Abstract
Background
Type 2 diabetes mellitus (DM) is a risk factor for cardiovascular diseases and is common among patients undergoing coronary artery bypass grafting (CABG) surgery. The main objective of our study was to investigate the impact of DM type 2, and its treatment subgroups, on short- and long-term mortality in patients with acute coronary syndrome (ACS) who undergo CABG.
Methods
The study included 1307 patients enrolled from the biennial Acute Coronary Syndrome Israeli Survey between 2000 and 2016, who were hospitalized for ACS and underwent CABG. Of them, 527 (40%) patients were with and 780 (60%) were without DM.
Results
Compared with the non-diabetic group, the diabetic group of patients comprised more women and had more comorbidities such as hypertension, dyslipidemia, renal impairment, peripheral vascular disease and prior ischemic heart disease. Overall 30-day mortality rate was similar between DM and non-DM patients (4.2% vs. 4%, p = 0.976). Ten-year mortality rate was higher in DM compared with non-diabetic patients (26.6% vs. 17.7%, log-rank p < 0.001), and higher in the subgroup of insulin-treated patients compared to non-insulin treated patients (31.5% vs. 25.6%, log-rank p = 0.019). Multivariable analysis showed that DM increased the mortality hazard by 1.61-fold, and insulin treatment among the diabetic patients increased the mortality hazard by 1.57-fold.
Conclusions
While type 2 DM did not influence the in-hospital mortality hazard, we showed that the presence of DM among patients with ACS referred to CABG, is a powerful risk factor for long-term mortality, especially when insulin was included in the diabetic treatment strategy.
Similar content being viewed by others
Background
Currently, overall cardiovascular disease affects approximately 32.2% of all type 2 diabetes mellitus (DM) patients worldwide, while cardiovascular disease is a major cause of mortality among people with type 2 DM, accounting for approximately half of all deaths [1]. Diabetic patients presenting with acute coronary syndrome (ACS) have poor prognoses due to the diffuse and rapidly progressive forms of atherosclerosis and multiple comorbidities [2].
Previous studies have demonstrated increased short- and long-term mortality in diabetic patients undergoing coronary artery bypass grafting (CABG) or even isolated valve surgery [3] compared with non-diabetic patients [4, 5], and more recent reports have shown a significant reduction in mortality among patients with diabetes [6]. However, none of these studies were performed on ACS patients.
The main objective of our study was to investigate the impact of DM type 2, and its treatment subgroups, on short- and long-term mortality in patients with ACS who undergo CABG.
Methods
Study design
The ACS Israeli Survey (ACSIS) is a voluntary biennial prospective national registry of all patients with ACS hospitalized in the 25 coronary care units and cardiology departments in all the public health hospitals in Israel over a 2-month period (from March to April) [7].
ACSIS is managed by the Working Group on Acute Cardiovascular Care of the Israel Heart Society, in participation with the Israeli Center for Cardiovascular Research. Demographic, historical, and clinical data from all patients were recorded on pre-specified forms. Patient management was at the discretion of the attending physicians. Admission and discharge diagnoses were recorded as determined by the attending physicians based on clinical, electrocardiographic, and biochemical criteria.
Study population
All patients in each medical center signed an informed consent form prior to participating in the ACSIS registry, and each center received approval from its institutional review board [8]. Between 2000 and 2016 (which included 8 consecutive registries), 1307 patients were hospitalized with ACS and underwent CABG and were included in the ACSIS registry. Of them, 527 (40%) patients had DM and 780 (60%) were without DM.
Clinical outcomes
Clinical outcomes included 30-day major adverse cardiovascular events (MACE: which included death, MI, and stroke), in-hospital complications, and long-term all-cause mortality.
Data collection and follow-up
All data from the 25 participating hospitals were collected and pooled into a designated database. All centers used standardized definitions for data collection, including demographic parameters, medical history, chronic and peri-procedural medical treatment, echocardiography measurements, procedure information and outcome measures. All patients were prospectively followed up for clinical events at 30 days and for late mortality. Mortality data were ascertained from the Israeli Ministry of Interior Population Registry through January 2018.
Statistical analysis
Data are presented as mean ± standard deviation for normal, or median for abnormal distribution. Continuous variables were tested with the Kolmogorov–Smirnov test for normal distribution. Categorical variables are given as frequencies and percentages. A Chi square test was used for comparison of categorical variables between the groups, a Student t-test was performed for comparison of normally distributed continuous variables, and a Mann–Whitney U test for non-normal distribution.
