Abstract
Background
Elevated endotoxin levels have been measured in ambient air around livestock farms, which is a cause of concern for neighbouring residents. There is clear evidence that occupational exposure to high concentrations of airborne endotoxin causes respiratory inflammation, respiratory symptoms and lung function decline. However, health effects of exposure to low levels of endotoxin are less well described. The aim of this systematic review is to summarize published associations between exposure to relatively low levels of airborne endotoxin and respiratory health endpoints.
Methods
Studies investigating respiratory effects of measured or modelled exposure to low levels of airborne endotoxin (average < 100 EU/m3) were eligible for inclusion. In total, 1362 articles were identified through a Pubmed database search, of which 31 articles were included in this review. Studies were included up to February 2017. Overview tables and forest plots were created, and study quality was assessed.
Results
Twenty-two included studies had a cross-sectional design, others were designed as longitudinal observational (n = 7) or experimental (n = 2) studies. Most studies (n = 23) were conducted in an occupational setting, some involved domestic or experimental exposure. Several studies reported statistically significant effects of exposure to low levels of endotoxin on respiratory symptoms and lung function. However, considerable heterogeneity existed in the outcomes of the included studies and no overall estimate could be provided by meta-analysis to quantify the possible relationship. Instead, a best evidence synthesis was performed among studies examining the exposure-response relationship between endotoxin and respiratory outcomes. Significant exposure-response relationships between endotoxin and symptoms and FEV1 were shown in several studies, with no conflicting findings in the studies included in the best evidence synthesis. Significantly different effects of endotoxin exposure were also seen in vulnerable subgroups (atopics and patients with broncho-obstructive disease) and smokers.
Conclusions
Respiratory health effects of exposure to low levels of airborne endotoxin (< 100 EU/m3) seem plausible. Future studies are needed to investigate ambient exposure to endotoxin and potential respiratory health effects, especially in vulnerable subgroups of the population.
Similar content being viewed by others
Background
Health effects of air pollution have mainly been studied in urban areas, where pollutant concentrations can be high due to emissions from industries and traffic. However, poor air quality in rural areas may also be of influence on people’s health. In the Netherlands, regions where air quality is influenced by emissions from livestock farms are densely populated [1, 2]. Since potential health effects of these emissions are relevant to all people living and working in these areas, the relationship between exposure and health is a current topic of research.
Over the last thirty years, a considerable amount of research has been performed to gain insight into the respiratory health risks of people occupationally exposed to high concentrations of organic dust and endotoxin [3,4,5,6]. Inhalation of endotoxin, a lipopolysaccharide component of the cell-wall of Gram-negative bacteria present in organic dust, induces an inflammatory response in the lungs [6,7,8,9]. Aerosolized endotoxin is absorbed onto the surface of particulate matter and thus transported through the air [7, 10]. By binding to the CD14/TLR4/MD2 receptor complex on macrophages it triggers the production of cytokines and proteins that cause inflammation [8, 9, 11]. When challenged with aerosolized endotoxin, people have shown a hundredfold increase in neutrophil levels and tripling of lymphocyte levels in bronchoalveolar fluid [12]. In 1987, Castellan et al. found a clear exposure–response relationship between endotoxin concentration and group mean percentage change in forced expiratory volume in one second (FEV1) in individuals experimentally exposed to endotoxin containing cotton dust [13]. The effects of exposure to endotoxin are predominantly respiratory, including decline in lung function and increased prevalence of chronic bronchitis and asthma-like syndrome [5, 14, 15]. In addition to adverse health effects, occupational endotoxin exposure in agricultural workers has also been implicated in protective effects on allergic sensitisation and hay fever [16, 17].
While respiratory health effects of exposure to high levels of endotoxin are well described, potential effects associated with low levels of exposure are less well established. However, interest in the possible adverse health effects of endotoxin exposure on non-occupationally exposed populations is growing [2, 18]. Ambient endotoxin concentrations in the proximity of livestock farms and bioaerosol levels near composting sites have been found to be in the lower range of exposure levels measured in several occupations [19, 20]. Since it is not clear whether effects observed at high exposure levels can be extrapolated to lower exposure levels, further research is warranted. These outcomes are interesting for governmental institutions in particular, in order to formulate guidelines to protect the public health and safety of their inhabitants. Currently, the Dutch Expert Committee on Occupational Safety (DECOS) of the Health Council recommends a health-based occupational exposure limit of 90 EU/m3 [21]. DECOS regards an exposure level of 90 EU/m3 as a NOEL (no observed effect level), based on the effects on FEV1 of six-hour exposure to endotoxins in the study by Castellan et al. [13]. Based on the occupational exposure limit, a tentative limit of 30 EU/m3 was recommended for the general population living in the surroundings of livestock farms [21, 22].
The aim of this systematic review is to investigate the possible respiratory health effects of exposure to low levels of airborne endotoxin in humans. Levels up to 100 EU/m3 are included since these levels can be compared to peak ambient levels of airborne endotoxin in livestock-dense areas [23, 24] We hypothesize that exposure to these concentrations of endotoxin can have modest, but negative effects on respiratory health.
Methods and design
Design
This systematic review was performed by the first author (A.F.) in collaboration with the last author (L.A.M.S) and was performed according to the steps of the PRISMA statement [25].
Information sources and search strategy
The Pubmed database was searched for relevant literature published until February 14th 2017. Search terms used to find eligible articles were based on the terms endotoxin, exposure, lung function and respiratory symptoms (such as cough, wheeze, chest tightness and shortness of breath). The full electronic search query is presented in Additional file 1: Supplement 1. Reference lists of all included studies and relevant literature reviews were searched for additional eligible articles.
Inclusion criteria
Studies were eligible for inclusion if measurements of airborne endotoxin concentrations were performed, through either active or passive air sampling methods. Studies which used modelling approaches based on air exposure measurements were also included. Respiratory outcomes (lung function measurement and/or respiratory symptoms) had to be defined and described. Only human experimental or observational studies were included, with full text written in English, Dutch, German or French and which were originally published in peer-reviewed journals. Case reports, literature reviews and non-human studies were excluded. Also studies with measurements of airborne endotoxin of only high levels of exposure (an average of >100 EU/m3) were excluded, as were studies where endotoxin was measured in dust reservoirs only. The exposure variable of interest was exposure to low levels of endotoxin (average < 100 EU/m3). The main outcome was the effect on respiratory health; both on pulmonary function and occurrence of respiratory symptoms (coughing, wheezing, shortness of breath, asthma, dyspnoea).
