Abstract
Background
There has been an interest in the relationship between ABO blood groups and infertility. Many studies have investigated the association of ABO blood groups with diminished ovarian reserve (DOR), ovarian hyperstimulation syndrome (OHSS), and outcomes of assisted reproductive technology (ART), with controversial results.
Methods
A systematic review and meta-analysis was conducted to evaluating the association of ABO blood groups with DOR, OHSS, and outcomes of ART.
Results
Thirteen studies performed between 2010 and 2018 were included in this meta-analysis. DOR, OHSS, live birth rate (LBR), clinical pregnancy rate (CPR), miscarriage rate (MR) were reported in 9, 2, 4, 3, 2 studies, respectively. The combined results showed similar risk of DOR among individuals with blood group A (RR, 0.98; 95% confidence interval [CI], 0.85, 1.13), B (RR, 0.96; 95% CI, 0.76, 1.20), AB (RR, 1.00; 95% CI, 0.76, 1.30), and non-O (RR, 0.94; 95% CI, 0.79, 1.11) as compared to those with blood group O. Meta-analysis showed that the incidences of OHSS were similar in women with blood group A (RR, 1.05; 95% CI, 0.66, 1.66), B (RR, 1.04; 95% CI, 0.46, 2.35), AB (RR, 0.51; 95% CI, 0.10, 2.56), non-O (RR, 1.02; 95% CI, 0.65, 1.57) with blood group O. As to the clinical outcomes, meta-analysis showed no difference in LBR among individuals with blood group A (RR, 1.27; 95% CI, 0.74, 2.17), B (RR, 1.47; 95% CI, 0.95, 2.29), AB (RR, 1.48; 95% CI, 0.76, 2.90), non-O (RR, 1.28; 95% CI, 0.83, 1.98) when compared to those with blood group O. Similarly, the results also found that there were no difference in CPR and MR between women with blood A (CPR: RR, 1.12), B (CPR: RR, 1.08), AB (CPR: RR, 1.05), non-O (CPR: RR, 1.05; MR: RR, 0.94) and blood group O.
Conclusions
ABO blood groups may not be associated with DOR, OHSS, LBR, CPR, and MR of ART. Infertility and ART outcomes are influenced by multiple factors. Blood groups should not be taken into account excessively during diagnosis and treatment of infertile women.
Similar content being viewed by others
Introduction
The ABO blood group system is a representation of the human blood group antigens expressed on the surface of red blood cells. The ABO blood group antigen plays an important role in immunology and organ transplantation [1]. Recently, studies have reported that ABO blood group was associated with the some gynecological diseases, such as endometriosis [2] and ovarian cancer [3].
Early in the 1960s, there was study that suggested that ABO blood group incompatibility might be an immune factor of infertility [4]. Many studies have reported a high risk of thrombosis in people with non-O blood group [5] [6]. Fifty years have passed, and some studies have explored the relationship between the ABO blood group and female infertility, including DOR, OHSS, RSA, etc. But unfortunately, the conclusions were inconsistent.
One study has observed the relationship between blood group and IVF-ET pregnancy outcomes and found that blood group B is associated with increased live birth rate [7]. However, subsequent studies have confirmed that ABO blood group is not related to the IVF pregnancy outcome [1, 8]. Some studies have also reported the relationship between the ABO blood group and ovarian reserve, and showed that blood group O is more likely to have diminished ovarian reserve (DOR) [9, 10]. Whereas some studies found that women with antigen B are more likely to have DOR [11], and subsequent studies found that ABO blood group was not related to ovarian reserve or ovarian response [12,13,14,15,16,17]. Research reports on the relationship between ABO blood group and ovarian hyperstimulation syndrome (OHSS) were also inconsistent [18, 19]. Binder found that blood group A is related to the occurrence of early-onset OHSS in IVF, but other studies did not showed a relationship between ABO blood group and early or late OHSS [20].
Although so many clinical trials have studied the relationship between ABO blood group and ovarian reserve, OHSS, and IVF outcomes, there is no systematic review and meta-analysis on this issue.
