used to remove potential outlier SNPs [6]. For each SNP, the F statistic was determined by executing beta2 /se2. F > 10 is considered to have sufficient strength for the selected IVs. In this study, all F statistics meet F > 10. Detailed information on IVs for all exposure is provided in the supplementary material.
GWAS summary of autoimmune liver disease and baseline characteristics of 29 candidate factors
IVs associated with AIH were obtained from the largest atlas of genetic associations for 220 human phenotypes to date, including 484,413 European controls and 821 European patients (GWAS ID: ebi-a-GCST90018785). The IVs associated with PBC were extracted from the largest international genome-wide meta-analysis of PBC to date, which included 16,489 European controls and 8021 European patients (GWAS ID: ebi-a-GCST90061440). For PSC, the IVs were obtained from the most extensive PSC GWAS conducted by the International PSC Study Group (IPSCSG), including 12,019 European controls and 2871 European patients (GWAS ID: ieu-a-1112). Twenty-nine potential risk factors were included in the analysis. Risk factors can be divided into six categories: lifestyle, related diseases, serum parameters, lipid metabolism, glucose metabolism, and obesity characteristics (Table 1). Lifestyle behaviors include smoking, alcohol consumption, coffee consumption, educational attainment, and household income. Related diseases include IBD, RA, SLE and cholelithiasis. Serum parameters include serum 25-Hydroxyvitamin D (vitamin D3), calcium, C-reactive protein (CRP), and testosterone. In addition, five traits related to lipid metabolism, four traits related to glucose metabolism and five traits related to obesity were analyzed. For each of the 29 modifiable potential risk factors examined, the F statistic of their respective genetic tools was greater than the empirical threshold of 10, indicating no potential weak tool bias. Ethical approval was obtained for all selected GWAS and informed consent was obtained from individuals.
Genetic correlation analysis
Linkage disequilibrium score (LDSC) regression was performed to determine the genetic correlation of 29 potential risk factors with AIH, PBC and PSC, as well as to evaluate the extent of sample overlap [44]. The regression intercept of bivariate LDSC reflected the sample overlap of trait pairs. GWAS summary statistics were filtered according to HapMap3 ref. Variants that were not SNPs (e.g., indels) and SNPs that were strand-ambiguous, repeated, and had a minor allele frequency (MAF) < 0.01 were excluded. The LDSC examines the association between test statistics and linkage disequilibrium to quantify the contribution of inflation from a true polygenic signal or bias [45]. This method can evaluate genetic correlation from GWAS summary statistics and is not biased by sample overlap [44]. The z-scores of each variant from Trait 1 are multiplied by the z-scores of each variant from Trait 2. The genetic covariance was estimated by regressing this product against the LD score. The genetic covariance normalized by SNP-heritability represents the genetic correlation. A Bonferroni-corrected P-value was set as 0.05/29 (1.72 × 10− 3). P < 1.72 × 10− 3 was defined as statistically significant association.
Univariable MR analyses
Univariate MR analyses used the random-effect inverse variance weighted (IVW) method as the primary analysis to estimate the association between modifiable risk factors and the risk of PBC, PSC, and AIH. In addition, MR-Egger and weighted median were utilized to refine the IVW estimates as they provide more robust estimates in a wider range of scenarios despite being less efficient (wider CIs). If the effect estimation in the IVW technique was significant and no contradictory outcomes were obtained in other methods, the causal connection was considered suggestive. In this study, multiple techniques for sensitivity analysis were implemented. First, the heterogeneity of IVs was evaluated using Cochran’s Q test. Specifically, heterogeneity was detected if the P value of the Cochran Q test was less than 0.05. Furthermore, the MR-Egger regression intercept, MR-PRESSO (global test), leave-one-out analysis, funnel plot and forest plot were employed to check for any potential pleiotropy and assessed the robustness of the results. When the p-value was less than 0.05, the MR analysis might support the premise that IVs had a direct effect on the outcome (conflicts with MR hypothesis III). Besides, leave-one-out analysis was performed to determine whether the causal estimate was driven by any single SNP. The power values were calculated online to enhance the robustness of the findings (https://shiny.cnsgenomics.com/mRnd/). All findings of MR analysis, sensitivity analysis, and visualization plots are available in the Supplementary Material.
Multivariable MR analyses
Multivariate MR was performed only for the phenotypes of interest (lipid metabolism, glucose metabolism, obesity characteristics). A Bonferroni-corrected P-value was set as 0.05/29 (1.72 × 10− 3). P values ranging from 1.72 × 10− 3 to 0.05 were classified as suggestive causal associations. Results are reported as ORs and corresponding 95% confidence intervals (CIs). All the analyses were undertaken using R 4.3.1 (R Foundation for statistical Computing, Vienna, Austria).
Results
LDSC regression analysis
MR estimates may violate causality provided there is a genetic correlation between exposure and outcome. In this study, no significant genetic correlation was observed between 29 potential risk factors and AIH, PBC and PSC. This suggested that MR estimates were not confounded by shared genetic components. Furthermore, the regression intercepts of the bivariate LDSC regressiom were not significantly deviated from zero. This reflected the extremely limited sample overlap between all exposures and outcomes. Detailed information regarding all genetic correlation results is available in the Supplementary Materials.