Study selection
Assessment of manuscripts for meeting the inclusion criteria was performed in a Mendeley database. Duplicates were removed and subsequently studies were selected based on title or abstract for full text-screening. In case a study was excluded based on full text screening, the reason for exclusion was listed. In case several publications reported measurements from the same series, the one with the most detailed methodology description and original values was included.
Data extraction
Extraction of data was performed systematically by summarizing information on author, publication year, country, study design, endotoxin measurement techniques, spirometry measurements, questionnaires and confounders in overview tables.
Studies were categorized according to characteristics of the sample population (i.e., occupationally exposed subjects, respiratory disease patient groups or general population). Results are presented by individual study, since the studies were too heterogeneous in terms of endpoint measurement and presentation of endotoxin exposure levels, population samples, settings, reported outcomes and data analysis techniques to compare the results. Therefore, a narrative synthesis was performed and a best evidence synthesis was conducted for suitable outcome variables (see data synthesis). Also, forest plots were constructed (using R, version 3.3.2) to improve readability and comparability of the results.
Methodological quality and risk of bias
Assessing quality of evidence and risk of bias in individual studies was performed using the NIH Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies [26]. This tool was designed to assess the methodological quality of cohort and cross-sectional studies. In this method, the quality of the studies is evaluated by rating fourteen items representing research question, study population and sample size, participation rate, timeframe, variation in exposure level, validity and reliability of exposure and outcome variables, blinding, loss to follow-up and confounding. Each item can be scored as ‘yes’, ‘no’, ‘not applicable’, ‘cannot determine’ or ‘not reported’. The overall scores of the different studies were presented as percentages to improve comparability. Studies with total scores ≥90% were considered strong, studies scoring 70–90% were considered of moderate quality and studies scoring below 70% were considered weak.
Data synthesis
Because of the heterogeneity of the included studies we refrained from performing a meta-analysis, but conducted a best evidence synthesis to come to some overall conclusions using the method described by Proper [27]. Only those articles that investigated the exposure-response relationship between endotoxin exposure and an outcome variable were included in the best evidence synthesis. Four outcome variables were selected for inclusion in the best evidence synthesis: wheeze, cough, (nocturnal) asthma symptoms and FEV1. Other reviews that applied this best evidence synthesis method considered results to be consistent when at least 75% of the studies showed statistically significant results in the same direction (defined according to p < 0.05) [27,28,29]. Originally three possible levels of evidence followed from this best evidence synthesis method, namely strong, moderate and insufficient evidence. In our evidence synthesis, we added the category ‘weak evidence’ in case the results could not be considered consistent according to Proper (not meeting the criterion of at least 75% significant results), but all studies showed results in one direction, of which at least two studies with significant results, and no conflicting findings existed for an outcome variable.
Results
Study selection
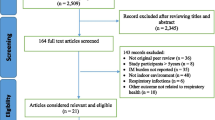
The search yielded a total of 1362 articles. In Fig. 1, a PRISMA flowchart of the study selection is presented. After removal of duplicates (n = 3) and selection on language (n = 40), 1319 articles remained. In total, 1153 articles were excluded based on title and abstract, leaving 166 articles to be assessed by screening the full text. Most of these articles were excluded because levels of exposure (n = 82), endotoxin level measurement techniques (n = 18) or study design (n = 28) did not match the inclusion criteria. Two studies were removed due to duplicate publication of the same endotoxin and outcome data [30, 31]. Reference lists of all included studies and 11 relevant literature reviews were searched for additional eligible articles, but did not yield additional studies for inclusion. In total, 31 articles were included in this systematic review.
Characteristics of included studies
Setting and population
An overview of the characteristics of the included studies is presented by publication date in Tables 1 and 2. Of the studies that were included most had a cross-sectional design, seven studies were set up longitudinally (with follow up periods between 5 days [32] and 11 years [33]) and two experimental studies [34, 35] were included. Eleven studies were performed in the United States, four in the Netherlands, three in Norway, two in Denmark, two in Switzerland, two in Sweden and one each in Australia, New Zealand, Germany, Canada, Pakistan, Poland and the UK. The included studies were performed between 1987 and 2016. Most of the studies examined endotoxin exposure among occupationally exposed subjects (n = 23), such as workers in wood, sewage and textile industries. Four studies focussed on susceptible populations, mostly children with asthma or adults with COPD. The remaining studies included children as a target population, except for one of the experimental studies where healthy adults were studied [35]. The number of included subjects ranged from 22 [36] to 3867 [37] subjects. In some cases the study was initiated because of specific reasons, such as a sudden increase in incidence of specific complaints reported by a group of workers. In some of these studies, other air pollutants than endotoxin were measured as well. We summarized important relationships between the other airborne agents and respiratory outcomes in Additional file 1: Supplement 2.
Measured pollutants
Dust was mostly collected with personal sampling techniques during working hours, alternatives used were area sampling and predictive calculations based on dispersion models [37, 38]. Endotoxin was measured using the Limulus Amebocyte Lysate (LAL) assay, which is the most accepted assay for endotoxin exposure measurements. The exposure agents measured in the included studies vary greatly. Some studies only reported measurement of the exposure to airborne endotoxin [39,40,41,42,43,44] whereas other studies included measurement of dust, bacteria, fungi and/or other airborne particles.
Health outcomes
Twenty-one studies performed spirometry measurements and included lung function values in their design, most of them included FEV1 and FVC as outcome measurements. All but three studies recorded symptoms through a questionnaire. Questionnaires used were often based on questions from the MRC, ATS, ECRHS or ISAAC questionnaires and the Organic Dust Questionnaire, but a number of other questionnaires were used as well as a source for the reporting of symptoms [35, 36, 40,41,42, 45,46,47,48,49,50,51]. The study by Horick et al. used monthly telephone calls to register respiratory symptoms [38].
