Abstract
Background
Accumulating data indicate that sensory nerve derived neuropeptides such as substance P and calcitonin gene related-protein (CGRP) can accelerate the progression of endometriosis via their respective receptors, so can agonists to their respective receptors receptor 1 (NK1R), receptor activity modifying protein 1 (RAMP-1) and calcitonin receptor-like receptor (CRLR). Adrenergic β2 receptor (ADRB2) agonists also can facilitate lesional progression. In contrast, women with endometriosis appear to have depressed vagal activity, concordant with reduced expression of α7 nicotinic acetylcholine receptor (α7nAChR). The roles of these receptors in adenomyosis are completely unknown.
Methods
Adenomyotic tissue samples from 30 women with adenomyosis and control endometrial tissue samples from 24 women without adenomyosis were collected and subjected to immunohistochemistry analysis of RAMP1, CRLR, NK1R, ADRB2 and α7nAChR, along with their demographic and clinical information. The extent of tissue fibrosis was evaluated by Masson trichrome staining.
Results
We found that the staining levels of NK1R, CRLR, RAMP1 and ADRB2 were all significantly elevated in adenomyotic lesions as compared with control endometrium. In contrast, α7nAChR staining levels were significantly reduced. The severity of dysmenorrhea correlated positively with lesional ADRB2 staining levels.
Conclusions
Our results suggest that SP, CGRP and noradrenaline may promote, while acetylcholine may stall, the progression of adenomyosis through their respective receptors on adenomyotic lesions. Additionally, through the activation of the hypothalamic-pituitary-adrenal (HPA)-sympatho-adrenal-medullary (SAM) axes and the lesional overexpression of ADRB2, adenomyosis-associated dysmenorrhea and adenomyotic lesions may be mutually promotional, forming a viscous feed-forward cycle.
Similar content being viewed by others
Introduction
Adenomyosis is a prevalent, benign gynecological condition characterized by infiltration of endometrial tissues into the myometrium [1]. Its presenting symptoms include a soft and diffusely enlarged uterus with pelvic pain, abnormal uterine bleeding (AUB), and subfertility [2,3,4,5], but its pathogenesis and pathophysiology are poorly understood [6,7,8]. While approximately one third of adenomyotic cases are asymptomatic [9], dysmenorrhea is the most prevalent symptom besides AUB [10].
Evidence accumulated in the last few years indicates that adenomyotic lesions, just like endometriotic lesions [11,12,13], are wounds undergoing repeated tissue injury and repair (ReTIAR) owing to cyclic bleeding of ectopic endometrium [14, 15]. It is well documented that neuromediators secreted by sensory and autonomic nerves are actively involved in all phases of tissue repair [16]. For example, neutrophins such as nerve growth factor (NGF) and their receptors are implicated in tissue repair and remodeling [17]. The neuropeptide substance P (SP) released by sensory nerves on the wounding site induces vasodilatation and vascular permeability that promote plasma extravasation [18, 19] via its receptor, neurokinin receptor 1 (NK1R) [20, 21]. Sensory nerve derived calcitonin gene related-protein (CGRP) also is implicated in vasodilatation and inflammation [22]. Both SP and CGRP can modulate collagen production during skin wound healing [23]. SP is known to accelerate the normal acute and chronic wound healing processes [24,25,26], while sensory denervation impairs cutaneous wound healing [27,28,29]. Similarly, sympathetic denervation by oxidopamine also leads to impaired wound healing which was associated with a decrease of neurogenic inflammation [30, 31].
As wounds, endometriotic lesions are highly vascularized, at least initially [32], and richly innervated [33, 34]. In fact, increased nerve fiber density is a notable feature of the lesional microenvironment, especially in deep endometriosis [35,36,37,38]. Similarly, adenomyotic lesions also are highly vascularized [14, 39, 40]. However, whether adenomyotic lesions are similarly innervated appears to be controversial [41,42,43]. Adenomyotic lesions also seem to have impaired sympathetic innervation [42].
We have previously reported that sensory nerve derived SP and CGRP can accelerate the progression of endometriosis via their respective receptors [44, 45], so can β2 adrenergic receptor (ADRB2) agonists [46] and an NK1R agonist [44]. More remarkably, we recently reported that lesional expression of ADRB2 correlated positively with the severity of dysmenorrhea in women with endometriosis, suggesting a positive feed-forward loop between pain and lesional progression [47]. These results clearly demonstrate that the promotional roles of neuropeptides, such as SP and CGRP, and neurotransmitters, such as adrenaline and noradrenaline, which are derived from sensory and sympathetic nerves, respectively, in the progression of endometriosis via their respective receptors.
One important nerve system that has not been investigated at all in adenomyosis is the vegus nerves. Women with endometriosis appear to have depressed vagal activity [48]. Vagotomy, which mimics reduced vagal activity, has recently been reported to accelerate the progression of endometriosis but vegus nerve stimulation substantially stalls the lesion progression in mouse [48].