Multivariable logistic regression analysis was used to identify factors associated with 30-day mortality. All statistically different variables (p < 0.1) in Table 1 and pre-specified variables were entered into the model. Long-term survival analysis was carried out using the Kaplan–Meier method, and comparison by the groups was tested using the log-rank test. Cox proportional hazard model was constructed to assess the association between DM and 10-year mortality adjusted to the following covariates: age, gender, hypertension, dyslipidemia, smoking, body mass index, renal impairment, prior myocardial infarction (MI), prior stroke, and congestive heart failure. Variables that were significant by the univariable analysis (p < 0.1) were included in the model. Results are presented as hazard ratio (HR), 95% confidence interval (CI) and p-value.
Statistical significance was assumed when the null hypothesis could be rejected at p < 0.05. All p-values reflect results of two-sided tests. Statistical analyses were conducted using R (version 3.4.1).
Results
Baseline characteristics
In our study cohort there were 780 non-diabetic patients, and 527 patients with DM type 2. Of them, 273 were treated with oral antihyperglycemic medications, 89 with insulin (with or without oral antihyperglycemic medications), and 165 with diet only. Presentation of the ACS was ST-segment elevation MI in 35%, non-ST-segment elevation MI in 45% and unstable angina pectoris in 20% (with no difference between DM and non-DM patients, p = 0.109). Compared with the non-diabetic group, the diabetic group of patients were more frequently women and had more comorbidities such as hypertension, dyslipidemia, renal impairment, peripheral vascular disease and prior ischemic heart disease (Tables 1 and 2). In addition to the antihyperglycemic medication, patients with DM were treated more frequently with platelet anti-aggregation therapy, angiotensin converting enzyme inhibitors or angiotensin II receptor blockers, calcium channel blockers, statins and diuretics (Table 1).
Early outcomes
Overall 30-day mortality rate was similar between the DM and non-DM patients (4.2% vs. 4%, p = 0.976), and between the subgroups of insulin-treated DM and non-insulin-treated DM (5.7% vs. 3.9%, p = 0.633). Other 30-day major events were similar between the DM and non-DM patients, such as stroke (0% vs. 0.3%, p = 0.658), recurrent MI (1.5% vs. 1.7%, p = 1.000) and MACE (p = 0.264). Major events were also similar between the non-insulin dependent and insulin-dependent DM patients: stroke (0% vs. 0%, p = 1.000), recurrent MI (0% vs. 1.8%, p = 0.415) and MACE (p = 0.615). These results were similarly consistent in the subgroups of the different ACS presentations and were reported as counts and crude event rates (Table 3).
Multivariable logistic regression analysis demonstrated that DM was not a predictor for death at 30-days after CABG (OR 0.98 95% CI 0.53–1.78, p = 0.955). The only significant variables that were associated with 30-day mortality rate were older age, male gender and dyslipidemia (Fig. 1).
Long-term mortality rate
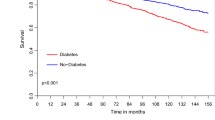
Kaplan–Meier survival analysis showed that mortality rates at 10 years of follow-up among patients with DM were significantly higher (26.6%) compared with those without DM who had ACS treated by CABG (17.7%; log-rank p-value < 0.001 for the overall difference during follow-up [Fig. 2a]). Consistent with the univariable findings, adjusted analysis, including a propensity score for the presence of DM, using a propensity score for confounders, also demonstrated a significantly higher risk for 10-year mortality among patients with, compared to those without DM (Fig. 2b). Additional independent predictors for long-term mortality among all study patients included: age > 65 years, male gender, hypertension, dyslipidemia and renal impairment (Table 4).
a Unadjusted 10-year survival curves by the presence of diabetes mellitus. b Hazard plot for survival at 10 years by the presence of diabetes mellitus, with propensity score adjustment. The covariates included in the model were: age, gender, hypertension, dyslipidemia, smoking, body mass index, renal impairment, prior MI, prior stroke and congestive heart failure. HR hazard ratio, MI myocardial infarction
Furthermore, long-term mortality was higher in the subgroup of insulin-treated patients compared to the subgroup of non-insulin treated patients with 10-year mortality rates of 31.5% vs. 25.6% (p = 0.019, Fig. 3). Interestingly, there were no significant differences in long-term mortality in DM patients treated with oral antihyperglycemic drugs or with diet only (Fig. 4).