Causal effects of the modifiable risk factors on AIH
Univariate MR Analysis showed that in the lifestyle segment, genetically predicted smoking initiation (OR = 1.637, 95%CI 1.055–2.540, P = 0.028) and lower coffee intake (OR = 0.359, 95%CI 0.131–0.985, P = 0.047) were suggestively associated with an increased risk of AIH (Fig. 2). No pleiotropy (MR-Egger regression intercept: P = 0.483; MR-PRESSO-Global Test: P = 0.198) or heterogeneity (Cochran’s Q test: P = 0.212) was detected for smoking initiation. No pleiotropy (MR-Egger regression intercept: P = 0.724; MR-PRESSO-Global Test: P = 0.712) or heterogeneity (Cochran’s Q test: P = 0.679) was detected for coffee intake. In the related disease plate, the genetically predicted extrahepatic autoimmune disease RA (OR = 1.620, 95%CI 1.423–1.843, P = 2.506 × 10− 13) was significantly associated with an increased risk of AIH. No pleiotropy (MR-Egger regression intercept: P = 0.304; MR-PRESSO-Global Test: P = 0.081) or heterogeneity (Cochran’s Q test: P = 0.074) was detected for RA. Genetically predicted cholelithiasis had a suggestive increased risk for AIH (OR = 1.134, 95%CI 1.023–1.257, P = 0.017). No pleiotropy (MR-Egger regression intercept: P = 0.312; MR-PRESSO-Global Test: P = 0.767) or heterogeneity (Cochran’s Q test: P = 0.783) was detected for cholelithiasis. In serum parameters profile, higher CRP (OR = 1.397, 95%CI 1.094–1.784, P = 0.007) was a suggestive adverse factor for AIH. Heterogeneity (Cochran’s Q test: P = 0.066) or pleiotropy (MR-Egger regression intercept: P = 0.269; MR-PRESSO-Global Test: P = 0.052) was not found in CRP. For the positive findings above, no outliers were identified with MR-PRESSSO and the leave-one-out plot as well as funnel plots. All findings of MR analysis, sensitivity analysis, and visualization plots are available in the Supplementary Materials.
Causal effects of the modifiable risk factors on PBC
Univariate analysis showed that genetically predicted smoking initiation (OR = 1.167, 95%CI 1.005–1.355, P = 0.043) was suggestively associated with an increased risk of PBC (Fig. 3). No pleiotropy (MR-Egger regression intercept: P = 0.164; MR-PRESSO-Global Test: P = 0.998) or heterogeneity (Cochran’s Q test: P = 0.999) was detected for smoking initiation. In the related disease plate, genetically predicted extrahepatic autoimmune diseases IBD (OR = 1.212, 95%CI 1.127–1.303, P = 2.015 × 10− 7) and RA (OR = 1.417, 95%CI 1.193–1.683, P = 7.193 × 10− 5) were significantly associated with increased risk of PBC. Heterogeneity was observed with a Cochran Q-derived P value < 0.05 for IBD and RA. As the random-effects IVW was used as main result, heterogeneity is acceptable. No pleiotropy was detected for IBD (MR-Egger regression intercept: P = 0.227; MR-PRESSO-Global Test: P = 0.097) and RA (MR-Egger regression intercept: P = 0.874; MR-PRESSO-Global Test: P = 0.117). Besides, genetically predicted SLE had a suggestive increased risk for PBC (OR = 1.086, 95%CI 1.017–1.160, P = 0.014). No pleiotropy was detected for SLE (MR-Egger regression intercept: P = 0.358). Heterogeneity was observed for SLE with a Cochran Q-derived P value < 0.05, but acceptable. In serum parameters profile, higher vitamin D3 (OR = 0.741, 95%CI 0.560–0.980, P = 0.036) and calcium (OR = 0.834, 95%CI 0.699–0.995, P = 0.044) levels were suggestive protective factors for PBC with possible heterogeneity (Cochran’s Q test: P = 2.140 × 10− 3 ;P = 2.244 × 10− 3, respectively). But no pleiotropy was detected for serum vitamin D3 (MR-Egger regression intercept: P = 0.813) and calcium (MR-Egger regression intercept: P = 0.419) levels. However, genetically predicted higher CRP had a suggestive increased risk for PBC (OR = 1.199, 95%CI 1.019–1.410, P = 0.028). Heterogeneity was observed for CRP (Cochran’s Q test: P = 4.545 × 10− 4), but no pleiotropy was detected (MR-Egger regression intercept: P = 0.747). Among obesity characteristics, higher waist circumference was suggestively associated with an increased risk of PBC (OR = 1.272, 95%CI 1.033–1.567, P = 0.024). No pleiotropy (MR-Egger regression intercept: P = 0.921; MR-PRESSO-Global Test: P = 0.053) but slight heterogeneity (Cochran’s Q test: P = 0.048) was detected for waist circumference. All findings of MR analysis, sensitivity analysis, and visualization plots are available in the Supplementary Materials.
Causal effects of the modifiable risk factors on PSC
Univariate MR Analysis showed that genetically predicted smoking initiation (OR = 0.630, 95%CI 0.462–0.860, P = 0.004) was suggestively associated with a decreased risk of PSC in the lifestyle segment (Fig. 4). No pleiotropy (MR-Egger regression intercept: P = 0.670; MR-PRESSO-Global Test: P = 0.196) or heterogeneity (Cochran’s Q test: P = 0.183) was detected for smoking initiation. In addition, genetically predicted IBD (OR = 1.252, 95%CI 1.164–1.346, P = 1.394 × 10− 9) and RA (OR = 1.543, 95%CI 1.279–1.861, P = 5.728 × 10− 6) were positively associated with an increased risk of PSC. No pleiotropy (MR-Egger regression intercept: P = 0.349) but heterogeneity (Cochran’s Q test: P = 8.392 × 10− 4) was detected for IBD. No pleiotropy (MR-Egger regression intercept: P = 0.635; MR-PRESSO-Global Test: P = 0.161) or heterogeneity (Cochran’s Q test: P = 0.149) was detected for RA. In the glucometabolic plate, genetically predicted glycosylated hemoglobin (HbA1c) (OR = 0.268, 95%CI 0.141–0.510, P = 6.172 × 10− 5) was a protective factor for PSC. No pleiotropy (MR-Egger regression intercept: P = 0.721; MR-PRESSO-Global Test: P = 0.190) or heterogeneity (Cochran’s Q test: P = 0.171) was detected for HbA1c. Besides, genetically predicted type 2 diabetes had a suggestive decreased risk for PSC (OR = 0.877, 95%CI 0.780–0.986, P = 0.028). No pleiotropy (MR-Egger regression intercept: P = 0.145; MR-PRESSO-Global Test: P = 0.242) or heterogeneity (Cochran’s Q test: P = 0.327) was detected for type 2 diabetes. Among obesity characteristics, higher BMI was suggestively associated with a decreased risk of PSC (OR = 0.777, 95%CI 0.626–0.964, P = 0.022). No pleiotropy (MR-Egger regression intercept: P = 0.585; MR-PRESSO-Global Test: P = 0.090) or heterogeneity (Cochran’s Q test: P = 0.104) was detected for BMI. All findings of MR analysis, sensitivity analysis, and visualization plots are available in the Supplementary Materials.