This study aimed to evaluate the association of ABO blood group and ovarian reserve, OHSS, and pregnancy outcomes of ART-treated women through a systematic review and meta-analysis of existing literature.
Methods
Search strategy
A systematic search of all published studies was performed using the PubMed, EMBASE, and Google Scholar from 1966 to April 2020. The key words used for the search were as follows: a term including blood group (ABO blood group, ABO blood type), a term included the infertility (diminished ovarian reserve, DOR, ovarian hyperstimulation syndrome, OHSS, assisted reproductive technology, ART, clinical pregnancy, live birth, miscarriage). These subsets were combined with “AND” to produce literatures related to our research subject. The cohort, retrospective, or prospective studies published in English was included in our study. Two authors independently reviewed the included literature. If there was a disagreement, the third author resolved it.
Study selection and data extraction
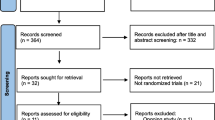
After reviewing the retrieved titles and abstracts, irrelevant literatures were excluded. Then the full text of studies that may meet the criteria were reviewed and checked for eligibility. Articles that satisfying the above inclusion criteria were selected. Figure 1 presents the results of this search. The quality of the included studies was assessed via the Newcastle-Ottawa Quality Assessment Scale. Data was extracted by two authors independently using pre-defined criteria (Number of cases with or without observational outcomes were clearly listed in the article, and the women were all underdoing ART treatment). If any discrepancies are found, the opinion of the third author will be sought. Data extraction includes research features and results data.
Statistical analysis
The present study used Review Manager version 5.3 for meta-analysis, and followed PRISMA guidelines whenever possible. Categorical variables were calculated using the Mantel-Haenszel statistical method and expressed as risk ratio (RR) values; Forest figures were used to graphically assess the heterogeneity of exposure effects, and I2 was used to statistically assess the heterogeneity of the entire study. A fixed or random-effect model was used to calculate the overall RR and its 95% confidence interval (CI). Because of the low power of the heterogeneity of the χ2 test when the sample size is small or the number of studies included is small, a P value of 0.1 instead of 0.05 was used to determine statistical significance.
Results
Studies selection and characteristics
The literature retrieval strategy described as above obtained a total of 454 articles after restricting language and research objects. By reviewing the title and abstract, 411 articles were excluded because they were not relevant. Of the remaining 43 articles, 30 were excluded for different reasons: 20 reviews, 8 meta-analysis, and 2 with incomplete data. Finally, 13 articles were included in the present study (Fig. 2).
The 13 eligible studies were published from 2010 to 2018, including 8 retrospective studies, 2 prospective studies, 2 cross-section studies, and one study without study type reported. The sample size ranged from 305 to 35,479. Of 13 studies, 9, 2, 4, 3, 2 studies reported the association of ABO blood group with DOR, OHSS, LBR, CPR, and MR, respectively (Table 1).
Meta-analysis
Our meta-analysis included 9 studies to assess the relationship between ABO blood group and DOR in infertile women undergoing ART treatment. The results showed the incidence of DOR in women with blood group O was no significantly different compared with those with blood group A (RR, 0.98; 95%CI 0.85, 1.13, P=0.80), blood group B (RR, 0.96; 95%CI 0.76, 1.20, P=0.71), blood group AB (RR, 1.00; 95%CI 0.76, 1.30, P=0.98), and non-O blood group (RR, 0.94; 95%CI 0.79, 1.11, P=0.43). The I2s, which were used to describe the heterogeneity of the included studies, were all > 75%. The results demonstrated highly heterogeneous between studies and the random effect model was used (Fig. 1).
Similarly, two studies were included to exam the relationship between ABO blood group and OHSS. The result of the meta-analysis showed similar incidence of OHSS in women with blood group A/ B/ AB/ non-O and women with blood group O. The I2s were all 0%, indicating good heterogeneity. The fixed-effect models were used and the combined RRs were (RR, 1.05; 95%CI 0.66, 1.66, P=0.85), (RR, 1.04; 95%CI 0.46, 2.35, P=0.92), (RR, 0.51; 95%CI 0.10, 2.56, P=0.42), (RR, 1.02; 95%CI 0.65, 1.57, P=0.95), respectively (Fig. 3).