In retrospect, this is not surprising, since in the last two decades it has been recognized that there is a complex and intricate crosstalk between the nervous and immune systems through a plethora of cytokines, neurotransmitters and hormones, which serves as counter-regulatory mechanisms capable of dampening inflammation and restoration of homeostasis [49, 50]. In fact, within the framework of cholinergic anti-inflammatory pathway [51], the vegus nerves are known play a pivotal role in anti-inflammation through the activation of the α7 nicotinic acetylcholine receptor (α7nAChR) [52, 53]. Incidentally, treatment with an α7nAChR agonist has been reported to suppress the formation of endometriotic lesions [54]. However, whether α7nAChR is expressed in endometriosis or adenomyosis is unclear.
In light of these findings, it is plausible that the neuropeptides and neurotransmitters may act on ectopic endometrium through their respective receptors. In this study, we hypothesized that adenomyotic lesions have increased immunoreactivity against NK1R, ADRB2, and CGRP receptors CRLR and RAMP1 but decreased immunoreactivity against α7nAChR. Since fibrosis is a notable feature of adenomyosis and a rough proxy for lesional progression [14, 55], we also evaluated the extent of lesion fibrosis to see whether it has any relationship with these receptors.
Materials and methods
This study was approved by the Institutional Ethics Review Board of the Shanghai OB/GYN Hospital. All tissue samples were obtained after written, full and informed consent from recruited subjects.
Human samples
Adenomyotic tissue samples were obtained, after informed written consent, during hysterectomy from premenopausal patients with ultrasonically, laparoscopically and histologically diagnosed adenomyosis (n = 30), who were admitted to the Shanghai OB/GYN Hospital, Fudan University, from January, 2018 to December, 2019. For controls, we also collected, after informed consent, endometrial tissue samples through curettage from 24 women with cervical intraepithelial neoplasia (CIN-III) but free of endometriosis, adenomyosis, and uterine fibroids, who were age- and menstrual phase-matched (in frequency) with patients with adenomyosis. The selection of the controls was based solely on age and menstrual phase besides disease status. None of the recruited subjects had any malignancy or other inflammatory disease, or received any hormonal or anti-platelet treatment for at least 3 months prior to the recruitment. Information on uterine size, severity of dysmenorrhea, amount of menses was also recorded.
Immunohistochemistry (IHC) analysis
Tissue samples were fixed with 10% formalin (w/v) and paraffin embedded. Serial 4-μm sections were obtained from each block, with the first resultant slide being stained with hematoxylin and eosin to confirm pathologic diagnosis [56], and the subsequent slides for IHC analysis for the two receptors of CGRP, i.e. receptor activity modifying protein 1 (RAMP-1) (1:100, ab203282, Abcam, Cambridge, UK), calcitonin receptor-like receptor (CRLR) (1:50, ab84467, Abcam), SP receptor NK1R (1:100, NB300–119, Novus, CO, USA), noradrenaline receptor ADRB2 (1:200, ab182136, Abcam), nicotinic acetylcholine receptor α7nAChR (1:200, ab10096, Abcam).
Routine deparaffinization and rehydration procedures were performed, as reported previously [44, 56]. For antigen retrieval, the slides were heated at 98 °C in a citrate buffer (pH 6.0) for a total of 30 min and then cooled to room temperature. The slides were then incubated with the primary antibodies overnight at 4 °C. After the slides were rinsed with PBS, the horse radish peroxidase-labeled secondary antibody Detection Reagent (Sunpoly-HII; BioSun Technology Co, Ltd., Shanghai, China) was added and incubated at room temperature for 30 min. The bound antibody complexes were stained with diaminobenzidine for 3 to 5 min or until appropriate for microscopic examination and then counterstained with hematoxylin for 30 s and mounted. Images were obtained with the microscope (Olympus BX53; Olympus, Tokyo, Japan) fitted with a digital camera (Olympus DP73; Olympus). For each marker from every patient, 3 slides were used, and 5 randomly selected images at 400× magnification of each slide were taken to get a mean optional density value by Image Pro-Plus 6.0.
Mouse brain (for RAMP1, CRLR, NK1R, and ADRB2) and mouse kidney (for α7nAChR) tissue samples were used as positive controls. For negative controls, mouse brain (for RAMP1, CRLR, NK1R, and ADRB2) and human endometrial (for α7nAChR) tissue samples were incubated with rabbit or mouse serum instead of primary antibodies (Supplementary Information Fig. S1).
Masson trichrome staining
Masson trichrome staining was used for the evaluation and semi-quantification of collagen fibers in endometriotic tissue samples. Tissue sections were deparaffinized in xylene and rehydrated in a graded alcohol series, then were immersed in Bouin’s solution at 37 °C for 2 h, which was made with saturated 75 mL of picric acid, 25 mL of 10% (w/v) formalin solution and 5 mL of acetic acid. Sections were stained using the Masson’s Trichrome Staining kit (Baso, Wuhan, China) following the manufacturer’s instructions. The proportion of the areas of the collagen fiber layer stained in blue relative to the entire field of the ectopic endometrium, calculated by the Image Pro-Plus 6.0, was taken as the percentage of fibrotic content in the tissues or lesions.