Multivariable MR analysis of AIH, PBC and PSC
Only the interrelated genetically predicted modifiable risk factors were adjusted for each other in the multivariate MR analysis model. Obesity features, glucose metabolism, and lipid metabolism are the key hereditary predicted modifiable risk factors that need to be adjusted. Consistent with the findings of univariate analysis, genetically predicted levels of TG (OR = 0.884, 95%CI 0.646–1.210, P = 0.442), HDL(OR = 0.919, 95%CI 0.685–1.233, P = 0.575), LDL (OR = 0.897, 95%CI 0.655–1.229, P = 0.500), and ApoA1 (OR = 0.870, 95%CI 0.656–1.154, P = 0.334) were not significantly causally associated with the risk of AIH after adjusting for genetically predicted HDL/LDL, TG/LDL, TG/HDL, and TG/LDL, respectively. Fasting glucose (OR = 1.230, 95%CI 0.593–2.550, P = 0.577), fasting insulin (OR = 2.101, 95%CI 0.731–6.036, P = 0.168), and HbA1c levels (OR = 0.483, 95%CI 0.186–1.253, P = 0.135), as well as genetically predicted type 2 diabetes (OR = 0.926, 95%CI 0.777–1.103, P = 0.388) were also not associated with the risk of AIH after correction for each other. In multivariate analysis, obesity parameters were reconfirmed to be independent from the risk of AIH.
Similar to the multivariate analysis of AIH, multivariate adjustment did not modify the conclusion that indicators of lipid and glucose metabolism had no association with PBC risk. Notably, higher waist circumference (OR = 1.578, 95%CI 0.236–4.537, P = 0.638), which was suggestively linked with an increased risk of PBC in univariate analysis, was no longer suggestively associated with PBC after adjusting for other obesity characteristics in the multivariate analysis.
Finally, in the multivariate analysis of PSC, after correction for genetically fasting glucose/fasting insulin/type 2 diabetes, the suggestive association of HbA1c (OR = 0.369, 95%CI 0.138–0.986, P = 0.046) with a decreased risk of PSC remained significant. However, higher BMI (OR = 0.763, 95%CI 0.344–1.691, P = 0.505), which was suggestively linked with an increased risk of PSC in univariate analysis, was no longer associated with PSC after adjusting for other obesity characteristics in the multivariate analysis.
Discussion
Significant changes have been reported in the epidemiology of AILD [1, 10]. AIH and PSC incidence and prevalence in Europe are on the rise. The prevalence of PBC is also increasing in Europe, North America and Asia Pacific [1, 5, 10]. Overall, although AILD is rare, its clinical burden is disproportionately high with regard to population incidence and prevalence [1]. Age, gender, and race also affect clinical outcomes [38]. Patient morbidity and mortality are mirrored in the high demand for gastroenterology, hepatology and organ transplantation services [46]. In this study, causal associations of 29 potential risk factors with AIH, PBC, and PSC were systematically explored by MR.
Smoking is associated with numerous autoimmune diseases, leading to diverse effects such as tissue damage, apoptosis, inflammation, and anti-estrogen effects [47]. Several previous large epidemiological studies have revealed a strong association between smoking, or a history of smoking, and the risk of PBC [48,49,50,51]. Notably, a history of smoking is particularly linked to the presence of advanced fibrosis in PBC patients [47, 52,53,54]. As the number of cigarettes smoked (pack-years) increased, so did the risk of developing advanced fibrosis in PBC patients [54]. Comparisons have shown a significant difference in liver inflammatory activity between smokers and non-smokers, with smokers exhibiting elevated levels of IL-10 and IFN-γ, reflective of a Th1 response [55]. Furthermore, hydrocarbons contained in cigarette smoke have been found to be associated with PBC population colonies [56]. The MR Analysis results in this study further confirmed that smoking is a suggestive risk factor for PBC. However, the link between smoking and AIH risk is still debated [57]. This study showed that genetically predicted smoking was suggestively associated with an increased risk of AIH. While further investigation is required to fully characterize and consolidate the aforementioned results, it is discernible that individuals with PBC and AIH have to be discouraged from smoking. Surprisingly, despite being under the same umbrella of AILD, the MR analysis results in the present study suggested that smoking was a potential protective factor for PSC patients. Moreover, previous retrospective studies have corroborated our findings. A UK cohort study by WEBB G J et al. found that non-smoking was linked to a lower prevalence of PSC [10]. Similarly, a case-control study by BOONSTRA K et al. indicated that smoking was associated with a reduced risk of developing PSC [58]. In the lifestyle panel, genetically predicted lower coffee intake was suggestively associated with an increased risk of AIH. This result aligns with previous studies indicating that individuals with AIH have lower lifetime coffee consumption than healthy controls [59].
In previous studies, several extra-hepatic autoimmune diseases, including SLE and RA, have been reported to be closely associated with AILD [60]. Notably, although AILD like AIH and PBC are considered rare, their co-existence with SLE in patients presenting with liver enzyme abnormalities is relatively common [61]. The overlap rates of SLE with AIH and PBC were 1.6-15% and 2.2-7.5%, respectively [62, 63]. Current MR results support a genetically predicted causal relationship between SLE and PBC. However, current findings suggest that genetically predicted SLE does not significantly alter the risk of AIH.