With regard to the pregnancy outcomes, the LBR of women with blood group A/B/AB/non-O were not different from women with blood group O. There were significant homogeneity in the studies because I2s were all > 75%. The random effect models were used and the combined RRs were 1.27 (95%CI 0.74, 2.17, P=0.38), 1.47 (95%CI 0.95, 2.29, P=0.09), 1.48 (95%CI 0.76, 2.90, P=0.25), 1.28 (95%CI 0.83, 1.98, P=0.27), respectively (Fig. 4). Compared with women with blood group O, women with blood group A/ B/ AB/ non-O had similar CPR. The combined RRs were 1.12 (95%CI 0.90, 1.38, P=0.31), 1.08 (95%CI 0.89, 1.30, P=0.43), 1.05 (95%CI 0.90, 1.24, P=0.52), 1.05 (95%CI 0.96, 1.15, P=0.27), respectively (Sup Fig. 1). At last, two studies compared the MR between women with blood group A and blood group non-O. There was good homogeneity (I2=0%, P=0.33), and the combined RR was 0.94 (95%CI 0.76, 1.18, P=0.62) with fix effect model (Sup Fig. 2).
All of the Meta results were summarized in Table 2.
The studies included in this meta-analysis scored medium to high basing on the Newcastle-Ottawa Scale, NOS (not shown). The meta-analysis funnel chart assessed the publication biases of included studies examining the association between ABO blood group and DOR, OHSS, pregnancy outcomes after ART. Due to their imperfect symmetrical shapes, these studies showed possible publication bias. (Sup Fig. 1.1–5.1).
Discussion
This study was the first meta-analysis of the association between ABO blood group and DOR, OHSS, and IVF pregnancy outcomes. The results suggested that there is no significant difference in the incidence of DOR, OHSS, LBR, CPR, and MR between blood group A/B/AB/non-O and blood group O.
The ABO blood group gene is located on chromosome 9q34 and has three main allele forms: the A allele, the B allele, and the O allele [22]. The A/B allele encodes a glycosyltransferase (A/B transferase), which catalyzes the transfer of nucleotide sugars to H antigens, thereby forming A/B blood group antigens [23]. The O allele has a single base deletion in the coding region near the N-terminus of the protein, the product is a protein with no enzyme activity, and the H antigen remains unchanged on the red blood cell [24].
Studies have found that the blood group was related to ovarian reserve. The possible explanations were speculated as following: (1) FSH and LH receptors are glycosylated proteins, which are essential for follicular development and maturation. The biological activities of FSH and LH are likely to be altered by the sugar transferase encoded by the O allele [23]. Besides, glycotransferase could affect the half-life and biological activity of LH [25]. (2) Some ovarian function related genes located near the ABO locus, such as nuclear receptor 5A1 (NR5A1) and transforming growth factor beta-receptor (TGFBR1) genes [26]. Therefore, these genes and ABO-group genes may recombine and be inherited together with ABO. (3) Haplotype DNA mutations can change the stability of folded proteins, thereby making certain allele combinations more commonly inherited together [27]. (4) In the last, other genetic factors such as FSH receptor polymorphism [28] and the fragile X mental retardation 1 gene (FMR1) rank carrier status [29] are associated with elevated FSH levels. Recent genome-wide association studies have identified about 20 loci related to the women menopause [30].
However, ABO antigens reflect ancient polymorphisms shared by many primates [31]. If there is a mutation or polymorphism of an unknown gene that causes DOR and is related to the ABO locus, it is likely to be younger than the ABO locus. Therefore, if there is such a mutation, although it may be scattered in some subgroups, it will not be scattered on all O antigen carriers. Therefore, any correlation between ABO blood group and ovarian reserve can be ignored.