Quality assessment
An overview of the quality assessment results is presented in Additional file 1: Supplement S3. All studies had well-described study objectives and most included detailed information on the study subjects. The main reason for scoring negative on study population description was the absence of the inclusion period. Eleven studies did not report the participation rate. All but one studies reported effect sizes, only Heldal et al. presented results otherwise [36]. Since most of the studies had a cross-sectional design, exposures were not measured prior to the outcomes. In case studies included cross-work shift or cross-week measurements of lung function values and in case of longitudinal studies, the item timeframe was scored positive. Twenty studies investigated effects of different levels of exposure. All studies used valid and reliable area or personal exposure measurements. Exposure assessment over time was scored positive when repeated personal full-work shift measurements were performed, area measurements were considered insufficient. Two studies used modelling to estimate personal endotoxin exposure, this was also regarded an accurate and reliable way of estimating exposure [37, 38]. Regarding outcome measures, spirometry measurements were considered valid and reliable, as was the use of validated questionnaires. Dang et al. were the only ones using an unvalidated questionnaire without performing additional spirometry measurements [49]. Blinding of outcome assessors was only applicable in non-occupational studies, as is reflected in the scoring of this criterion. Four studies did not perform/report correction for confounding. Since not all scoring items were applicable for all included studies, a percentage of the maximum score was calculated for each included study. The percentages of total scores varied between 55% and 100%. Most studies were considered moderate based on their score (n = 13), others were considered strong (n = 9) or weak (n = 9).
Findings
Main results of questionnaires, spirometry and dose-response relationships are combined and summarised in Table 3.
Questionnaire
Respiratory symptoms recorded by most studies included cough, wheezing, shortness of breath, chest tightness and nocturnal asthma symptoms. As presented in Additional file 1: Supplement S4.1, ten studies reported a significantly higher prevalence of respiratory symptoms among exposed subjects when compared to unexposed or lower exposed controls. The definition of exposure differed between studies, ranging from only endotoxin exposure (measured) to exposure to various bioaerosols. The symptoms that were found to be significantly more prevalent among exposed subjects were cough [41, 49, 51,52,53,54], wheeze [49, 55, 56], shortness of breath [41, 49, 52, 55], (work-related) chest tightness [34, 49, 54, 56], chronic bronchitis [53] and (work-related) asthmatic symptoms [41, 56, 57]. Fransman et al. found that plywood workers exposed to 23 EU/m3 endotoxin had significantly more attacks of shortness of breath with wheezing than unexposed controls and that workers employed > 6.5 years had significantly more asthma, shortness of breath and wheezing when compared to members of the general population [55]. Smit et al. also showed a significant positive association between length of employment and lower respiratory tract (LRT) symptoms [41]. Two studies found that respiratory symptoms lessened during holidays/days off; 53–83% of respiratory symptoms lessened during holidays in one study [55], another study found a PR of 2.84 (95%CI 1.56–5.18) for decline in symptoms of wheeze during holidays [56]. Shiryaeva et al. found that the highest frequency of symptoms was present on Mondays and that symptoms decreased gradually over the week, wheeze and chest tightness decreased significantly [58].
One study among textile yarn workers exposed to different kinds of humidifiers (endotoxin levels 0.18–0.64 EU/m3) did not find a significant difference in the reporting of symptoms among subpopulations [32]. Three other studies did not find a significant difference in prevalence of respiratory symptoms among exposed subjects when compared to controls [42, 59, 60]. Two studies did not find any difference in exposure levels of endotoxin between subjects with and without respiratory complaints [36, 50]. In the study by Zock et al. among potato processing workers, subjects exposed to 21 EU/m3 (AM) seemed to have more symptoms of respiratory symptoms than the group exposed to 56 EU/m3 (AM) [39]. Exposure to 7.40 EU/m3 for 1 h in an experimental setting did not significantly influence the prevalence of cough symptoms [35].
The forest plots in Fig. 2 show a summary of the effects of exposure (to endotoxin and other bioaerosols) on respiratory symptoms presented in the included studies. The odds ratios (or exp.(beta) for symptom score) of asthma, chest tightness, cough and wheeze appear to be higher in subjects exposed to bioaerosols, albeit with wide confidence intervals and often not significant. Figure 3 shows the odds ratios for different symptoms for an increase in exposure of 1 unit of log-transformed endotoxin.
Forest plots presenting the odds ratio (OR) for asthma (a), chest tightness (b), cough (c) and wheeze (d) – exposure to multiple bioaerosols. Prevalence is calculated for exposed vs non-exposed subjects or high vs low exposed subjects. *OR for dose-dependent relationship with endotoxin (no other bioaerosols included in calculations). °non-occupational study
Spirometry
The results for the different outcomes of spirometry measurements are summarised in Additional file 1: Supplement S4.2. Three studies found a significant difference in pre-shift lung function values between exposed subjects and controls (exposure definition differed between studies), where exposed subjects had lower values for FEV1 and/or FVC [44, 51, 53]. The baseline FVC recorded by one study was 84.7% of predicted for woodworkers exposed to 24–43 EU/m3 endotoxin compared to 94.9% for controls (p = 0.0001), for FEV1 comparable outcomes were found [53].
Six studies presented significant cross-work shift declines of FEV1 and/or FVC among exposed subjects [32, 39, 43, 46, 53, 58]. Mean absolute decrease in FEV1 was found to be 0.06–0.12 L among potato processing workers exposed to 56 EU/m3 [39], another study found an mean decrease of 0.07–0.10 L among textile yarn workers exposed to spray-humidifiers associated with endotoxin levels of 0.64 EU/m3 [32]. The latter study found a significant decrease in FEV1 over the workday but also a decreased FEV1 level on Friday when compared to Monday. Dahlqvist et al. found that subjects with a period of employment > 18 years had a significantly larger change in MMEF over the workweek than subjects employed < 6 years [52]. Another study that performed cross-week analyses did not find significant lung function decline over the workweek [58]. In terms of cross-work shift decline in percentage predicted lung function, one study found a cross-work shift decrease of 6.34% in FEV1 among woodworkers exposed to 24–43 EU/m3 whereas controls had a decrease of 1.78% (p < 0.001) [53].