Statistical analysis
The comparison of distributions of continuous variables between two groups was made using the Wilcoxon’s test. Multivariate linear regression analyses were used to determine whether age, menstrual phase, parity, presence or absence of uterine fibroids, presence of absence of deep endometriosis, and group identity (adenomyosis or control) were associated with immunostaining levels of different markers in human samples. Pearson’s correlation coefficient was used to gauge the correlation between to continuous variables, and Spearman’s rank correlation coefficient was used to evaluate the correlation between one continuous variable and one ordinal variable. The normality assumption was checked by plotting the regression residuals using Q-Q plot. P-values of < 0.05 were considered statistically significant. All computations were made with R 4.0.2 [57] (www.r-project.org).
Results
The characteristics of the recruited patients with adenomyosis and the control subjects are listed in Table 1. As shown in the table, the two groups of patients were comparable in age and menstrual phase. However, patients with adenomyosis had significantly lower parity, heavier menses and more severe dysmenorrhea.
We carried out immunohistochemistry analysis for CGRP receptors RAMP1 and CRLR, SP receptor NK1R, adrenergic receptor ADRB2, and α7nChR for adenomyotic lesions, as well as the extent of lesion fibrosis by Masson trichrome staining.
We found that RAMP-1, CRLR, NK1R, ADRB2 and α7nAChR staining was seen in both epithelial and stromal cells of adenomyotic lesions and control endometrial tissues, The staining of RAMP-1, CRLR, NK1R and α7nAChR was seen in both epithelial and stromal cells and localized in the cytomembrane. The ADRB2 immunoreactivity was seen in both epithelial and stromal cells and was localized in the cytoplasm (Fig. 1). Overall, the staining of RAMP-1, CRLR, NK1R, and ADRB2 in adenomyotic lesions was much intense than control endometrium. In contrast, the staining of α7nAChR was much weaker in adenomyotic lesions than control endometrium (Fig. 1).
Representative photomicrographs of immunohistochemistry analysis of calcitonin receptor like receptor (CRLR), receptor activity modifying protein 1 (RAMP-1), neurokinin 1 receptor (NK1R), adrenergic receptor β2 (ADRB2), and α7 nicotinic acetylcholine receptor (α7nAChR) in the epithelial and stromal components in control) and adenomyotic tissues. Magnification: × 400. Scale bar = 50 μm
We found that the staining levels of RAMP1, CRLR, NK1R, and ADRB2 were significantly elevated in both the epithelial and stromal components in adenomyotic lesions as compared with control endometrium (all p-values < 3.0 × 10− 9; Fig. 2a-h). In contrast, the staining levels of α7nAChR were significantly reduced in both compared with control endometrium as compared with the control endometrium (both p-values < 7.1 × 10− 10; Fig. 2i, j). For all markers, the staining levels in the epithelial and stromal components were highly positively correlated (all r ≥ 0.82, all p < 3.7 × 10− 14). Multiple linear regression incorporating age, menstrual phase, parity, presence or absence of uterine fibroids, presence or absence of deep endometriosis, presence or absence of ovarian endometriomas, and group identity (adenomyosis or control) confirmed that adenomyotic lesions were associated with higher staining of RAMP1, CRLR, NK1R, ADRB2 but lower staining of α7nAChR in both epithelial and stromal components (all p-values < 1.2 × 10− 12, all R2’s ≥ 0.63).
Summary of immunohistochemistry analyses by boxplots. Epithelial a and stromal b staining of RAMP1, epithelial c and stromal d staining of CRLR, epithelial e and stromal f staining of NK1R, epithelial g and stromal h staining of ADRB2, and epithelial i and stromal j staining of α7nAChR. In all figures, the dashed line represents the regression line. The comparison was made between patients with adenomyosis and controls (Wilcoxon’s rank test). Symbols for statistical significance levels: **: p < 0.01; ***: p < 0.001
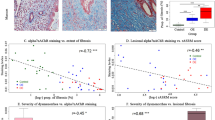
Consistent with previously reported [14], adenomyotic lesions had significantly higher extent of fibrosis than control endometrium (p = 1.4 × 10− 9; Fig. 3a). Multiple linear regression incorporating age, menstrual phase, parity, presence or absence of uterine fibroids, presence or absence of deep endometriosis, presence or absence of ovarian endometriomas, and group identity (adenomyosis or control) confirmed that adenomyotic lesions were associated with higher fibrotic content (p = 1.6 × 10− 9, R2 = 0.58). In addition, the uterine size correlated positively with the extent of fibrosis (r = 0.73, p = 1.2 × 10− 9; Fig. 3b). While no relationship between lesional staining levels and the amount of menses (all p-values > 0.21), the lesional staining levels of ADRB2 in both epithelial and stromal components correlated positively with the severity of dysmenorrhea (Spearman’s r = 0.62, p = 0.0004, and r = 0.65, p = 0.0002, respectively; Fig. 3c, d). In both epithelial and stromal components, we found that the staining levels of α7nAChR correlated negatively with that of NK1R, CRLR, RAMP1, and ADRB2 (all r’s < − 0.68, all p’s < 8.9 × 10− 8).