Correspondingly, a good ovarian reserve is likely to have a high response to hyper- ovulation, even develop to OHSS. In addition, previous studies have suggested that women with blood group A are more likely to have ovulation disorders, and ovulation disorders are considered to be a high-risk factor for OHSS [18]. Women of blood group A are more likely to develop early-onset OHSS, and the incidence of early-onset OHSS of blood group O is lower. This finding may be due to the 25% lower plasma concentrations of Von Willebrand factor and coagulation factor VIII in individuals with blood group O compared with the blood group A [32]. However, the increase of coagulation factors in plasma is one of the high-risk factors for the OHSS, but it is not an absolute cause. Endothelial dysfunction was another important factor for OHSS. Endothelial dysfunction increases the permeability of capillaries, resulting in fluid loss into the third space, and subsequent changes in metabolism and hematology [33]. For people with non-O blood group, two-thirds of the overall change in VWF concentration seems to be genetically determined, with higher concentrations of VWF and factor VIII [34]. According to reports, in many different clinical situations such as HELLP [35] and OHSS, VWF levels are elevated and endothelial cell dysfunction is present.
Regarding the relationship between the ABO blood group and pregnancy outcomes, previous studies have found that a certain blood group is less likely to achieve a successful pregnancy. As mentioned above, the ABO blood group is the main determinant of plasma von Willebrand factor and factor VIII levels, and hemoglobin and factor VIII levels increase in individuals with non-O blood groups [36, 37]. Recently, one study proposed that the ADAMTS13-von Willebrand factor pathway play a key role in normal pregnancy and pathogenesis of preeclampsia [38]. Multiple studies have also shown that certain ABO blood group gene polymorphisms are associated with increased levels of some immune and inflammatory mediators [39, 40], which are associated with early embryo implantation and subsequent placental implantation [41, 42]. Therefore, it is reasonable to believe that the immune or inflammatory environment associated with certain ABO blood groups may affect the embryo implantation and subsequent embryo growth during IVF.
With regard to the relationship between ABO blood group and DOR, OHSS, pregnancy outcomes, the conclusions were inconsistent, and even opposite. The contradiction may be due to the racial differences between the study populations. Even the study populations were from the same country; there may be different results, which may be related to the difference in the blood group composition ratio of the study population.
There were several limitations in our present study. The biggest one was that 11 out of the 13 included studies were retrospective studies. When evaluating some outcomes such as MR and OHSS, relatively few studies were included. Secondly, there was highly variable study characteristic: the diagnosis standard for DOR, population racial (Ethnicity and race also affect the distribution of different blood groups [43]). Thirdly, some studies did not adjust for some possible confounding factors, such as smoking history, body mass index, and history of ovarian surgery.
In conclusion
The present study indicated that ABO blood groups might not be associated with the incidence of DOR, OHSS, LBR, CPR, and MR after ART treatment. Infertility and ART outcomes are influenced by multiple factors. Blood groups should not be taken into account excessively during diagnosis and treatment of infertile women. Further well-designed clinical studies are needed to confirm the association between ABO blood group and women infertility.
Availability of data and materials
All data is available in this paper.
Abbreviations
- ART:
-
Assisted reproductive technology
- DOR:
-
Diminished ovarian reserve
- OHSS:
-
Ovarian hyperstimulation syndrome
- RR:
-
Risk ratio
- CPR:
-
Clinical pregnancy rate
- LBR:
-
Live birth rate
- MR:
-
Miscarriage rate
- CI:
-
Confidence interval
References
Pereira N, Patel HH, Stone LD, Christos PJ, Elias RT, Spandorfer SD, et al. Association between ABO blood type and live-birth outcomes in single-embryo transfer cycles. Fertil Steril. 2017;108:791–797. Available from: http://dx.doi.org/https://doi.org/10.1016/j.fertnstert.2017.08.019.
Malekzadeh F, Moini A, Amirchaghmaghi E, Daliri L, Akhoond MR, Talebi M, et al. The association between ABO and Rh blood groups and risk of endometriosis in iranian women. Int J Fertil Steril. 2018;12:213–7.
Poole EM, Gates MA, High BA, Chanock SJ, Cramer DW, Cunningham JM, et al. ABO blood group and risk of epithelial ovarian cancer within the ovarian Cancer association consortium. Cancer Causes Control. 2012;23:1805–10.