Exposure to 7.40 EU/m3 for 1 h in an experimental setting did not lead to significant changes in lung function parameters among 48 healthy volunteers [35].
Four studies found no significant effect of exposure to bioaerosols on lung function parameters among exposed subjects [42, 48, 54, 60]. Another study among 97 paper mill workers showed no significant difference in yearly decline of lung function between low and high exposed groups (endotoxin levels ranged between 6 and 370 EU/m3) [33]. A longitudinal study also found no significant changes in lung function after 5 years of exposure to endotoxin levels of 28 EU/m3 among refuse derived fuel workers [40].
Dose-response relationship
Eighteen of the included studies performed analyses to study the dose-dependent exposure-response relationship between endotoxin exposure and respiratory health effects, the results are presented in Additional file 1: Supplement S4.3. Symptoms that occurred significantly more often with increasing levels of endotoxin exposure were cough [51], asthmatic symptoms [37, 45, 47, 57], wheeze [37, 38, 47] and usual phlegm [54]. One study found an OR of 2.042 (95%CI 1.029–4.042) for nocturnal asthma symptoms for every 1 EU/m3 increase in endotoxin exposure [45]. A relative risk of 5.56 (95%CI 1.19–26.03) for wheeze was found for every 0.4 log10 endotoxin increase in personal exposure in another study [38].
Five studies found a significant drop in FEV1 levels associated with an increase in endotoxin exposure [39, 43, 45, 46, 53]. One study found that children exposed to higher levels of endotoxin had significantly lower levels of evening FEV1, with a decrease of 316 ml per 1 EU/m3 increase (95%CI -597 to − 36 ml, p = 0.036) [45]. Cyprowski et al. found a 42 ml decrease in FEV1 per 1 EU/m3 increase in exposure (p = 0.044) [43].
Subgroup analyses
Wheeze, nocturnal cough and other asthmatic symptoms were more prevalent among children of atopic parents in a big German study where endotoxin exposure (median 0.064 EU/m3) was modelled using dispersion models. Per one log unit increase in endotoxin exposure the OR for asthmatic symptoms among children with atopic parents was 1.15 (95%CI 1.03–1.29) [37].
One study among asthmatic school children found that subjects with baseline FEV1 < 80% of predicted had significant associations with endotoxin exposure, predicted FEV1 values dropped with 7.7% (95%CI -12.3 to − 3.3%) for every 2.19 EU/m3 increase in exposure [46]. Another study among asthmatic school children found that airborne endotoxin was associated with increased maximum symptom-days only in subjects with non-atopic asthma. For atopics, there was an inverted U-shaped relationship between school air endotoxin and maximum symptom-days (plateau at 230 EU/m3) [47].
In an occupational study, 60% of exposed asthmatic workers reported that their asthma seemed worse at work, while none of the non-exposed asthmatic subjects reported this [49]. A second occupational study found that atopic exposed subjects had a significantly higher proportion with symptoms at work (PR 3.2 (95%CI 1.6–6.2), p < 0.001) than non-atopics [50]. Another study showed that there were no significant differences in respiratory outcomes related to exposure between atopic and non-atopic subjects [58].
In the study by Schlünssen et al., asthma symptoms were found to be associated with endotoxin in non-smokers (OR 10.1;1.7–59.7), whereas this was not found for smokers (OR 0.5; 0.1–2.8) [57]. Dahlqvist et al. found no differences in the distribution of symptoms between smokers and non-smokers [52]. In one study, non-smokers showed larger across work shift declines than smokers: for FEV1 the across work shift difference was − 0.1% (95%CI -3.6;3.5) for smokers and − 1.8% (95%CI -4.5,1.0) for non-smokers [39]. On the contrary, in another study smokers showed an across work shift decline of 1.12% (SD 9.5) for FVC and 2.26% (SD 12.1) for FEV1, whereas non-smokers showed an across work shift decline of 0.53% (SD 11.9) for FVC and 0.73% (SD 12.5) for FEV1 [40]. Similarly, smokers had a mean cross work shift decline in FEV1 of 0.93% (SD 5.24), this was 0.72% (SD 6.31) for former smokers and 0.41% (SD7.52) for non-smokers in a second study [58]. Yet another study showed comparable lung function declines for smokers and non-smokers [33].
One study found that smokers exposed to 3–11 EU/m3 endotoxin had significantly lower lung function values than non-exposed smokers. For ex-smokers, no significant difference was found according to exposure [42].
An overview of the results among subgroups is provided in Additional file 1: Supplement S4.4.
Best evidence synthesis
In Additional file 1: Supplement S5, an overview of the best evidence synthesis is presented.
For wheeze, there were two strong studies and one study of moderate quality showing a significant increase in complaints when personal endotoxin exposure increased [37, 38, 47]. Four other studies also showed dose-dependent increase of wheeze, although their results did not reach significance, which may be due to limited sample sizes in some of these studies [57, 58, 61, 62]. The evidence for the effects of endotoxin exposure on wheeze symptoms could be classified as weak, since less than 75% of the results found reached significance, but none of the studies showed results in the opposite direction.
For nocturnal asthma symptoms, there were one strong study and three studies of moderate quality showing a significant dose-dependent increase of symptoms [37, 45, 47, 57]. Two other studies (in < 100 subjects) showed an increase of symptoms as well, but their results did not reach significance [61, 62]. This was considered weak evidence for effects of exposure to endotoxin on asthma complaints since less than 75% of the results reached significance. No studies mentioned evidence for improvement of asthma symptoms related to endotoxin exposure, however.
Only one study, with a design classified as weak, found a significant dose-dependent effect of exposure to endotoxin on symptoms of cough [36]. Another strong study did suggest the same effect but did not reach statistical significance [58]. On the contrary, Zock et al. found that subjects exposed to lower levels of endotoxin had a higher prevalence of cough symptoms than subjects exposed to higher levels of endotoxin [39]. Overall, insufficient evidence was found to state an effect of endotoxin exposure on symptoms of cough.