a Boxplot showing the summary results of the extent of fibrosis in adenomyotic lesions and control endometrium (Wilcoxon’s test). b Scatter plot showing the relationship between the extent of lesional fibrosis and uterine size (in cm3). Data from the women with adenomyosis (in maroon color) and control patients (in royal blue color) are represented in different colors. Pearson’s correlation coefficient, along with its statistical significance level, is also shown. The dashed line represents the regression line. c and d Boxplots showing, respectively, the difference in epithelial and stromal ADRB2 in women with adenomyosis complaining different severity of dysmenorrhea. For panels c and d, the Spearman’s correlation coefficient and its statistical significance level are shown. Symbols for statistical significance levels: **: p < 0.01; ***: p < 0.001
Discussion
In adenomyosis, the role of nervous systems has been traditionally investigated in the context of pain, in that the hyperinnervation within or surrounding adenomyotic lesions is often taken as evidence to support the notion of increased nociception, nociceptor activation and thus enhanced perception of pain. Indeed, it has been reported that the PGP9.5-positive nerve fiber density in the basal layer of the endometrium or myometrium is significantly increased in women with adenomyosis complaining pain, and that neurofilament (NF)-positive, but not PGP9.5-positive, nerve fibers are found in the basal layer of the endometrium and myometrium in women with adenomyosis [41]. While Mechsner and her associates also found that adenomyotic lesions are not innervated [42], a more recent study, however, found that NF-positive nerve fiber density is increased in adenomyotic lesions as compared with controls [43]. Hence whether or not sensory nerve-derived SP and CGRP play any role in lesional progression in adenomyosis remains unclear. Similarly, whether adrenergic receptors or nicotinic AChRs play any role in lesional progression is completely unknown.
Surprisingly, however, the role of receptors for neuropeptides/neurotransmitters secreted by sensory, sympathetic and vegal nerves in adenomyotic lesions has, to our best knowledge, never been investigated. This is unfortunate, since, first, the endometrial-myometrial interface (EMI), which is known to play a role in adenomyosis [58], is richly innervated [59]. In addition, neuropeptides such as SP and CGRP and their receptors have recently been shown to play a promotional role in endometriosis progression and fibrogenesis [44, 45], a feature also shared by adenomyosis [14, 55]. The promotional role of adrenergic signaling also has been implicated in endometriosis [46, 60]. Moreover, the potential of α7nAChR agonists as therapeutics has been shown [54], which provides a cue that the AChR signaling pathway or, more broadly, the vagal activity, or lack thereof, may play some roles in adenomyotic progression and fibrogenesis.
In essence, this study provides, for the first time, a survey of the expression patterns and levels of SP receptor NK1R, CGRP receptors CRLR and RAMP1, adrenergic receptor ADRB2, and acetylcholine receptor α7nAChR in adenomyotic lesions. We found that the staining levels of CRLR, RAMP1, NK1R and ADRB2 are all significantly elevated in adenomyotic lesions as compared with control endometrium. In contrast, α7nAChR staining levels were significantly reduced. One notable result is the positive correlation between the severity of dysmenorrhea and lesional ADRB2 staining levels. This suggests that, similar to endometriosis [47], there may also exist a similar feed-forward loop in adenomyosis.
As adenomyotic and endometriotic lesions are both ectopic endometrium and share the same hallmark of cyclic bleeding [12], our results suggest that, as in endometriosis, sensory nerve-derived neuropeptides such as SP and CGRP and sympathetic nerve-derived neurotransmitters such as noradrenaline may be actively involved in the promotion of adenomyosis progression through their respective receptors on adenomyotic lesions. In contrast, vagus nerve derived neurotransmitter acetylcholine might stall the progression. Additionally, through the activation of the hypothalamic-pituitary-adrenal (HPA)-sympatho-adrenal-medullary (SAM) axes and the lesional overexpression of ADRB2, adenomyosis-associated dysmenorrhea and adenomyotic lesions may be mutually promotional, forming a viscous feed-forward cycle.
Our findings of lesional overexpression of NK1R and CRLR/RAMP1 are broadly consistent with the documented roles of SP/CGRP and their receptors in wound healing and fibrogenesis. SP is known to facilitate the normal acute and chronic wound healing processes [24,25,26]. In contrast, sensory denervation impairs cutaneous wound healing through increased apoptosis and reduced proliferation [27, 28]. In addition, our findings are in agreement with the report that NK1R is expressed in endometriotic lesions, especially in peritoneal lesions [61]. SP enhances, while NK1R antagonism reduces, endometrial stromal cell viability, and treatment of endometrial cells with TNFα induces NK1R expression [61]. Increased nerve fiber density in adenomyotic myometrium [41, 43] may result in elevated SP/CGRP concentration within lesions.