Behrman SJ, Buettner-Janusch J, Heglar R, Gershowitz H, Tew WL. ABO(H) blood incompatibility as a cause of infertility: a new concept. Am J Obstet Gynecol. 1960;79:847–55.
Dentali F, Sironi A, Ageno W, Turato S, Bonfanti C, Frattini F, et al. Non-O blood type is the commonest genetic risk factor for VTE: results from a meta-analysis of the literature. Semin Thromb Hemost. 2012;38:535–47.
Franchini M, Mannucci PM. ABO blood group and thrombotic vascular disease. Thromb Haemost. 2014;112:1103–9.
Goldsammler M, Jindal SK, Kallen A, Mmbaga N, Pal L. Blood type predicts live birth in the infertile population. J Assist Reprod Genet. 2015;32:551–5.
Di Nisio M, Ponzano A, Tiboni GM, Guglielmi MD, Rutjes AWS, Porreca E. Non-O blood group and outcomes of in vitro fertilization. J Assist Reprod Genet. 2018;35:1289–94.
Nejat EJ, Jindal S, Berger D, Buyuk E, Lalioti M, Pal L. Implications of blood type for ovarian reserve. Hum Reprod. 2011;26:2513–7.
Mu L, Jin W, Yang H, Chen X, Pan J, Lin J, et al. ABO blood type is associated with ovarian reserve in Chinese women with subfertility. Oncotarget. 2016;7:50908–13.
Lin S, Li R, Chi H, Huang S, Zhang H, Zheng X, et al. Effect of ABO blood type on ovarian reserve in Chinese women. Fertil Steril. 2014;102:1729–1732.e2. Available from: http://dx.doi.org/https://doi.org/10.1016/j.fertnstert.2014.09.008.
de Mouzon J, Hazout A, SB MC-B, PC-B. Reply: threshold values of follicle count for the definition of PCO. Hum Reprod. 2012;27:1544–5.
Timberlake KS, Foley KL, Hurst BS, Matthews ML, Usadi RS, Marshburn PB. Association of blood type and patient characteristics with ovarian reserve. Fertil Steril.; 2013;100:1735–1739. Available from: http://dx.doi.org/https://doi.org/10.1016/j.fertnstert.2013.08.027.
Şengül Ö, Dilbaz B, Yerebasmaz N, Dede S, Altinbaş Ş, Erkaya S. Only female age, and not blood type, is associated with ovarian reserve. Int J Fertil Steril. 2014;8:143–6.
Spitzer D, Corn C, Stadler J, Wirleitner B, Schuff M, Vanderzwalmen P, et al. Implications of blood type for ovarian reserve and infertility - impact on oocyte yield in IVF patients. Geburtshilfe Frauenheilkd. 2014;74:928–32.
Awartani K, Al Ghabshi R, Al Shankiti H, Al Dossari M, Coskun S. Association of blood groups with ovarian reserve and outcome of in vitro fertilization treatment. Ann Saudi Med. 2016;36:116–20.
Deng J, Jia M, Cheng X, Yan Z, Fan D, Tian X. ABO blood group and ovarian reserve: a meta-analysis and systematic review. Oncotarget. 2017;8:25628–36.
Binder H, Flegel WA, Emran J, Müller A, Cupisti S, Beckmann MW, et al. Blood group a: an overseen risk factor for early-onset ovarian hyperstimulation syndrome? Reprod Biomed Online 2008;17:185–189. Available from: http://dx.doi.org/https://doi.org/10.1016/S1472-6483(10)60193-9.
Binder H, Flegel WA, Emran J, Müller A, Dittrich R, Beckmann MW, et al. Association of blood group a with early-onset ovarian hyperstimulation syndrome. Transfus Clin Biol. 2008;15:395–401.
Bellver J, Ferrando M, Garrido N, Pellicer A. Blood group and ovarian hyperstimulation syndrome. Fertil Steril. 2010;93:270–1.