There were also several studies investigating the dose-dependent effects of exposure to endotoxin on FEV1 levels. Four strong studies and one study of moderate quality found significant declines of FEV1 (cross-work shift or cross-day) in relation to increasing endotoxin exposure [39, 43, 45, 46, 53]. Two other studies (in 70 salmon workers and 128 cotton workers) found non-significant declines in FEV1 with increasing endotoxin exposure [48, 58]. Overall, evidence regarding the effect of endotoxin on decline in FEV1 was considered to be weak since less than 75% of the results were significant, although multiple strong studies support the hypothesis that FEV1 declines with higher endotoxin exposure and no studies found results in the opposite direction.
Discussion
This review systematically summarizes the current knowledge on the respiratory effects of exposure to low levels of endotoxin. To our knowledge, no previous systematic review presented health effects of exposure to airborne endotoxin at levels that can be found in polluted ambient air, for instance near large-scale livestock farms or composting sites. Overall, negative effects on lung function and an increase in respiratory symptoms seem present although the evidence found was inconsistent in several ways.
By performing a best evidence synthesis we attempted to rate the level of evidence of the results found. Through this synthesis we could conclude that there is weak evidence regarding effects of low levels of airborne endotoxin on FEV1 values, although multiple strong studies showed significantly decreasing FEV1 values related to higher endotoxin exposures. For other outcomes too, only weak or insufficient evidence was found. This was mainly due to a lack of statistically significant findings, as many studies were underpowered, in particular for studying dichotomous outcomes. Still, most of the included studies did suggest negative effects of exposure to airborne endotoxin on wheeze, cough and (sleep-related) asthma symptoms. Apart from the exposure-response associations included in the best evidence synthesis, several other studies indicated that exposure to airborne endotoxin can have respiratory effects at these levels of exposure. Overall, twelve out of eighteen studies found statistically significant dose-dependent effects of exposure to endotoxin on respiratory symptoms and/or lung function values.
Strengths and limitations
One of the limitations concerning this review is the use of only one database in the search for relevant literature. Although Pubmed is widely used and expected to include almost all relevant literature on the topic of interest by the authors, it might be that relevant literature was not identified because of the exclusion of other databases. Another limitation is the inclusion of mostly cross-sectional observational studies and the strength of evidence must be interpreted against that background, the findings of this study remain descriptive. Further research in the field of respiratory inflammation related to endotoxin exposure at low levels would strengthen the evidence, as would investigation to certain biomarkers to prove a causal relationship. Since the nature of the included studies was quite heterogeneous and the statistical methods, sample populations and exposure and outcome definitions varied too much in the different studies, a meta-analysis, or meta-regression, could not be performed. A limitation in the assessment of the quality of the studies is the absence of clear cut-off points for considering a study design strong, moderate or weak. To overcome this, overall quality scores were compared by calculating percentages and strict cut-off points were formulated. Other limitations of the approach in this systematic review are the influences of multiple testing, selective reporting and publication bias.
A strength in the design of this review is the systematic approach and conduction of the inclusion and assessment of the relevant literature and data extraction of the included articles. By this systematic approach, chances of missing relevant literature or data was minimalized. The careful quality assessment, which was conducted by two researchers to optimise critical appraisal, is another strength of this study and improves the interpretation of the results of the different included studies. Although most of the included studies were designed cross-sectionally, several longitudinal follow-up studies were included. Inclusion of these articles gives insight in longer term changes in lung function and adds to the clinical relevance of this review. Another strength of this review is the inclusion of only actual measured levels of airborne endotoxin, enabling the nearest approximation of the true exposure of the included subjects. The only other exposure measurement method which was acceptable was modelling of personal endotoxin exposures based on measured airborne endotoxin levels. Hoopmann et al. used a dispersion model to predict personal endotoxin exposures by using endotoxin emission measurements of neighbouring livestock farms. All studies used the functional LAL assay to measure endotoxin exposure. Although within-laboratory precision of the assay is good, variation between laboratories may be substantial, in particular if different extraction and analysis procedures are used [21]. Underestimation of endotoxin levels, especially when using older protocols, may have resulted in the inclusion of studies with true mean endotoxin levels above 100 EU/m3, although most studies had mean exposure levels far below this threshold. The best evidence synthesis was conducted to strengthen the statements on the evidence of the reported results. Only dose-dependent exposure-response relationships were used in the best evidence synthesis in order to rely only on those results that were fully attributable to exposure to endotoxin.
Significant respiratory effects of other airborne agents
This review aimed to summarize associations between endotoxin and respiratory health, but it should be noted that airborne endotoxin levels are generally correlated with other bioaerosol components such as fungi and bacteria. Ambient air contains multiple agents, and exclusive exposure to endotoxin is only found in experimental research. Although all the included studies considered endotoxin exposure as a potential cause of the respiratory outcomes, other possible causative agents were often considered as well and we came across interesting findings regarding other bioaerosol exposures, as shown in Additional file 1: Table S2.
(Dis)agreement with current scientific literature
The search for a relationship between organic dusts and disease is an ongoing challenge given the inherent aspect of exposure to multiple agents and the difficulty to prove causal relationships in observational epidemiological studies. However, the findings of this review are in line with previous research findings in higher exposed populations. Several occupational studies among farmers have shown increased prevalence of respiratory diseases related to exposure to endotoxin [3,4,5,6] and studies experimenting with direct inhalation of endotoxin have shown an inflammatory response in the airways [12]. In addition, Radon et al. investigated the prevalence of respiratory symptoms among inhabitants of rural areas. They found that the number of animal houses in the neighbourhood was a predictor of self-reported wheeze and decreased FEV1 [18]. More recently, a Dutch study revealed a relationship between living in the vicinity of a large number of neighbouring farms and lower MMEF values and also between ammonia and particulate air pollution and lower FEV1 values, potentially related to endotoxin exposure [2].
From our results it seems that individuals with atopy or a chronic lung disease might be more susceptible to effects of exposure to endotoxin. This is in line with the findings of a study among COPD patients presented by Borlée et al. in 2015 [1]. Here, COPD patients living in the vicinity of livestock farms were found to have more exacerbations and use more medication. More evidence should be sought to confirm that patients with asthma or COPD and atopics form a vulnerable subgroup for the effects of exposure to airborne endotoxin.