Despite the fact that this study provides, to our best knowledge, the first survey of the some important neuropeptide and neurotransmitter receptors in adenomyotic lesions, our study has several limitations. The most conspicuousl one is that our study is limited by the use of histologic and immunohistochemistry analyses only and lacks molecular data. In addition, we did not evaluate the role of receptors for other neuropeptides such as vasoactive intestinal peptide (VIP) since sensory nerves also secrete neuropeptides other than SP and CGRP. Along the same line, we did not evaluate other adrenergic receptors, such as ADRB1 and ADRB3, nor did we evaluate other acetylcholine receptors such as muscarinic receptors (mAChRs) and other nicotinic AChRs. Neither did we evaluate receptors for other neurotransmitters secreted by glutamatergic, dopaminergic, serotonergic, and GABAergic neurons. These receptors may play important roles in adenomyosis progression and in causing adenomyosis-related symptomology. For example, loss of GABAergic inhibition in mice with induced adenomyosis may exacerbate pain [62]. Similarly, dopaminergic signaling may also be crucial in the prevention or hindrance of adenomyosis [63, 64], especially in view of the evidence that dopamine D2 receptor (DRD2) signaling is seemingly depressed in the development of endometriosis [65,66,67]. Future studies are needed to elucidate their involvement, if any, in adenomyosis.
Pain, AUB and infertility are three major complaints that prompt women with adenomyosis to seek medical attention. Pain and infertility themselves are known to be potent stressors, causing anxiety and depression [68, 69]. Closely associated with lower quality of life [70], AUB also can induce psychological stress, depression, and anxiety [71, 72]. In particular, adenomyosis-associated pain can be intense and debilitating and typically chronic and uncontrollable, and the psychological stress thus induced appears to contain all the ingredients for exerting a potent negative effect on women with adenomyosis [73]. As a result, it is likely to induce systemic activation of the HPA and the SAM axes, resulting in increased release of glucocorticoids and catecholamines. The catecholamines, especially adrenaline and noradrenaline, would activate the ADRB2/CREB signaling pathway in lesions, inducing angiogenesis and proliferation and leading to accelerated progression of adenomyosis as in endometriosis [46]. The accelerated progression may further exacerbate pain, effectively forming a vicious cycle. This may explain as why the lesional ADRB2 staining was associated with the severity of dysmenorrhea.
Conclusions
We found increased lesional staining levels of CRLR, RAMP1, NK1R and ADRB2 but decreased staining levels of α7nAChR in adenomyotic lesions as compared with control endometrium. In particular, the severity of dysmenorrhea correlated positively with the lesional ADRB2 staining levels. Our results suggest that sensory nerve-derived neuropeptides such as SP and CGRP and sympathetic nerve-derived neurotransmitters such as noradrenaline may promote the development of adenomyosis through their respective receptors on adenomyotic lesions. In contrast, vagus nerve derived neurotransmitter acetylcholine may stall the progression of adenomyosis. Our data also suggest that, similar to endometriosis [47], there may also exist a feed-forward loop in adenomyosis. Above all, our results suggest that receptors of neuropeptides and neurotransmitters may play roles in the development of adenomyosis and its related symptomology. However, our study also unveils another layer of complex wrinkles in adenomyosis that are in need of further investigation.
Availability of data and materials
The de-identified supporting data are available from the senior author upon written and reasonable request.
References
Vercellini P, Vigano P, Somigliana E, Daguati R, Abbiati A, Fedele L. Adenomyosis: epidemiological factors. Best Pract Res Clin Obstet Gynaecol. 2006;20:465–77.
Farquhar C, Brosens I. Medical and surgical management of adenomyosis. Best Pract Res Clin Obstet Gynaecol 2006.
Harada T, Khine YM, Kaponis A, Nikellis T, Decavalas G, Taniguchi F. The impact of Adenomyosis on Women's fertility. Obstet Gynecol Surv. 2016;71:557–68.
Vercellini P, Consonni D, Dridi D, Bracco B, Frattaruolo MP, Somigliana E. Uterine adenomyosis and in vitro fertilization outcome: a systematic review and meta-analysis. Hum Reprod. 2014;29:964–77.
Gordts S, Grimbizis G, Campo R. Symptoms and classification of uterine adenomyosis, including the place of hysteroscopy in diagnosis. Fertil Steril 2018;109:380–388 e1.
Bergeron C, Amant F, Ferenczy A. Pathology and physiopathology of adenomyosis. Best Pract Res Clin Obstet Gynaecol. 2006;20:511–21.
Vannuccini S, Tosti C, Carmona F, Huang SJ, Chapron C, Guo SW, et al. Pathogenesis of adenomyosis: an update on molecular mechanisms. Reprod BioMed Online. 2017;35:592–601.
Garcia-Solares J, Donnez J, Donnez O, Dolmans MM. Pathogenesis of uterine adenomyosis: invagination or metaplasia? Fertil Steril. 2018;109:371–9.