Discussion
Our observational real-world study investigated the impact of type 2 DM on early- and long-term mortality in patients after ACS treated by CABG. First, we found that diabetic and non-diabetic patients, and insulin-dependent and non-insulin-dependent DM patients, had similar in-hospital outcomes. Second, our principal finding was that the long-term mortality rate of diabetic patients was higher than that of non-diabetic patients, and mortality was even higher when the diabetic treatment strategy included insulin.
Long-term mortality
While not performed on an ACS population, prior studies of DM versus non-DM in patients who underwent CABG have shown inconsistent results regarding long-term mortality. As in our results, Marui et al. reported an increase in 3- and 5-year mortality rates (11% vs. 9.7% and 19.6% vs. 16.2%, respectively) [9], and Koshizaka et al. reported significant differences in 5-year mortality rates (15.5% vs. 8.5%) in diabetic compared to non-diabetic patients [10]. Wit et al. reported significantly higher 3-year mortality rates in patients with insulin-treated compared with non-insulin-treated DM and non-diabetic patients (16.7% vs. 8.7% vs. 6.3%) [11]. A previous report of our group among all CABG patients showed a 5-year mortality rate of 15.3% among diabetic patients and a 9.3% rate among non-diabetic patients [5]. In contrast, Onuma et al. reported slightly increasing mortality rates in diabetic compared with non-diabetic patients 5 years after CABG: 8.6% vs. 7.1% [12], and Kappetein et al. reported non-significant differences between diabetic and non-diabetic patients at 5 years: 12.9% vs. 10.9% [13]. While in the general population DM is associated with excess mortality, compared with the general population without DM, with a hazard ratio of 1.15 at 5 years [14], we reported a greater impact of DM on patients who underwent CABG (HR of 1.44 at 5 years and 1.61 at 10 years). We assume that this higher impact of DM in our cohort, compared to the natural history of DM in the general population, is due to accelerated coronary artery disease (CAD) [15].
Operative mortality
We report here that in-hospital mortality among diabetic and non-diabetic patients after ACS was 4.2% and 4%, respectively. Furthermore, we have shown that DM was not associated with short-term cardiovascular events after CABG. Although patients with stable CAD were also studied, other comparisons between DM and non-DM patients who underwent CABG showed no difference in early outcomes. Abizaid et al. reported similar in-hospital mortality rates between diabetic and non-diabetic patients (2.1% vs. 1.2%) [16]. Marui et al. found no differences in 30-day mortality (0.9% vs. 1.2%) [9], as did Carson et al. (3.7% vs. 2.7%) [4]. Likewise, Li et al. reported similar post-CABG mortality rates for diabetic and non-diabetic patients (2% vs. 1.9%) [17]. Although diabetic patients in our series were older and had more comorbidities, differences in early mortality rates did not reach statistical significance.
Cardiovascular risk factors and ACS
The DM group in our study included patients who were treated either with insulin, oral antihyperglycemic drugs or with diet. Interestingly, not only overt diabetes, but also genetic predisposition to type 2 DM was significantly associated with a greater severity of coronary atheromatous burden in patients with ACS, independently of traditional risk factors [18]. There were substantial differences between the DM and non-DM groups in our cohort, with many of the unfavorable clinical characteristics (gender, comorbidities, and lower left ventricle ejection fraction) being more common in the DM group. We attempted to overcome some of the clinical differences through statistical adjustment of important variables.
We reported that the prevalence of women was higher in the DM group. The fact that diabetic women presented with more comorbidities is in keeping with recent findings showing that, in both percutaneous and surgical revascularization, women presented with worse outcomes at 1 year; albeit there were no gender differences at 5 years of follow-up [19]. Previous studies have shown that women were shown to have significantly smaller epicardial coronary arteries than men, even after adjustment for age, body habitus, and left ventricular mass [20, 21]. Consequently, diverse and gender-specific pathophysiological processes may contribute to different outcomes seen in women as compared to men.
While DM increases the risk of heart failure, mostly due to CAD, in some cases it is secondary to diabetic cardiomyopathy [22]. Insulin-dependent DM patients have more comorbidities than non-insulin dependent patients, as reported in this study. Although the presence of insulin treatment is indeed a marker for more advanced disease, its underlying biological mechanism has not been fully elucidated. It may be related to the impact of a procoagulant imbalance, chronic exposure to high glucose levels, and direct effects of hyperinsulinemia. Interestingly, endogenous hyperinsulinemia has been associated with increased long-term mortality following MI in patients without diabetes [23]. Further studies are required to examine whether insulin-dependent diabetic patients should be included in risk stratification algorithms for patients who undergo CABG, and also whether they require more intense cardiovascular protective therapies with the newly available anti-diabetic drugs.