Living near a farm was also associated with a lower prevalence of allergic rhinitis [1, 63]. Several studies in occupationally exposed farming populations have shown a dual effect of endotoxin with both negative and protective effects, but these populations were exposed to average endotoxin levels above 100 EU/m3 [16, 17]. Our focus on lung function and symptoms led to inclusion of studies showing adverse effects of endotoxin exposure. Furthermore, most studies that showed protective effects of endotoxin in homes analyzed endotoxin concentrations in house dust samples, whereas our systematic review only includes airborne endotoxin levels [64, 65].
Future perspectives
This study adds to the knowledge in the field by summarizing all the evidence available on respiratory effects of exposure to low levels of endotoxin, but future research is needed to strengthen the evidence. Endotoxin is known to originate from rural activities such as farming and composting and adds to air pollution in areas with a high density of these sources. Since possible effects are suggested by this review and other studies, endotoxin in ambient air should be seriously considered and investigated in larger populations. Large studies focussing on long-term exposed individuals are expected to give the best results. Since it is impossible to measure airborne personal exposure for a large group of individuals, modelling of personal exposure seems to be a good way to predict average long-term exposure levels. If a relationship between endotoxin and respiratory complaints becomes more evident, safety measures should be considered in order to protect inhabitants of areas with increased levels of these air pollutants. Another interesting topic for future research would be the effect of exposure to low levels of endotoxin on specific vulnerable subgroups, such as broncho-obstructive patients or atopics, since the respiratory effects seem different in these groups than in the general population.
Conclusion
Respiratory health effects of exposure to low levels of airborne endotoxin are found in multiple studies. More research regarding this relationship is needed in order to be able to inform/advise neighbouring residents of livestock farms and form guidelines and policies on ambient exposure to endotoxin. Special attention should be given to respiratory effects of endotoxin exposure in vulnerable subgroups, such as patients with broncho-obstructive disease.
Abbreviations
- ATS-DLD:
-
American Thoracic Society Division of Lung Disease
- COPD:
-
Chronic Obstructive Pulmonary Disease
- ECRHS:
-
European Community Respiratory Health Survey
- EU:
-
Endotoxin Units
- FeNO:
-
Fractional exhaled nitric oxide
- FEV1 :
-
Forced Expiratory Volume, in first second
- FVC:
-
Forced Vital Capacity
- ISAAC:
-
International Study of Asthma and Allergies in Childhood
- LAL:
-
Limulus Amebocyte Lysate
- LRT:
-
Lower respiratory tract
- MEF:
-
Midexpiratory flow
- MMEF:
-
Maximum midexpiratory flow (=FEF25–75)
- MRC:
-
Medical Research Council
- MWF:
-
Metal working fluids
- NIH:
-
National Institutes of Health
- PEF:
-
Peak expiratory flow
- PM:
-
Particulate matter
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- VC:
-
Vital capacity
References
Borlée F, Yzermans CJ, Van Dijk CE, Heederik D, Smit LAM. Increased respiratory symptoms in COPD patients living in the vicinity of livestock farms. Eur Respir J. 2015;46(6):1605–14.
Borlée F, Yzermans C, Aalders B, Rooijackers J, Krop E, Maassen C, et al. Air pollution from livestock farms is associated with airway obstruction in neighboring residents. Am J Respir Crit Care Med. 2017;196:1152–61.
Basinas I, Sigsgaard T, Heederik D, Takai H, Omland Ø, Andersen NT, et al. Exposure to inhalable dust and endotoxin among Danish livestock farmers: results from the SUS cohort study. J Environ Monit. 2012;14:604.
Basinas I, Sigsgaard T, Kromhout H, Heederik D, Wouters IM, Schlü Nssen V. A comprehensive review of levels and determinants of personal exposure to dust and endotoxin in livestock farming. J Expo Sci Environ Epidemiol. 2015;25(10):123–37.
Eduard W, Pearce N, Douwes J. Chronic Bronchitis, COPD, and lung function in farmers: the role of biological agents. Chest. 2009;136(3)
Douwes J, Thorne P, Pearce N, Heederik D. Bioaerosol Health effects and exposure assessment: progress and prospects. Ann Occup Hyg. 2003;47(3):187–200.
Soukup JM, Becker S. Human alveolar macrophage responses to air pollution particulates are associated with insoluble components of coarse material, including particulate endotoxin. Toxicol Appl Pharmacol. 2001;171(1):20–6.
Kumar S, Adhikari A. Dose-dependent immunomodulating effects of endotoxin in allergic airway inflammation. Innate Immun. 2017;23(3):249–57.
Xu H, Liew LN, Kuo IC, Huang CH, Goh DLM, Chua KY. The modulatory effects of lipopolysaccharide-stimulated B cells on differential T-cell polarization. Immunology. 2008;125(2):218–28.
Schins RPF, Lightbody JH, Borm PJA, Shi T, Donaldson K, Stone V. Inflammatory Effects of coarse and fine particulate matter in relation to chemical and biological constituents. Toxicol Appl Pharmacol. 2004;195:1–11.
Sigsgaard T, Bonefeld-Jørgensen EC, Hoffmann HJ, Bønløkke J, Krüger T. Microbial cell wall agents as an occupational hazard. Toxicol Appl Pharmacol. 2005;207(2):310–9.
Sandstrom T, Bjermer L, Rylander R. Lipopolysaccharide (LPS) inhalation in healthy subjects increases neutrophils, lymphocytes and fibronectin levels in bronchoalveolar lavage fluid. Eur Respir J. 1992;5(8):992–6.
Castellan RM, Olenchock SA, Kinsley KB, Hankinson JL. Inhaled endotoxin and decreased spirometric values. An exposure-response relation for cotton dust. N Engl J Med. 1987;317(10):605–10.
Bolund ACS, Miller MR, Basinas I, Elholm G, Omland O, Sigsgaard T, et al. The effect of occupational farming on lung function development in young adults: a 15-year follow-up study. Occup Environ Med. 2015;72(10):707–13.
Omland O. Exposure and Respiratory health in farming in temperate zones--a review of the literature. Ann Agric Environ Med. 2002;9(2):119–36.
Elholm G, Schlünssen V, Doekes G, Basinas I, Bolund A, Hjort C, et al. High exposure to endotoxin in farming is associated with less new-onset pollen sensitisation. Occup Env Med. 2018;75(2):139–47.