Benson RC, Sneeden VD. Adenomyosis: a reappraisal of symptomatology. Am J Obstet Gynecol 1958;76:1044–1057; discussion 57-61.
Li X, Liu X, Guo SW. Clinical profiles of 710 premenopausal women with adenomyosis who underwent hysterectomy. J Obstet Gynaecol Res. 2014;40:485–94.
Zhang Q, Duan J, Liu X, Guo SW. Platelets drive smooth muscle metaplasia and fibrogenesis in endometriosis through epithelial-mesenchymal transition and fibroblast-to-myofibroblast transdifferentiation. Mol Cell Endocrinol. 2016;428:1–16.
Guo SW. Fibrogenesis resulting from cyclic bleeding: the holy grail of the natural history of ectopic endometrium. Hum Reprod. 2018.
Guo SW, Ding D, Shen M, Liu X. Dating Endometriotic ovarian cysts based on the content of cyst fluid and its potential clinical implications. Reprod Sci. 2015;22:873–83.
Liu X, Shen M, Qi Q, Zhang H, Guo SW. Corroborating evidence for platelet-induced epithelial-mesenchymal transition and fibroblast-to-myofibroblast transdifferentiation in the development of adenomyosis. Hum Reprod. 2016;31:734–49.
Shen M, Liu X, Zhang H, Guo SW. Transforming growth factor beta1 signaling coincides with epithelial-mesenchymal transition and fibroblast-to-myofibroblast transdifferentiation in the development of adenomyosis in mice. Hum Reprod. 2016;31:355–69.
Laverdet B, Danigo A, Girard D, Magy L, Demiot C, Desmouliere A. Skin innervation: important roles during normal and pathological cutaneous repair. Histol Histopathol. 2015;30:875–92.
Micera A, Lambiase A, Stampachiacchiere B, Bonini S, Bonini S, Levi-Schaffer F. Nerve growth factor and tissue repair remodeling: trkA (NGFR) and p75(NTR), two receptors one fate. Cytokine Growth Factor Rev. 2007;18:245–56.
Hughes SR, Williams TJ, Brain SD. Evidence that endogenous nitric oxide modulates oedema formation induced by substance P. Eur J Pharmacol. 1990;191:481–4.
Holzer P. Neurogenic vasodilatation and plasma leakage in the skin. Gen Pharmacol. 1998;30:5–11.
Ansel JC, Brown JR, Payan DG, Brown MA. Substance P selectively activates TNF-alpha gene expression in murine mast cells. J Immunol. 1993;150:4478–85.
Columbo M, Horowitz EM, Kagey-Sobotka A, Lichtenstein LM. Substance P activates the release of histamine from human skin mast cells through a pertussis toxin-sensitive and protein kinase C-dependent mechanism. Clin Immunol Immunopathol. 1996;81:68–73.
Brain SD. Sensory neuropeptides: their role in inflammation and wound healing. Immunopharmacology. 1997;37:133–52.
Cheret J, Lebonvallet N, Buhe V, Carre JL, Misery L, Le Gall-Ianotto C. Influence of sensory neuropeptides on human cutaneous wound healing process. J Dermatol Sci. 2014;74:193–203.
Kant V, Kumar D, Kumar D, Prasad R, Gopal A, Pathak NN, et al. Topical application of substance P promotes wound healing in streptozotocin-induced diabetic rats. Cytokine. 2015;73:144–55.
Leal EC, Carvalho E, Tellechea A, Kafanas A, Tecilazich F, Kearney C, et al. Substance P promotes wound healing in diabetes by modulating inflammation and macrophage phenotype. Am J Pathol. 2015;185:1638–48.
Yang L, Di G, Qi X, Qu M, Wang Y, Duan H, et al. Substance P promotes diabetic corneal epithelial wound healing through molecular mechanisms mediated via the neurokinin-1 receptor. Diabetes. 2014;63:4262–74.
Smith PG, Liu M. Impaired cutaneous wound healing after sensory denervation in developing rats: effects on cell proliferation and apoptosis. Cell Tissue Res. 2002;307:281–91.
Engin C, Demirkan F, Ayhan S, Atabay K, Baran NK. Delayed effect of denervation on wound contraction in rat skin. Plast Reconstr Surg. 1996;98:1063–7.
Buckley G, Wong J, Metcalfe AD, Ferguson MW. Denervation affects regenerative responses in MRL/MpJ and repair in C57BL/6 ear wounds. J Anat. 2012;220:3–12.
Kim LR, Whelpdale K, Zurowski M, Pomeranz B. Sympathetic denervation impairs epidermal healing in cutaneous wounds. Wound Repair Regen. 1998;6:194–201.
Souza BR, Cardoso JF, Amadeu TP, Desmouliere A, Costa AM. Sympathetic denervation accelerates wound contraction but delays reepithelialization in rats. Wound Repair Regen. 2005;13:498–505.
Nisolle M, Casanas-Roux F, Anaf V, Mine JM, Donnez J. Morphometric study of the stromal vascularization in peritoneal endometriosis. Fertil Steril. 1993;59:681–4.