The most common co-existing AILD in RA is PBC, with a prevalence of 3.8–6.3% [64, 65]. While the incidence of RA in PBC is reported to be 1.8–13%, about 50% of patients with PBC show RF positive [66,67,68]. Genetic investigations have revealed shared genes between RA and PBC such as HLA-DQB1, STAT4, IRF5, MMEL1, and CTLA4 [69]. In addition, in patients with AIH, the prevalence of RA ranges from 1.6–5.4% [70]. Furthermore, a proteome-wide MR has highlighted AIF1 and HLA-DQA2 as targets for PSC and RA [71]. This study suggests a causal connection between genetically predicted RA and the susceptibility to PBC, AIH, and PSC. Patients with overlapping diseases are typically diagnosed with RA before AILD, underscoring the importance of screening for AMA and ANA in RA patients presenting with cholestatic elevated liver enzymes, as the co-occurrence of these conditions can impact the prognosis and management of patients.
Both ulcerative colitis (UC) and Crohn disease (CD) are associated with a variety of hepatobiliary symptoms. The majority of patients with PSC are initially diagnosed with extensive colitis [72]. Currently, there is an ongoing discussion regarding whether PSC represents an extraintestinal manifestation of IBD or if PSC and IBD are distinct entities that share a common susceptibility leading to a dual phenotype [19, 73]. This study reinforces the causative link between genetically predicted IBD and PSC. Given that PSC-IBD is associated with a higher risk of malignancy [74], timely identification of high-risk individuals and the implementation of appropriate surveillance strategies are crucial in managing this complex relationship. Furthermore, the findings of this study support a causative relationship between genetically predicted IBD and PBC risk, aligning with earlier studies conducted by Zhang and Zhao et al [75, 76]. Disruption of intestinal permeability in IBD may lead to bacterial translocation, bile duct cell activation, and liver inflammation, ultimately contributing to the onset of PBC [77]. To further elucidate the causal interplay between IBD and PBC, it is imperative to conduct long-term prospective studies.
The serum indicators analyzed in this MR Study highlighted Vitamin D3 as a potential protective factor against PBC, indicating a reduced risk associated with this nutrient. In patients with PBC, Vitamin D3 deficiency is a common occurrence. Several studies have identified its correlation with poor response to UDCA, increased risk of cirrhosis development, liver-related mortality, and the necessity for liver transplantation [28, 32, 34]. Therefore, these findings imply that Vitamin D3 supplementation could serve as a cost-effective strategy for early intervention in the management of PBC [38].
Regarding data sources and study design, this study presents several advantages. First, the study avoided the bias inherent in conventional observational epidemiological studies by utilizing univariate and multivariate multimodal MR analyzes to evaluate the causal link between two complicated genetic variables. Second, this study is the first to systematically analyze multiple modifiable causal risk factors for AILD.
The study still has a few shortcomings. Firstly, in this study, there are IVW results whose power value failed to exceed 80%, potentially impacting the credibility of the results. Furthermore, the Causal Analysis Using Summary Effect estimates (CAUSE) method should be employed to confirm whether the detected horizontal pleiotropy is relevant or irrelevant. CAUSE can avoid more false positives induced by correlated horizontal pleiotropy than other methods [78].
Conclusion
Evidence on the causal relationship between 29 genetically predicted modifiable risk factors and the risk of PSC, PBC, and AIH is provided by this study. These findings provide fresh perspectives on the management and prevention strategies for AILD.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Trivedi PJ, Hirschfield GM. Recent advances in clinical practice: epidemiology of autoimmune liver diseases. Gut. 2021;70(10):1989–2003. 10.1136. PubMed PMID: 34266966; PubMed Central PMCID: PMCQ1. /gutjnl-2020-322362.
Horst AK, Kumashie KG, Neumann K, Diehl L, Tiegs G. Antigen presentation, autoantibody production, and therapeutic targets in autoimmune liver disease. Cell Mol Immunol. 2021;18(1). https://doi.org/10.1038/s41423-020-00568-6. PubMed PMID: 33110250; PubMed Central PMCID: PMCQ1.
Shah RA, Kowdley KV. Current and potential treatments for primary biliary cholangitis. Lancet Gastroenterol Hepatol. 2020;5(3):306–15. https://doi.org/10.1016/S2468-1253. (19)30343-7. PubMed PMID: 31806572; PubMed Central PMCID: PMCQ1.
Floreani A, Restrepo-Jiménez P, Secchi MF, De Martin S, Leung PSC, Krawitt E, et al. Etiopathogenesis of autoimmune hepatitis. J Autoimmun. 2018;95:133–43. https://doi.org/10.1016/j.jaut.2018.10.020. PubMed PMID: 30385083; PubMed Central PMCID: PMCQ1.
Lamba M, Ngu JH, Stedman CAM. Trends in incidence of Autoimmune Liver diseases and increasing incidence of Autoimmune Hepatitis. Clin Gastroenterol Hepatol. 2021;19(3). https://doi.org/10.1016/j.cgh.2020.05.061. PubMed PMID: 32526342; PubMed Central PMCID: PMCQ1.
Yokoda RT, Carey EJ. Primary biliary cholangitis and primary sclerosing Cholangitis. Am J Gastroenterol. 2019;114(10):1593–605. https://doi.org/10.14309/ajg.0000000000000268. PubMed PMID: 31169523; PubMed Central PMCID: PMCQ1.
Lleo A, Wang G-Q, Gershwin ME, Hirschfield GM. Primary biliary cholangitis. Lancet. 2020;396(10266):1915–26. https://doi.org/10.1016/S0140-6736. (20)31607-X. PubMed PMID: 33308474; PubMed Central PMCID: PMCQ1.
Barberio B, Massimi D, Cazzagon N, Zingone F, Ford AC, Savarino EV. Prevalence of primary sclerosing cholangitis in patients with inflammatory bowel disease: a systematic review and Meta-analysis. Gastroenterology. 2021;161(6):1865–77. https://doi.org/10.1053/j.gastro.2021.08.032. PubMed PMID: 34425093; PubMed Central PMCID: PMCQ1.
Vesterhus M, Karlsen TH. Emerging therapies in primary sclerosing cholangitis: pathophysiological basis and clinical opportunities. J Gastroenterol. 2020;55(6):588–614. https://doi.org/10.1007/s00535-020-01681-z. PubMed PMID: 32222826; PubMed Central PMCID: PMCQ1.