Pereira N, Levine BA, Karipcin FS, Elias RT, Spandorfer SD, Rosenwaks Z. ABO blood type and variation in serum markers of ovarian reserch. Fertil Steril. 2013;100(3):S155.
Yazer MH. What a difference 2 nucleotides make: a short review of ABO genetics. Transfus Med Rev. 2005;19:200–9.
Palcic MM, Seto NOL, Hindsgaul O. Natural and recombinant a and B gene encoded glycosyltransferases. Transfus Med. 2001;11:315–23.
Yamamoto FI, Clausen H, White T, Marken J, Hakomori SI. Molecular genetic basis of the histo-blood group ABO system. Nature. 1990;345:229–33.
Dharmesh SM, Baenziger JU. Estrogen modulates expression of the glycosyltransferases that synthesize sulfated oligosaccharides on lutropin. Proc Natl Acad Sci U S A. 1993;90:11127–31.
Lourenço D, Brauner R, Lin L, De Perdigo A, Weryha G, Mihaela Muresan RB, Guerra-Junior G, Maciel-Guerra AT, Achermann JC, AB KME. Mutations in NR5A1 associated with ovarian insufficiency. N Engl J Med. 2009;360:1200–10.
Clark AG. The role of haplotypes in candidate gene studies. Genet Epidemiol. 2004;27:321–33.
Huang W, Cao Y, Shi L. Effects of FSHR polymorphisms on premature ovarian insufficiency in human beings: a meta-analysis. Reprod Biol Endocrinol. 2019;17:1–6.
Peprah E. Understanding decreased fertility in women carriers of the FMR1 premutation: a possible mechanism for fragile X-associated primary ovarian insufficiency (FXPOI). Reprod Health. 2014;11:67.
Wood MA, Rajkovic A. Genomic markers of ovarian reserve. Semin Reprod Med. 2013;31:399–415.
Ségurel L, Thompson EE, Flutre T, Lovstad J, Venkat A, Margulis SW, et al. The ABO blood group is a trans-species polymorphism in primates. Proc Natl Acad Sci U S A. 2012;109:18493–8.
O’Donnell J, Laffan MA. The relationship between ABO histo-blood group, factor VIII and von Willebrand factor. Transfus Med. 2001;11:343–51.
Soares SR, Gómez R, Simón C, García-Velasco JA, Pellicer A. Targeting the vascular endothelial growth factor system to prevent ovarian hyperstimulation syndrome. Hum Reprod Update. 2008;14:321–33.
Davies JA, Collins PW, Hathaway LS, Bowen DJ. Effect of von Willebrand factor Y/C1584 on in vivo protein level and function and interaction with ABO blood group. Blood. 2007;109:2840–6.
Hulstein JJJ, van Runnard Heimel PJ, Franx A, Lenting PJ, Bruinse HW, Silence K, de Groot PhG FR. Acute activation of the endothelium results in increased levels of active von Willebrand factor in hemolysis, elevated liver enzymes and low platelets (HELLP) syndrome. J Thromb Haemost. 2006:2569–75.
O’Donnell J, Boulton FE, Manning RA, Laffan MA. Amount of H antigen expressed on circulating von Willebrand factor is modified by ABO blood group genotype and is a major determinant of plasma von Willebrand factor antigen levels. Arterioscler Thromb Vasc Biol. 2002;22:335–41.
Schleef M, Strobel E, Dick A, Frank J, Schramm W, Spannagl M. Relationship between ABO and secretor genotype with plasma levels of factor VIII and von Willebrand factor in thrombosis patients and control individuals. Br J Haematol. 2005;128:100–7.
Xiao J, Feng Y, Li X, Li W, Fan L, Liu J, et al. Expression of ADAMTS13 in Normal and abnormal placentae and its potential role in angiogenesis and placenta development. Arterioscler Thromb Vasc Biol. 2017;37:1748–56.
Paré G, Chasman DI, Kellogg M, Zee RYL, Rifai N, Badola S, et al. Novel association of ABO histo-blood group antigen with soluble ICAM-1: results of a genome-wide association study of 6,578 women. PLoS Genet. 2008;4.