Berkley KJ, Dmitrieva N, Curtis KS, Papka RE. Innervation of ectopic endometrium in a rat model of endometriosis. Proc Natl Acad Sci U S A. 2004;101:11094–8.
Tokushige N, Markham R, Russell P, Fraser IS. Nerve fibres in peritoneal endometriosis. Hum Reprod. 2006;21:3001–7.
Wang G, Tokushige N, Markham R, Fraser IS. Rich innervation of deep infiltrating endometriosis. Hum Reprod. 2009;24:827–34.
Wang G, Tokushige N, Russell P, Dubinovsky S, Markham R, Fraser IS. Hyperinnervation in intestinal deep infiltrating endometriosis. J Minim Invasive Gynecol. 2009;16:713–9.
Anaf V, El Nakadi I, De Moor V, Chapron C, Pistofidis G, Noel JC. Increased nerve density in deep infiltrating endometriotic nodules. Gynecol Obstet Investig. 2011;71:112–7.
Anaf V, Simon P, El Nakadi I, Fayt I, Simonart T, Buxant F, et al. Hyperalgesia, nerve infiltration and nerve growth factor expression in deep adenomyotic nodules, peritoneal and ovarian endometriosis. Hum Reprod. 2002;17:1895–900.
Nie J, Liu X, Zheng Y, Geng JG, Guo SW. Increased immunoreactivity to SLIT/ROBO1 and its correlation with severity of dysmenorrhea in adenomyosis. Fertil Steril. 2011;95:1164–7.
Harmsen MJ, Wong CFC, Mijatovic V, Griffioen AW, Groenman F, Hehenkamp WJK, et al. Role of angiogenesis in adenomyosis-associated abnormal uterine bleeding and subfertility: a systematic review. Hum Reprod Update. 2019;25:647–71.
Zhang X, Lu B, Huang X, Xu H, Zhou C, Lin J. Innervation of endometrium and myometrium in women with painful adenomyosis and uterine fibroids. Fertil Steril. 2010;94:730–7.
Barcena de Arellano ML, Oldeweme J, Arnold J, Schneider A, Mechsner S. Remodeling of estrogen-dependent sympathetic nerve fibers seems to be disturbed in adenomyosis. Fertil Steril. 2013;100:801–9.
Choi YJ, Chang JA, Kim YA, Chang SH, Chun KC, Koh JW. Innervation in women with uterine myoma and adenomyosis. Obstet Gynecol Sci. 2015;58:150–6.
Liu X, Yan D, Guo SW. Sensory nerve-derived neuropeptides accelerate the development and fibrogenesis of endometriosis. Hum Reprod. 2019;34:452–68.
Yan D, Liu X, Guo SW. Neuropeptides substance P and calcitonin gene related peptide accelerate the development and Fibrogenesis of endometriosis. Sci Rep. 2019;9:2698.
Long Q, Liu X, Qi Q, Guo SW. Chronic stress accelerates the development of endometriosis in mouse through adrenergic receptor beta2. Hum Reprod. 2016;31:2506–19.
Ding D, Wang X, Chen Y, Benagiano G, Liu X, Guo S-W. Evidence in support for the progressive nature of ovarian endometriomas. J Clin Endocrinol Metab 2020;In press.
Hao M, Liu X, Rong P, Li S, Guo S-W. Reduced Vagal Tone in Women with Endometriosis and Auricular Vagus Nerve Stimulation as a Potential Therapeutic Approach. Sci Rep 2020;In press. .
Steinman L. Elaborate interactions between the immune and nervous systems. Nat Immunol. 2004;5:575–81.
Sternberg EM. Neural regulation of innate immunity: a coordinated nonspecific host response to pathogens. Nat Rev Immunol. 2006;6:318–28.
Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–9.
He X, Zhao M, Bi X, Sun L, Yu X, Zhao M, et al. Novel strategies and underlying protective mechanisms of modulation of vagal activity in cardiovascular diseases. Br J Pharmacol. 2015;172:5489–500.
Chavan SS, Pavlov VA, Tracey KJ. Mechanisms and therapeutic relevance of Neuro-immune communication. Immunity. 2017;46:927–42.
Yamada-Nomoto K, Yoshino O, Akiyama I, Ushijima A, Ono Y, Shima T, et al. Alpha-7 nicotinic acetylcholine receptor (nAChR) agonist inhibits the development of endometriosis by regulating inflammation. Am J Reprod Immunol. 2016;76:491–8.
Liu X, Ding D, Ren Y, Guo SW. Transvaginal Elastosonography as an imaging technique for diagnosing Adenomyosis. Reprod Sci. 2018;25:498–514.
Ding D, Liu X, Duan J, Guo SW. Platelets are an unindicted culprit in the development of endometriosis: clinical and experimental evidence. Hum Reprod. 2015;30:812–32.
Team RDC. R: a language and environment for statistical computing. In. Vienna: R Foundation for Statistical Computing; 2016.