Smit LAM, Heederik D, Doekes G, Blom C, Van Zweden I, Wouters IM. Exposure-response analysis of allergy and respiratory symptoms in endotoxin-exposed adults. Eur Respir J. 2008;31(6):1241–8.
Radon K, Schulze A, Ehrenstein V, Van Strien RT, Praml G, Nowak D. Environmental exposure to confined animal feeding operations and respiratory health of neighboring residents. Epidemiology. 2007;18(3):300–8.
de Rooij MMT, Heederik DJJ, Borlée F, Hoek G, Wouters IM. Spatial and temporal variation in Endotoxin and PM10 concentrations in ambient air in a livestock dense area. Environ Res. 2017;153:161–70.
Pearson C, Littlewood E, Douglas P, Robertson S, Gant T, Hansell A. Exposures and health outcomes in relation to bioaerosol emissions from composting facilities: a systematic review of occupational and community studies. J Toxicol Env Heal B Crit Rev. 2015;18(1):43–69.
Health Council of the Netherlands. Endotoxins. Health-based recommended occupational exposure limit. Hague: Health Council of the Netherlands. 2010/04OSH; 2010.
Gezondheidsraad. Gezondheidsrisico’s rond veehouderijen. 2012. Available from: https://www.gezondheidsraad.nl/sites/default/files/201227Gezondheidsrisicoveehouderijen.pdf. Accessed 1 Feb 2017.
Thorne P, Ansley A, Perry SS. Concentrations of bioaerosols, odors and hydrogen sulfide inside and downwind from two types of swine livestock operations. J Occup Env Hyg. 2009;6(4):211–20.
Heederik D, Opstal-vanWinden AJ, Smit LAM, Wouters IM, Hooiveld M, IJzermans CJ, et al. Potential effects of intensive livestockfarming on neighboring residents' health. Utrecht: IRAS Universiteit Utrecht, NIVEL, RIVM. 2011; p. 204.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. BMJ. 2009;339:b2700.
NIH Quality assessment Tool for Observational Cohort and Cross-Sectional Studies. National Institutes of Health. Available from: https://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools/cohort. Accessed 1 Feb 2017.
Proper KI, Singh AS, Mechelen van W, Chinapaw MJM. Sedentary behaviors and health outcomes among adults. A Systematic Review of Prospective Studies. Am J Prev Med. 2011;40(2):174–82.
Proper K, Koning M, Van der Beek A, Hildebrandt V, Bosscher R, Van Mechelen W. The effectiveness of worksite physical activity programs on physical activity, physical fıtness and health. Clin J Sport Med. 2003;13(2):106–17.
Hoogendoorn W, van Poppel M, Bongers P, Koes B, Bouter L. Systematic review of psychosocial factors at work and private life as risk factors for back pain. Spine (Phila Pa 1976). 2000;25:2114–25.
Udeni Alwis K, Mandryk J, Hocking A. Exposure to Biohazards in Wood Dust: Bacteria, Fungi, Endotoxins, and (1-->3)-B-D-GlucansAppl Occup Environ Hyg 1999;14(9):598–608.
Mandryk J, Alwis KU, Hocking AD. Effects of personal exposures on pulmonary function and work-related symptoms among sawmill workers. Ann Occup Hyg. 2000;44(4):281–9.
Kateman E, Heederik D, Pal TM, Smeets M, Smid T, Spitteler M. Relationship of airborne microorganisms with the lung function and leucocyte levels of workers with a history of humidifier fever. Scand J Work Environ Health. 1990;16(6):428–33.
Sigsgaard T, Jensen LD, Abell A, Wurtz H, Thomsen G. Endotoxins isolated from the air of a Danish paper mill and the relation to change in lung function: an 11-year follow-up. Am J Ind Med. 2004;46(4):327–32.
Kennedy SM, Copes R, Bartlett KH, Brauer M. Point-of-sale glass bottle recycling: indoor airborne exposures and symptoms among employees. Occup Environ Med. 2004;61(7):628–35.
Schiffman SS, Studwell CE, Landerman LR, Berman K, Sundy JS. Symptomatic effects of exposure to diluted air sampled from a swine confinement atmosphere on healthy human subjects. Environ Health Perspect. 2005;113(5):567–76.
Heldal KK, Eduard W. Associations between acute symptoms and bioaerosol exposure during the collection of household waste. Am J Ind Med. 2004;46(3):253–60.
Hoopmann M, Hehl O, Neisel F, Werfel T. Associations between bioaerosols coming from livestock facilities and asthmatic symptoms in children. Gesundheitswesen. 2006;68(8–9):575–84.
Horick N, Weller E, Milton DK, Gold DR, Li R, Spiegelman D. Home Endotoxin exposure and wheeze in infants: correction for bias due to exposure measurement error. Environ Health Perspect. 2006;114(1):135–40.
Zock JP, Hollander A, Heederik D, Douwes J. Acute lung function changes and low endotoxin exposures in the potato processing industry. Am J Ind Med. 1998;33(4):384–91.
Mahar S. Worker Health in refuse-derived fuel plants, a five-year followup. Arh Hig Rada Toksikol. 2002;53(3):191–6.
Smit LAM, Spaan S, Heederik D. Endotoxin exposure and symptoms in wastewater treatment workers. Am J Ind Med. 2005;48(1):30–9.
Widmeier S, Bernard A, Tschopp A, Jeggli S, Dumont X, Hilfiker S, et al. Surfactant protein a, exposure to endotoxin, and asthma in garbage collectors and in wastewater workers. Inhal Toxicol. 2007;19(4):351–60.
Cyprowski M, Sobala W, Buczynska A, Szadkowska-Stanczyk I. Endotoxin exposure and changes in short-term pulmonary function among sewage workers. Int J Occup Med Environ Health. 2015;28(5):803–11.
Ghani N, Khalid A, Tahir A. Cross-sectional Study on the endotoxin exposure and lung function impairment in the workers of textile industry near Lahore, Pakistan. J Pak Med Assoc. 2016;66(7):803–14.
Rabinovitch N, Liu AH, Zhang L, Rodes CE, Foarde K, Dutton SJ, et al. Importance of the personal endotoxin cloud in school-age children with asthma. J Allergy Clin Immunol. 2005;116(5):1053–7.