Qi L, Cornelis MC, Kraft P, Jensen M, van Dam RM, Sun Q, et al. Genetic variants in ABO blood group region, plasma soluble E-selectin levels and risk of type 2 diabetes. Hum Mol Genet. 2010;19:1856–62.
Alikani M. Epithelial cadherin distribution in abnormal human pre-implantation embryos. Hum Reprod. 2005;20:3369–75.
Staun-Ram E, Shalev E. Human trophoblast function during the implantation process. Reprod Biol Endocrinol. 2005;3:1–12.
Garratty G, Glynn SA, McEntire R. ABO and Rh(D) phenotype frequencies of different racial/ethnic groups in the United States. Transfusion. 2004;44:703–6.
Acknowledgements
There was no acknowledgement.
Funding
This study was funded by the Technology Innovation Guidance program of Hunan province.
Author information
Authors and Affiliations
Contributions
Jing Zhao participated in the design of the study and the acquisition of data, performed the statistical analysis, drafted the article and revised it critically. Yanping Li contributed to conception and design. Jie Hao conducted acquisition of data and analysis and interpretation of data. Bin Xu and Yonggang Wang participated in the interpretation of the data and the revision of the article. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
No applicable.
Competing interests
The authors declare that they have no conflict of interest
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Fig. S1
Forest plot showing the results of meta-analysis of studies assessing the association of ABO blood groups with CPR. Fig. S2 Forest plot showing the results of meta-analysis of studies assessing the association of ABO blood groups with MR.
Additional file 2: Fig. S1.1
Funnel plot of analysis for the association of blood group A/O and DOR, showing the results of Eggers to assess publication bias. Fig. S1.2 Funnel plot of analysis for the association of blood group B/O and DOR, showing the results of Eggers to assess publication bias. Fig. S1.3 Funnel plot of analysis for the association of blood group AB/O and DOR, showing the results of Eggers to assess publication bias. Fig. S1.4 Funnel plot of analysis for the association of blood group non-O/O and DOR, showing the results of Eggers to assess publication bias.
Additional file 3: Fig. S2.1
Funnel plot of analysis for the association of blood group A/O and OHSS, showing the results of Eggers to assess publication bias. Fig. S2.2 Funnel plot of analysis for the association of blood group B/O and OHSS, showing the results of Eggers to assess publication bias. Fig. S2.3 Funnel plot of analysis for the association of blood group AB/O and OHSS, showing the results of Eggers to assess publication bias. Fig. S2.4 Funnel plot of analysis for the association of blood group non-O/O and OHSS, showing the results of Eggers to assess publication bias.
Additional file 4: Fig. S3.1
Funnel plot of analysis for the association of blood group A/O and LBR, showing the results of Eggers to assess publication bias. Fig. S3.2 Funnel plot of analysis for the association of blood group B/O and LBR, showing the results of Eggers to assess publication bias. Fig. S3.3 Funnel plot of analysis for the association of blood group AB/O and LBR, showing the results of Eggers to assess publication bias. Fig. S3.4 Funnel plot of analysis for the association of blood group non-O/O and LBR, showing the results of Eggers to assess publication bias.
Additional file 5: Fig. S4.1
Funnel plot of analysis for the association of blood group A/O and CPR, showing the results of Eggers to assess publication bias. Fig. S4.2 Funnel plot of analysis for the association of blood group B/O and CPR, showing the results of Eggers to assess publication bias. Fig. S4.3 Funnel plot of analysis for the association of blood group AB/O and CPR, showing the results of Eggers to assess publication bias. Fig. S4.4 Funnel plot of analysis for the association of blood group non-O/O and CPR, showing the results of Eggers to assess publication bias.
Additional file 6: Fig. S5.1
Funnel plot of analysis for the association of blood group non-O/O and MR, showing the results of Eggers to assess publication bias.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhao, J., Yao, Z., Hao, J. et al. Association of ABO blood groups with ovarian reserve, and outcomes after assisted reproductive technology: systematic review and meta-analyses. Reprod Biol Endocrinol 19, 20 (2021). https://doi.org/10.1186/s12958-020-00685-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12958-020-00685-x