Webb GJ, Ryan RP, Marshall TP, Hirschfield GM. The epidemiology of UK Autoimmune Liver Disease varies with Geographic Latitude. Clin Gastroenterol Hepatol. 2021;19(12):2587–96. https://doi.org/10.1016/j.cgh.2021.01.029. PubMed PMID: 33493696; PubMed Central PMCID: PMCQ1.
Trivella J, John BV, Levy C. Primary biliary cholangitis: Epidemiology, prognosis, and treatment. Hepatol Commun. 2023;7(6). 10.1097/. HC9.0000000000000179. PubMed PMID: 37267215; PubMed Central PMCID: PMCQ2.
Liu S-P, Bian Z-H, Zhao Z-B, Wang J, Zhang W, Leung PSC, et al. Animal models of Autoimmune Liver diseases: a Comprehensive Review. Clin Rev Allergy Immunol. 2020;58(2):252–71. https://doi.org/10.1007/s12016-020-08778-6. PubMed PMID: 32076943; PubMed Central PMCID: PMCQ1.
Sy AM, Ferreira RD, John BV. Hepatocellular Carcinoma in primary biliary cholangitis. Clin Liver Dis. 2022;26(4):691–704. https://doi.org/10.1016/j.cld.2022.06. .011. PubMed PMID: 36270724; PubMed Central PMCID: PMCQ2.
Chang C, Tanaka A, Bowlus C, Gershwin ME. The use of biologics in the treatment of autoimmune liver disease. Expert Opin Investig Drugs. 2020;29(4):385–98. PubMed PMID: 32102572; PubMed Central PMCID: PMCQ1.
Hirschfield GM, Dyson JK, Alexander GJM, Chapman MH, Collier J, Hübscher S, et al. The British Society of Gastroenterology/UK-PBC primary biliary cholangitis treatment and management guidelines. Gut. 2018;67(9):1568–94. https://doi.org/10.1136/gutjnl-2017-315259. PubMed PMID: 29593060; PubMed Central PMCID: PMCQ1.
Lindor KD, Bowlus CL, Boyer J, Levy C, Mayo M. Primary biliary cholangitis: 2021 practice guidance update from the American Association for the study of Liver diseases. Hepatology (Baltimore MD). 2022;75(4):1012–3. https://doi.org/10.1002/hep.32117. PubMed PMID: 34431119; PubMed Central PMCID: PMCQ1.
Carey EJ. Progress in primary biliary cholangitis. N Engl J Med. 2018;378(23):2234–5. 10.1056/. NEJMe1804945. PubMed PMID: 29874531; PubMed Central PMCID: PMCQ1.
Huang L-X, Wang Z-L, Jin R, Chen H-S, Feng B. Incomplete response to ursodeoxycholic acid in primary biliary cholangitis: criteria, epidemiology, and possible mechanisms. Expert Rev Gastroenterol Hepatol. 2022;16(11–12):1065–78. PubMed PMID: 36469627; PubMed Central PMCID: PMCQ2.
Rogler G, Singh A, Kavanaugh A, Rubin DT. Extraintestinal manifestations of Inflammatory Bowel Disease: current concepts, treatment, and implications for Disease Management. Gastroenterology. 2021;161(4):1118–32. https://doi.org/10.1053/j.gastro.2021.07.042. PubMed PMID: 34358489; PubMed Central PMCID: PMCQ1.
Floreani A, De Martin S, Secchi MF, Cazzagon N. Extrahepatic autoimmunity in autoimmune liver disease. Eur J Intern Med. 2019;59:1–7. https://doi.org/10.1016/j.ejim.2018.10.014. PubMed PMID: 30360943; PubMed Central PMCID: PMCQ1.
Floreani A, Franceschet I, Cazzagon N. Primary biliary cirrhosis: overlaps with other autoimmune disorders. Semin Liver Dis. 2014;34(3):352 – 60. doi: 10.1055/s-0034-1383734. PubMed PMID: 25057958; PubMed Central PMCID: PMCQ2.
Muratori P, Fabbri A, Lalanne C, Lenzi M, Muratori L. Autoimmune liver disease and concomitant extrahepatic autoimmune disease. Eur J Gastroenterol Hepatol. 2015;27(10):1175–9. 10.1097/MEG.0000000000000424. PubMed PMID: 26148248; PubMed Central PMCID: PMCQ4.
Parés A, Guañabens N. Bone fractures in primary biliary cholangitis. J Intern Med. 2023;294(2):159–60. https://doi.org/10.1111/joim.13644. PubMed PMID: 37165698; PubMed Central PMCID: PMCQ1.
Schönau J, Wester A, Schattenberg JM, Hagström H. Risk of fractures and postfracture mortality in 3980 people with primary biliary cholangitis: a population-based cohort study. J Intern Med. 2023;294(2):164–77. https://doi.org/10.1111/joim.13624. PubMed PMID: 36823685; PubMed Central PMCID: PMCQ1.
Danford CJ, Trivedi HD, Papamichael K, Tapper EB, Bonder A. Osteoporosis in primary biliary cholangitis. World J Gastroenterol. 2018;24(31):3513–20. https://doi.org/10.3748/wjg.v24.i31.3513. PubMed PMID: 30131657; PubMed Central PMCID: PMCQ2.
Seki A, Ikeda F, Miyatake H, Takaguchi K, Hayashi S, Osawa T, et al. Risk of secondary osteoporosis due to lobular cholestasis in non-cirrhotic primary biliary cholangitis. J Gastroenterol Hepatol. 2017;32(9):1611–6. https://doi.org/10.1111/jgh.13746. PubMed PMID: 28114749; PubMed Central PMCID: PMCQ2.
Parés A, Guañabens N. Primary biliary cholangitis and bone disease. Best Pract Res Clin Gastroenterol. 2018;34–35:63–70. https://doi.org/10.1016/j.bpg.2018.06. .005. PubMed PMID: 30343712; PubMed Central PMCID: PMCQ3.