Limitations
A selection bias could have been introduced by the fact that, while primarily the ACSIS registry included patients admitted only to cardiology wards and intensive cardiac care units nationwide, in the main it did not include patients hospitalized in internal medicine wards. There was insufficient anatomical information regarding the complexity of CAD, the specific artery involved, and the surgical techniques performed. Therefore, it is difficult to draw conclusions regarding the association between specific interventions in native arteries or grafts and clinical outcomes. Information regarding patients treated with insulin analogs compared with human insulin was lacking in the ACSIS registry, and therefore we could not draw any conclusions regarding specific treatment that could improve cardiovascular morbidity in insulin-dependent DM patients.
Conclusions and clinical implications
We have shown that the presence of DM among patients with ACS who are referred to CABG is a powerful risk factor for long-term mortality, especially if the diabetic treatment strategy includes insulin. Accordingly, the high-risk population of insulin-dependent DM may require specific and/or more intense cardiovascular protective therapies after CABG. Further studies are needed to examine whether novel interventions, such as GLP-1 analogs or SGLT2 inhibitors, could improve the long-term outcomes of these patients.
Availability of data and materials
Data collected from the ACSIS national registry.
Abbreviations
- DM:
-
Diabetes mellitus
- CAD:
-
Coronary artery disease
- ACS:
-
Acute coronary syndrome
- CABG:
-
Coronary artery bypass grafting
- ACSIS:
-
The Acute Coronary Syndrome Israeli Survey
- MACE:
-
Major adverse cardiovascular events
- MI:
-
Myocardial infarction
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
References
Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc Diabetol. 2018;17(1):83.
Amsterdam EA, Wenger NK, Brindis RG, Casey DE Jr, Ganiats TG, Holmes DR Jr, Jaffe AS, Jneid H, Kelly RF, Kontos MC, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130(25):2354–94.
Ram E, Kogan A, Levin S, Fisman EZ, Tenenbaum A, Raanani E, Sternik L. Type 2 diabetes mellitus increases long-term mortality risk after isolated surgical aortic valve replacement. Cardiovasc Diabetol. 2019;18(1):31.
Carson JL, Scholz PM, Chen AY, Peterson ED, Gold J, Schneider SH. Diabetes mellitus increases short-term mortality and morbidity in patients undergoing coronary artery bypass graft surgery. J Am Coll Cardiol. 2002;40(3):418–23.
Kogan A, Ram E, Levin S, Fisman EZ, Tenenbaum A, Raanani E, Sternik L. Impact of type 2 diabetes mellitus on short- and long-term mortality after coronary artery bypass surgery. Cardiovasc Diabetol. 2018;17(1):151.
Rawshani A, Rawshani A, Franzen S, Sattar N, Eliasson B, Svensson AM, Zethelius B, Miftaraj M, McGuire DK, Rosengren A, et al. Risk factors, mortality, and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2018;379(7):633–44.
Behar S, Battler A, Porath A, Leor J, Grossman E, Hasin Y, Mittelman M, Feigenberg Z, Rahima-Maoz C, Green M, et al. A prospective national survey of management and clinical outcome of acute myocardial infarction in Israel, 2000. Isr Med Assoc J. 2003;5(4):249–54.
Kornowski R. The ACSIS Registry and primary angioplasty following coronary bypass surgery. Catheter Cardiovas Interv. 2011;78(4):537–9.
Marui A, Kimura T, Nishiwaki N, Mitsudo K, Komiya T, Hanyu M, Shiomi H, Tanaka S, Sakata R. Investigators CR-KPCRC-: five-year outcomes of percutaneous versus surgical coronary revascularization in patients with diabetes mellitus (from the CREDO-Kyoto PCI/CABG Registry Cohort-2). Am J Cardiol. 2015;115(8):1063–72.
Koshizaka M, Lopes RD, Reyes EM, Gibson CM, Schulte PJ, Hafley GE, Hernandez AF, Green JB, Kouchoukos NT, Califf RM, et al. Long-term clinical and angiographic outcomes in patients with diabetes undergoing coronary artery bypass graft surgery: results from the Project of Ex-vivo Vein Graft Engineering via Transfection IV trial. Am Heart J. 2015;169(1):175–84.