Delfino RJ, Staimer N, Tjoa T, Gillen DL. Relations of exhaled nitric oxide and FEV1 to personal endotoxin exposure in schoolchildren with asthma. Occup Environ Med. 2015;72(12):830–6.
Lai PS, Sheehan WJ, Gaffin JM, Petty CR, Coull BA, Gold DR, et al. School endotoxin exposure and asthma morbidity in inner-city children. Chest. 2015;148(5):1251–8.
Kawamoto MM, Garabrant DH, Held J, Balmes JR, Patzman J, Dimick DV, et al. Respiratory effects of cotton dust exposure in the cotton garnetting industry. Am J Ind Med. 1987;11(5):505–15.
Dang B, Chen L, Mueller C, Dunn KH, Almaguer D, Roberts JL, et al. Ocular and respiratory symptoms among lifeguards at a hotel indoor waterpark resort. J Occup Environ Med. 2010;52(2):207–13.
Renstrom A, Olsson M, Hedren M, Johansson SGO, van Hage M. Pet shop workers: exposure, sensitization, and work-related symptoms. Allergy. 2011;66(8):1081–7.
Heldal KK, Madso L, Eduard W. Airway inflammation among compost workers exposed to actinomycetes spores. Ann Agric Environ Med. 2015;22(2):253–8.
Dahlqvist M, Johard U, Alexandersson R, Bergstrom B, Ekholm U, Eklund A, et al. Lung function and precipitating antibodies in low exposed wood trimmers in Sweden. Am J Ind Med. 1992;21(4):549–59.
Mandryk J, Alwis KU, Hocking AD. Work-related symptoms and dose-response relationships for personal exposures and pulmonary function among woodworkers. Am J Ind Med. 1999;35(5):481–90.
Sprince NL, Thorne PS, Popendorf W, Zwerling C, Miller ER, DeKoster JA. Respiratory symptoms and lung function abnormalities among machine operators in automobile production. Am J Ind Med. 1997;31(4):403–13.
Fransman W, McLean D, Douwes J, Demers PA, Leung V, Pearce N. Respiratory Symptoms and occupational exposures in New Zealand plywood mill workers. Ann Occup Hyg. 2003;47(4):287–95.
Meza F, Chen L, Hudson N. Investigation of respiratory and dermal symptoms associated with metal working fluids at an aircraft engine manufacturing facility. Am J Ind Med. 2013;56(12):1394–401.
Schlunssen V, Madsen AM, Skov S, Sigsgaard T. Does the use of biofuels affect respiratory health among male Danish energy plant workers? Occup Environ Med. 2011;68(7):467–73.
Shiryaeva O, Aasmoe L, Straume B, Olsen A-H, Ovrum A, Kramvik E, et al. Respiratory effects of bioaerosols: exposure-response study among salmon-processing workers. Am J Ind Med. 2014;57(3):276–85.
Wouters IM, Hilhorst SKM, Kleppe P, Doekes G, Douwes J, Peretz C, et al. Upper airway inflammation and respiratory symptoms in domestic waste collectors. Occup Environ Med. 2002;59(2):106–12.
Rusca S, Charriere N, Droz PO, Oppliger A. Effects of bioaerosol exposure on work-related symptoms among Swiss sawmill workers. Int Arch Occup Environ Health. 2008;81(4):415–21.
Ramagopal M, Wang Z, Black K, Hernandez M, Stambler AA, Emoekpere OH, et al. Improved exposure characterization with robotic (PIPER) sampling and association with children’s respiratory symptoms, asthma and eczema. J Expo Sci Environ Epidemiol. 2014;24(4):421–7.
Bose S, Rivera-Mariani F, Chen R, Williams D, Belli A, Aloe C, et al. Domestic exposure to endotoxin and respiratory morbidity in former smokers with COPD. Indoor Air. 2016;26(5):734–42.
Smit LAM, Hooiveld M, van der Sman-de Beer F, Opstal-van Winden AWJ, Beekhuizen J, Wouters IM, et al. Air pollution from livestock farms, and asthma, allergic rhinitis and COPD among neighbouring residents. Occup Environ Med. 2014;71(1):134–40.
Braun-Fahrländer C, Riedler J, Herz U, Eder W, Waser M, Grize L, et al. Environmental exposure to endotoxin and its relation to asthma in school-age children. N Engl J Med. 2002;347(12):869–77.
Stein MM, Hrusch CL, Gozdz J, Igartua C, Pivniouk V, Murray SE, et al. Innate immunity and asthma risk in Amish and Hutterite farm children. N Engl J Med. 2016;375(5):411–21.
Acknowledgements
Not applicable.
Funding
This study was funded by the Dutch Ministry of Infrastructure and the Environment (grant no.: DGAN-ANK / 16151555).
Availability of data and materials
All data generated or analysed during this study are included in this published article and its additional files.
Author information
Authors and Affiliations
Contributions
AF and LS made the search query. AF performed study selection, data extraction and analysis, quality assessment of the included studies and the best evidence synthesis. LS supervised the whole process and performed quality assessment of the included studies. All authors contributed to interpretation of the data, read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1:
Supplement 1. Pubmed search strategy. Supplement 2. Study incentives and other airborne exposures found to be associated with the outcome variables. Table S2. Study incentives and other airborne exposures found to be associated with the outcome variables. Supplement 3: Quality Assessment of included studies. Table S3. Quality Assessment of included studies based on the NIH Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. Supplement 4. Results tables Table S4.1. Overview of results – questionnaire outcomes among subjects exposed to bioaerosols. Table S4.2. Overview of results – spirometry outcomes among subjects exposed to bioaerosols. Table S4.3. Overview of results – exposure-response relationships between endotoxin exposure and respiratory outcomes. Table S4.4 Analysis of effects of endotoxin exposure on respiratory health in subgroups. Supplement 5: Best evidence synthesis. (DOCX 56 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Farokhi, A., Heederik, D. & Smit, L.A.M. Respiratory health effects of exposure to low levels of airborne endotoxin – a systematic review. Environ Health 17, 14 (2018). https://doi.org/10.1186/s12940-018-0360-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12940-018-0360-7