Smyk DS, Orfanidou T, Invernizzi P, Bogdanos DP, Lenzi M. Vitamin D in autoimmune liver disease. Clin Res Hepatol Gastroenterol. 2013;37(5):535–45. https://doi.org/10.1016/j.clinre.2013.05.016. PubMed PMID: 23845396; PubMed Central PMCID: PMCQ3.
Vogel A, Strassburg CP, Manns MP. Genetic association of vitamin D receptor polymorphisms with primary biliary cirrhosis and autoimmune hepatitis. Hepatology (Baltimore MD). 2002;35(1):126–31. PubMed PMID: 11786968; PubMed Central PMCID: PMCQ1.
Wang Z, Peng C, Wang P, Sui J, Wang Y, Sun G, et al. Serum vitamin D level is related to disease progression in primary biliary cholangitis. Scand J Gastroenterol. 2020;55(11):1333–40. PubMed PMID: 33021858; PubMed Central PMCID: PMCQ4.
Xu H, Wu Z, Feng F, Li Y, Zhang S. Low vitamin D concentrations and BMI are causal factors for primary biliary cholangitis: a mendelian randomization study. Front Immunol. 2022;13:1055953. https://doi.org/10.3389/fimmu.2022.1055953. PubMed PMID: 36605198; PubMed Central PMCID: PMCQ1.
Ebadi M, Ip S, Lytvyak E, Asghari S, Rider E, Mason A, et al. Vitamin D is Associated with Clinical outcomes in patients with primary biliary cholangitis. Nutrients. 2022;14(4). https://doi.org/10.3390/nu14040878. PubMed PMID: 35215528; PubMed Central PMCID: PMCQ1.
Kempinska-Podhorodecka A, Milkiewicz M, Wasik U, Ligocka J, Zawadzki M, Krawczyk M, et al. Decreased expression of vitamin D receptor affects an Immune response in primary biliary cholangitis via the VDR-miRNA155-SOCS1 pathway. Int J Mol Sci. 2017;18(2). https://doi.org/10.3390/ijms18020289. PubMed PMID: 28146070; PubMed Central PMCID: PMCQ1.
Kempinska-Podhorodecka A, Adamowicz M, Chmielarz M, Janik MK, Milkiewicz P, Milkiewicz M. Vitamin-D receptor-gene polymorphisms affect quality of life in patients with Autoimmune Liver diseases. Nutrients. 2020;12(8). https://doi.org/10.3390/nu12082244. PubMed PMID: 32727130; PubMed Central PMCID: PMCQ1.
Gong L, Wei F, Gonzalez FJ, Li G. Hepatic fibrosis: targeting peroxisome proliferator-activated receptor alpha from mechanism to medicines. Hepatology (Baltimore MD). 2023;78(5):1625–53. https://doi.org/10.1097/HEP.0000000000000182. PubMed PMID: 36626642; PubMed Central PMCID: PMCQ1.
Kempińska-Podhorodecka A, Abramczyk J, Cielica E, Huła B, Maciejowska H, Banales J, et al. Effect of low testosterone levels on the expression of Proliferator-activated receptor alpha in female patients with primary biliary cholangitis. Cells. 2023;12(18). https://doi.org/10.3390/cells12182273. PubMed PMID: 37759496; PubMed Central PMCID: PMCQ2.
Wu J, Fan X, Song Y. The causal effect of bioavailable testosterone on primary biliary cholangitis in female patients: a bidirectional mendelian randomization analysis. Dig Liver Dis. 2023;55(8):1091–7. https://doi.org/10.1016/j.dld.2023.02.020. PubMed PMID: 36922303; PubMed Central PMCID: PMCQ2.
Schwinge D, Schramm C. Sex-related factors in autoimmune liver diseases. Semin Immunopathol. 2019;41(2):165–75. https://doi.org/10.1007/s00281-018-0715-8. PubMed PMID: 30276446; PubMed Central PMCID: PMCQ1.
Sekula P, Del Greco MF, Pattaro C, Köttgen A. Mendelian randomization as an Approach to assess causality using Observational Data. J Am Soc Nephrol. 2016;27(11):3253–65. PubMed PMID: 27486138; PubMed Central PMCID: PMCQ1.
Boef AGC, Dekkers OM, le Cessie S. Mendelian randomization studies: a review of the approaches used and the quality of reporting. Int J Epidemiol. 2015;44(2):496–511. https://doi.org/10.1093/ije/dyv071. PubMed PMID: 25953784; PubMed Central PMCID: PMCQ1.
Birney E. Mendelian randomization. Cold Spring Harb Perspect Med. 2022;12(4). https://doi.org/10.1101/cshperspect.a041302. PubMed PMID: 34872952; PubMed Central PMCID: PMCQ2.
Bowden J, Holmes MV. Meta-analysis and mendelian randomization: a review. Res Synth Methods. 2019;10(4):486–96. https://doi.org/10.1002/jrsm.1346. PubMed PMID: 30861319; PubMed Central PMCID: PMCQ1.
Sanderson E. Multivariable mendelian randomization and mediation. Cold Spring Harb Perspect Med. 2021;11(2). https://doi.org/10.1101/cshperspect.a038984. PubMed PMID: 32341063; PubMed Central PMCID: PMCQ2.
Bulik-Sullivan B, Finucane HK, Anttila V, Gusev A, Day FR, Loh P-R, et al. An atlas of genetic correlations across human diseases and traits. Nat Genet. 2015;47(11):1236–41. https://doi.org/10.1038/ng.3406. PubMed PMID: 26414676; PubMed Central PMCID: PMCQ1.
Bulik-Sullivan BK, Loh P-R, Finucane HK, Ripke S, Yang J, Patterson N, et al. LD score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat Genet. 2015;47(3):291–5. https://doi.org/10.1038/ng.3211. PubMed PMID: 25642630; PubMed Central PMCID: PMCQ1.