Hao M, Liu X, Guo SW. Adenomyosis Resulting from Mechanically or Thermally Induced Endometrial-Myometrial Interface Disruption in Mouse and Its Possible Prevention. . Reprod BioMed Online 2020;https://doi.org/10.1016/j.rbmo.2020.07.023, published online: July 28, 2020. .
Krantz KE. Innervation of the human uterus. Ann N Y Acad Sci. 1959;75:770–84.
Guo SW, Zhang Q, Liu X. Social psychogenic stress promotes the development of endometriosis in mouse. Reprod BioMed Online. 2017;34:225–39.
McKinnon BD, Evers J, Bersinger NA, Mueller MD. Induction of the neurokinin 1 receptor by TNFalpha in endometriotic tissue provides the potential for neurogenic control over endometriotic lesion growth. J Clin Endocrinol Metab. 2013;98:2469–77.
Chen Y, Zhu B, Zhang H, Ding D, Liu X, Guo SW. Possible loss of GABAergic inhibition in mice with induced Adenomyosis and treatment with Epigallocatechin-3-Gallate attenuates the loss with improved Hyperalgesia. Reprod Sci. 2014;21:869–82.
Singtripop T, Mori T, Park MK, Sakamoto S, Kawashima S. Development of uterine adenomyosis after treatment with dopamine antagonists in mice. Life Sci. 1991;49:201–6.
Kelly MA, Rubinstein M, Asa SL, Zhang G, Saez C, Bunzow JR, et al. Pituitary lactotroph hyperplasia and chronic hyperprolactinemia in dopamine D2 receptor-deficient mice. Neuron. 1997;19:103–13.
Novella-Maestre E, Carda C, Ruiz-Sauri A, Garcia-Velasco JA, Simon C, Pellicer A. Identification and quantification of dopamine receptor 2 in human eutopic and ectopic endometrium: a novel molecular target for endometriosis therapy. Biol Reprod. 2010;83:866–73.
Richards EG, Zheng Y, Shenoy CC, Ainsworth AJ, Delaney AA, Jones TL, et al. KLF11 is an epigenetic mediator of DRD2/dopaminergic signaling in endometriosis. Reprod Sci. 2017;24:1129–38.
Yin B, Jiang H, Liu X, Guo SW. Enriched environment decelerates the development of endometriosis in mouse. Reprod Sci. 2020;27:1423–35.
Siedentopf F, Tariverdian N, Rucke M, Kentenich H, Arck PC. Immune status, psychosocial distress and reduced quality of life in infertile patients with endometriosis. Am J Reprod Immunol. 2008;60:449–61.
Tariverdian N, Rucke M, Szekeres-Bartho J, Blois SM, Karpf EF, Sedlmayr P, et al. Neuroendocrine circuitry and endometriosis: progesterone derivative dampens corticotropin-releasing hormone-induced inflammation by peritoneal cells in vitro. J Mol Med (Berl). 2010;88:267–78.
Karlsson TS, Marions LB, Edlund MG. Heavy menstrual bleeding significantly affects quality of life. Acta Obstet Gynecol Scand. 2014;93:52–7.
Shapley M, Jordan K, Croft PR. Increased vaginal bleeding and psychological distress: a longitudinal study of their relationship in the community. BJOG. 2003;110:548–54.
Strine TW, Chapman DP, Ahluwalia IB. Menstrual-related problems and psychological distress among women in the United States. J Women's Health (Larchmt). 2005;14:316–23.
Karatsoreos IN, McEwen BS. Annual research review: the neurobiology and physiology of resilience and adaptation across the life course. J Child Psychol Psychiatry. 2013;54:337–47.
Acknowledgements
The authors would like to thank Dr. Dingmin Yan for her technical assistance.
Funding
This research was supported in part by grants 81771553 (SWG), 81671436 (XSL) and 81871144 (XSL) from the National Natural Science Foundation of China, grant 2020S001 (XX) from the Bureau of Science and Technology, Zhenhai District, Ningbo, Zhejiang Province, grant 2017ZZ01016 from the Science and Technology Commission of Shanghai Municipality, and grant SHDC2020CR2062B from Shanghai Shenkang Center for Hospital Development.
Author information
Authors and Affiliations
Contributions
S.W.G. conceived and designed the study, performed data analysis and data interpretation, and drafted the manuscript. X.X. and X.C. performed all the experiments and carried out initial data analysis. X.L. was involved in patient recruitment and the data interpretation and discussion. All participated in the writing and approved the final version of the manuscript. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Institutional Ethics Review Board of the Shanghai OB/GYN Hospital, Fudan University. All tissue samples were obtained after written, full and informed consent from recruited subjects.
Consent for publication
All authors have approved the final version of this manuscript and consent for its publication.
Competing interests
All authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Xu, X., Cai, X., Liu, X. et al. Possible involvement of neuropeptide and neurotransmitter receptors in Adenomyosis. Reprod Biol Endocrinol 19, 25 (2021). https://doi.org/10.1186/s12958-021-00711-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12958-021-00711-6