Wit MA, de Mulder M, Jansen EK, Umans VA. Diabetes mellitus and its impact on long-term outcomes after coronary artery bypass graft surgery. Acta Diabetol. 2013;50(2):123–8.
Onuma Y, Wykrzykowska JJ, Garg S, Vranckx P, Serruys PW, Arts I, Investigators II. 5-Year follow-up of coronary revascularization in diabetic patients with multivessel coronary artery disease: insights from ARTS (arterial revascularization therapy study)-II and ARTS-I trials. JACC Cardiovasc Interv. 2011;4(3):317–23.
Kappetein AP, Head SJ, Morice MC, Banning AP, Serruys PW, Mohr FW, Dawkins KD, Mack MJ, Investigators S. Treatment of complex coronary artery disease in patients with diabetes: 5-year results comparing outcomes of bypass surgery and percutaneous coronary intervention in the SYNTAX trial. Eur J Cardio Thorac Surg. 2013;43(5):1006–13.
Tancredi M, Rosengren A, Svensson AM, Kosiborod M, Pivodic A, Gudbjornsdottir S, Wedel H, Clements M, Dahlqvist S, Lind M. Excess mortality among persons with type 2 diabetes. N Engl J Med. 2015;373(18):1720–32.
Lathief S, Inzucchi SE. Approach to diabetes management in patients with CVD. Trends Cardiovasc Med. 2016;26(2):165–79.
Abizaid A, Costa MA, Centemero M, Abizaid AS, Legrand VM, Limet RV, Schuler G, Mohr FW, Lindeboom W, Sousa AG, et al. Clinical and economic impact of diabetes mellitus on percutaneous and surgical treatment of multivessel coronary disease patients: insights from the Arterial Revascularization Therapy Study (ARTS) trial. Circulation. 2001;104(5):533–8.
Li Z, Amsterdam EA, Young JN, Hoegh H, Armstrong EJ. Contemporary outcomes of coronary artery bypass grafting among patients with insulin-treated and non-insulin-treated diabetes. Ann Thorac Surg. 2015;100(6):2262–9.
Zheng Q, Jiang J, Huo Y, Chen D. Genetic predisposition to type 2 diabetes is associated with severity of coronary artery disease in patients with acute coronary syndromes. Cardiovasc Diabetol. 2019;18(1):131.
Huckaby LV, Seese LM, Sultan I, Gleason TG, Wang Y, Thoma F, Kilic A. The impact of sex on outcomes after revascularization for multivessel coronary disease. Ann Thorac Surg. 2020. https://doi.org/10.1016/j.athoracsur.2020.02.026.
Hiteshi AK, Li D, Gao Y, Chen A, Flores F, Mao SS, Budoff MJ. Gender differences in coronary artery diameter are not related to body habitus or left ventricular mass. Clin Cardiol. 2014;37(10):605–9.
Murthy VL, Naya M, Taqueti VR, Foster CR, Gaber M, Hainer J, Dorbala S, Blankstein R, Rimoldi O, Camici PG, et al. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation. 2014;129(24):2518–27.
Meagher P, Adam M, Civitarese R, Bugyei-Twum A, Connelly KA. Heart failure with preserved ejection fraction in diabetes: mechanisms and management. Can J Cardiol. 2018;34(5):632–43.
Kragelund C, Snorgaard O, Kober L, Bengtsson B, Ottesen M, Hojberg S, Olesen C, Kjaergaard JJ, Carlsen J, Torp-Petersen C, et al. Hyperinsulinaemia is associated with increased long-term mortality following acute myocardial infarction in non-diabetic patients. Eur Heart J. 2004;25(21):1891–7.
Acknowledgements
Not applicable.
Funding
None.
Author information
Authors and Affiliations
Contributions
ER: wrote the research project and the full manuscript; LS: study revision and interpretation. RK, ZI: data collection and interpretation; EZF, AT, EZ: study revision and editing; YP: study revision and interpretation; ER: wrote the research project and study revision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All patients in each medical center signed an informed consent form prior to participating in the ACSIS registry, and each center received approval from its institutional review board.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ram, E., Sternik, L., Klempfner, R. et al. Type 2 diabetes mellitus increases the mortality risk after acute coronary syndrome treated with coronary artery bypass surgery. Cardiovasc Diabetol 19, 86 (2020). https://doi.org/10.1186/s12933-020-01069-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-020-01069-6