Wang R, Tang R, Li B, Ma X, Schnabl B, Tilg H. Gut microbiome, liver immunology, and liver diseases. Cell Mol Immunol. 2021;18(1). https://doi.org/10.1038/s41423-020-00592-6. PubMed PMID: 33318628; PubMed Central PMCID: PMCQ1.
Zein CO. Clearing the smoke in chronic liver diseases. Hepatology (Baltimore MD). 2010;51(5):1487–90. 10.1002. /hep.23694. PubMed PMID: 20432251; PubMed Central PMCID: PMCQ1.
Gershwin ME, Selmi C, Worman HJ, Gold EB, Watnik M, Utts J, et al. Risk factors and comorbidities in primary biliary cirrhosis: a controlled interview-based study of 1032 patients. Hepatology (Baltimore MD). 2005;42(5):1194–202. PubMed PMID: 16250040; PubMed Central PMCID: PMCQ1.
Parikh-Patel A, Gold EB, Worman H, Krivy KE, Gershwin ME. Risk factors for primary biliary cirrhosis in a cohort of patients from the United States. Hepatology (Baltimore MD). 2001;33(1):16–21. PubMed PMID: 11124815; PubMed Central PMCID: PMCQ1.
Prince MI, Ducker SJ, James OFW. Case-control studies of risk factors for primary biliary cirrhosis in two United Kingdom populations. Gut. 2010;59(4):508–12. doi: 10.1136/gut.2009.184218. PubMed PMID: 20332522; PubMed Central PMCID: PMCQ1.
Howel D, Fischbacher CM, Bhopal RS, Gray J, Metcalf JV, James OF. An exploratory population-based case-control study of primary biliary cirrhosis. Hepatology (Baltimore MD). 2000;31(5):1055–60. PubMed PMID: 10796879; PubMed Central PMCID: PMCQ1.
Corpechot C, Gaouar F, Chrétien Y, Johanet C, Chazouillères O, Poupon R. Smoking as an independent risk factor of liver fibrosis in primary biliary cirrhosis. J Hepatol. 2012;56(1):218–24. https://doi.org/10.1016/j.jhep.2011.03.031. PubMed PMID: 21703179; PubMed Central PMCID: PMCQ1.
Tsochatzis E, Papatheodoridis GV, Manolakopoulos S, Tiniakos DG, Manesis EK, Archimandritis AJ. Smoking is associated with steatosis and severe fibrosis in chronic hepatitis C but not B. Scand J Gastroenterol. 2009;44(6):752–9. PubMed PMID: 19296398; PubMed Central PMCID: PMCQ4.
Zein CO, Beatty K, Post AB, Logan L, Debanne S, McCullough AJ. Smoking and increased severity of hepatic fibrosis in primary biliary cirrhosis: a cross validated retrospective assessment. Hepatology (Baltimore MD). 2006;44(6):1564–71. PubMed PMID: 17133468; PubMed Central PMCID: PMCQ1.
Whetzel CA, Corwin EJ, Klein LC. Disruption in Th1/Th2 immune response in young adult smokers. Addict Behav. 2007;32(1):1–8. PubMed PMID: 16644136; PubMed Central PMCID: PMCQ1.
Ala A, Stanca CM, Bu-Ghanim M, Ahmado I, Branch AD, Schiano TD, et al. Increased prevalence of primary biliary cirrhosis near Superfund toxic waste sites. Hepatology (Baltimore MD). 2006;43(3):525–31. PubMed PMID: 16496326; PubMed Central PMCID: PMCQ1.
Bose T. Bitter correlationship between autoimmune hepatitis and smoking. Med Hypotheses. 2015;84(2):118–21. https://doi.org/10.1016/j.mehy.2014.12. .006. PubMed PMID: 25543266; PubMed Central PMCID: PMCQ2.
Boonstra K, de Vries EMG, van Geloven N, van Erpecum KJ, Spanier M, Poen AC, et al. Risk factors for primary sclerosing cholangitis. Liver International: Official J Int Association Study Liver. 2016;36(1):84–91. PubMed PMID: 26077553; PubMed Central PMCID: PMCQ1.
Lammert C, Chalasani SN, Green K, Atkinson E, McCauley B, Lazaridis KN. Patients with Autoimmune Hepatitis Report Lower Lifetime Coffee Consumption. Dig Dis Sci. 2022;67(6):2594–9. https://doi.org/10.1007/s10620-021-06989-1. PubMed PMID: 33939140; PubMed Central PMCID: PMCQ3.
Huang W, Jin T, Zheng W, Yin Q, Yan Q, Pan H, et al. Identifying the genetic association between systemic lupus erythematosus and the risk of autoimmune liver diseases. J Autoimmun. 2024;145:103188. https://doi.org/10.1016/j.jaut.2024.103188. PubMed PMID: 38458076; PubMed Central PMCID: PMCQ1.
González-Regueiro JA, Cruz-Contreras M, Merayo-Chalico J, Barrera-Vargas A, Ruiz-Margáin A, Campos-Murguía A, et al. Hepatic manifestations in systemic lupus erythematosus. Lupus. 2020;29(8):813–24. doi: 10.1177/0961203320923398. PubMed PMID: 32390496; PubMed Central PMCID: PMCQ3.
Heijke R, Ahmad A, Frodlund M, Wirestam L, Dahlström Ö, Dahle C, et al. Usefulness of Clinical and Laboratory Criteria for diagnosing Autoimmune Liver Disease among patients with systemic Lupus Erythematosus: an observational study. J Clin Med. 2021;10(17). https://doi.org/10.3390/jcm10173820. PubMed PMID: 34501268; PubMed Central PMCID: PMCQ2.
Takahashi A, Abe K, Saito R, Iwadate H, Okai K, Katsushima F, et al. Liver dysfunction in patients with systemic lupus erythematosus. Intern Med. 2013;52(13):1461–5. PubMed PMID: 23812192; PubMed Central PMCID: PMCQ4.
Radovanović-Dinić B, Tešić-Rajković S, Zivkovic V, Grgov S. Clinical connection between rheumatoid arthritis and liver damage. Rheumatol Int. 2018;38(5):715 – 24. doi: 10.1007/s00296-018-4021-5. PubMed PMID: 29627896; PubMed Central PMCID: PMCQ2.
Fan J, Jiang T, He D. Genetic link between rheumatoid arthritis and autoimmune liver diseases: a two-sample mendelian randomization study. Semin Arthritis Rheum. 2023;58:152142. https://doi.org/10.1016/j.semarthrit.2022.152142. PubMed PMID: 36446255; PubMed Central PMCID: PMCQ2.
Marasini B, Gagetta M, Rossi V, Ferrari P. Rheumatic disorders and primary biliary cirrhosis: an appraisal of 170 Italian patients. Ann Rheum Dis. 2001;60(11):1046–9. PubMed PMID: 11602476; PubMed Central PMCID: PMCQ1.
Wang L, Zhang F-C, Chen H, Zhang X, Xu D, Li Y-Z, et al. Connective tissue diseases in primary biliary cirrhosis: a population-based cohort study. World J Gastroenterol. 2013;19(31):5131–7. https://doi.org/10.3748/wjg.v19.i31.5131. PubMed PMID: 23964148; PubMed Central PMCID: PMCQ2.
Floreani A, Franceschet I, Cazzagon N, Spinazzè A, Buja A, Furlan P, et al. Extrahepatic autoimmune conditions associated with primary biliary cirrhosis. Clin Rev Allergy Immunol. 2015;48(2–3):192–7. https://doi.org/10.1007/s12016-014-8427-x. PubMed PMID: 24809534; PubMed Central PMCID: PMCQ1.
Smyk DS, Bogdanos DP, Mytilinaiou MG, Burroughs AK, Rigopoulou EI. Rheumatoid arthritis and primary biliary cirrhosis: cause, consequence, or coincidence? Arthritis. 2012;2012:391567. https://doi.org/10.1155/2012/391567. PubMed PMID: 23150824.
Wong G-W, Yeong T, Lawrence D, Yeoman AD, Verma S, Heneghan MA. Concurrent extrahepatic autoimmunity in autoimmune hepatitis: implications for diagnosis, clinical course and long-term outcomes. Liver International: Official J Int Association Study Liver. 2017;37(3):449–57. https://doi.org/10.1111/liv.13236. PubMed PMID: 27541063; PubMed Central PMCID: PMCQ1.
Chen L, Zhao Y, Li M, Lv G. Proteome-wide mendelian randomization highlights AIF1 and HLA-DQA2 as targets for primary sclerosing cholangitis. Hepatol Int. 2023. https://doi.org/10.1007/s12072-023-10608-8. PubMed PMID: 37950809; PubMed Central PMCID: PMCQ1.
Harbord M, Annese V, Vavricka SR, Allez M, Barreiro-de Acosta M, Boberg KM, et al. The first European evidence-based Consensus on extra-intestinal manifestations in inflammatory bowel disease. J Crohns Colitis. 2016;10(3):239–54. https://doi.org/10.1093/ecco-jcc/jjv213. PubMed PMID: 26614685; PubMed Central PMCID: PMCQ1.
van Munster KN, Bergquist A, Ponsioen CY. Inflammatory bowel disease and primary sclerosing cholangitis: one disease or two? J Hepatol. 2024;80(1):155–68. https://doi.org/10.1016/j.jhep.2023.09.031. PubMed PMID: 37940453; PubMed Central PMCID: PMCQ1.
Kim YS, Hurley EH, Park Y, Ko S. Primary sclerosing cholangitis (PSC) and inflammatory bowel disease (IBD): a condition exemplifying the crosstalk of the gut-liver axis. Exp Mol Med. 2023;55(7):1380–7. https://doi.org/10.1038/s12276-023-01042-9. PubMed PMID: 37464092; PubMed Central PMCID: PMCQ1.
Zhang H, Chen L, Fan Z, Lv G. The causal effects of inflammatory bowel disease on primary biliary cholangitis: a bidirectional two-sample mendelian randomization study. Liver International: Official J Int Association Study Liver. 2023;43(8):1741–8. https://doi.org/10.1111/liv.15616. PubMed PMID: 37283182; PubMed Central PMCID: PMCQ1.
Zhao J, Li K, Liao X, Zhao Q. Causal associations between inflammatory bowel disease and primary biliary cholangitis: a two-sample bidirectional mendelian randomization study. Sci Rep. 2023;13(1):10950. https://doi.org/10.1038/s41598-023-35785-2. PubMed PMID: 37414807; PubMed Central PMCID: PMCQ2.
Efe C, Purnak T, Ozaslan E, Ozbalkan Z, Karaaslan Y, Altiparmak E, et al. Autoimmune liver disease in patients with systemic lupus erythematosus: a retrospective analysis of 147 cases. Scand J Gastroenterol. 2011;46(6):732–7. https://doi.org/10.3109/00365521.2011.558114. PubMed PMID: 21348808; PubMed Central PMCID: PMCQ4.
Morrison J, Knoblauch N, Marcus JH, Stephens M, He X. Mendelian randomization accounting for correlated and uncorrelated pleiotropic effects using genome-wide summary statistics. Nat Genet. 2020;52(7):740–7. https://doi.org/10.1038/s41588-020-0631-4. PubMed PMID: 32451458; PubMed Central PMCID: PMCQ1.
Acknowledgements
We sincerely thank the National Natural Science Foundation of China (81971997) for supporting this work.
Funding
This study was supported by the National Natural Science Foundation of China (81971997).
Author information
Authors and Affiliations
Contributions
Weize Gao and Chong Peng contributed equally to this work. Weize Gao and Chong Peng designed the study, run the program and wrote the manuscript. Zhan Wang and Yongxin Li contributed significantly to revise and manuscript preparation. Mingjun Liu guided the whole process of this work with constructive discussions.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Gao, W., Peng, C., Wang, Z. et al. Genetic association and causal relationship between multiple modifiable risk factors and autoimmune liver disease: a two-sample mendelian randomization study. J Transl Med 22, 425 (2024). https://doi.org/10.1186/s12967-024-05247-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-024-05247-y