Abstract
Background
Little is known about factors that affect the composition of contracted blood clots in specific diseases. We investigated the content of polyhedral erythrocytes (polyhedrocytes) formed in blood clots and its determinants in type 2 diabetes (T2D) patients.
Methods
In 97 patients with long-standing T2D [median HbA1c, 6.4% (interquartile range 5.9–7.8)], we measured in vitro the composition of blood clots, including a clot area covered by polyhedrocytes using scanning electron microscopy and the erythrocyte compression index (ECI), defined as a ratio of the mean polyhedrocyte area to the mean native erythrocyte area. Moreover, plasma fibrin clot permeability (Ks), clot lysis time (CLT), thrombin generation, oxidative stress [total protein carbonyl (total PC), total antioxidant capacity and thiobarbituric acid reactive substances (TBARS)], and platelet activation markers were determined. The impact of glucose concentration on polyhedrocytes formation was assessed in vitro.
Results
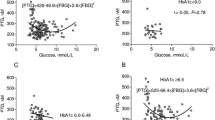
Polyhedrocytes content in contracted clots was positively correlated with glucose (r = 0.24, p = 0.028), glycated hemoglobin (r = 0.40, p = 0.024), total cholesterol (r = 0.22, p = 0.044), TBARS (r = 0.60, p = 0.0027), P-selectin (r = 0.54, p = 0.0078) and platelet factor-4, PF4 (r = 0.59, p = 0.0032), but not with thrombin generation, platelet count, Ks or CLT. Patients who formed more polyhedrocytes (≥ 10th percentile) (n = 83, 85.6%) had higher glucose (+ 15.7%, p = 0.018), fibrinogen (+ 16.6%, p = 0.004), lower red blood cell distribution width (RDW, − 8.8%, p = 0.034), reduced plasma clot density (− 21.8% Ks, p = 0.011) and impaired fibrinolysis (+ 6.5% CLT, p = 0.037) when compared to patients with lesser amount of polyhedrocytes (< 10th percentile). ECI and the content of polyhedrocytes were strongly associated with total PC (r = 0.79, p = 0.036 and r = 0.67, p = 0.0004, respectively). In vitro an increase of glucose concentration by 10 mmol/L was associated with 94% higher polyhedrocytes content (p = 0.033) when compared to the baseline (7.1 mM). After adjustment for age, sex and fibrinogen, multiple regression analysis showed that RDW was the only independent predictor of polyhedrocytes content in T2D (OR = 0.61, 95% CI 0.39–0.92).
Conclusions
Poor glycemic control, together with enhanced platelet activation and oxidative stress, increase the content of polyhedrocytes in blood clots generated in T2D patients.
Similar content being viewed by others
Introduction
Type 2 diabetes (T2D) is associated with a hypercoagulable state involving increased platelet activation, thrombin generation and unfavorable fibrin clot properties [1,2,3,4]. T2D patients are at high risk of arterial thromboembolism [1, 2, 5] and associated mortality [6,7,8,9]. T2D patients present significant changes in the morphology of their erythrocytes and in the nature of the fibrin formed upon the addition of thrombin [10]. Platelet count is usually within the reference range, however platelet activity is increased [11] as reflected among others by elevated platelet factor 4 or P-selectin in T2D patients [12,13,14]. Moreover, platelet contractile force has been shown to be increased in T2D patients [15]. During clot formation platelets activated by thrombin form a platelet–fibrin mesh constituting the basic structure of thrombus in vivo or whole blood clot in vitro [16]. Such clots undergo volume shrinkage [17] that is called clot contraction or retraction [18]. Cellular blood components, in particular red blood cells (RBCs), platelets, and plasma fibrinogen concentration influence the rate and extent of clot contraction [18]. It has been postulated that RBCs enhance functional coagulation properties and platelet aggregation. RBC aggregation and decreased deformability are the dominant hematological abnormalities in T2D subjects and may lead to the development of microvascular complications [19]. Reduced clot contraction has been demonstrated in subjects with a lower platelet count and/or dysfunction, elevated hematocrit, leukocytosis, increased plasma fibrinogen, and other changes in blood composition that may affect platelet function and properties of blood clots [20] [21]. It has been suggested that defective clot retraction contributes to arterial and venous thrombosis [22]. Cines et al. have demonstrated that contracted blood clots and arterial thrombi developed a tessellated structure of polyhedral erythrocytes, called polyhedrocytes [18]. Polyhedrocytes were observed in the interior of the blood clot, where the RBCs were tightly compressed, with meshwork of fibrin and platelets on its surface [18, 23]. It has been postulated that polyhedrocytes provide impermeable seal, due to minimal interstitial space, which stems bleeding and promotes fibrinolysis resistance [18, 23]. We have reported that polyhedrocytes are observed in 20% of thrombi obtained from epicardial arteries of ST-elevation myocardial infarction patients [18, 23, 24]. Formation of polyhedrocytes in arterial thrombi has been associated with higher RBC count and both lower platelet count and plasma fibrinogen, but not with cardiovascular risk factors or the ischemia time [25].
To our knowledge, there have been no reports on determinants of polyhedrocyte formation in clots of patients with T2D. RBCs are extremely sensitive to oxidative stress or cytokine upregulation. This usually accompanies systemic inflammation in most diseases, including T2D [26]. We hypothesized that poor metabolic control, along with prothrombotic plasma clot phenotype, enhanced platelet activation and oxidative stress observed in T2D patients with high cardiovascular risk, are associated with higher content of polyhedrocytes.
Research design and methods
We enrolled 97 consecutive T2D patients with high cardiovascular risk hospitalized from August 2015 to April 2016 in John Paul II Hospital in Krakow, Poland and 30 apparently healthy controls. All patients fulfilled the American Diabetes Association criteria for diagnosis of T2D and were treated for at least 12 months. The exclusion criteria were arterial or venous thromboembolic events within previous 6 months, current anticoagulant therapy, known cancer, signs of acute infection, chronic inflammatory disorders (e.g. rheumatoid arthritis), liver injury (alanine or asparagine transaminase > 1.5 times above the upper limit of the reference range), estimated glomerular filtration rate (eGFR) < 30 mL/min, and pregnancy. Current smoking was defined as smoking at least one cigarette daily. Arterial hypertension was defined as a systolic and/or diastolic blood pressure of ≥ 140 mmHg or ≥ 90 mmHg, respectively or a history of arterial hypertension with or without taking antihypertensive agents. Coronary artery disease (CAD) was established based on a documented history of myocardial infarction (MI) or a positive result of ECG stress test or gated single photon emission computed tomography with Tc-99 m-MIBI (SPECT) or a coronary angiography. Previous MI was established based on medical records.
The Jagiellonian University Ethical Committee approved the study and all the participants provided their written informed consent.
Routine laboratory investigations
Fasting venous blood was drawn between 7 and 10 A.M. and was kept at a room temperature. Blood samples were collected into citrated tubes (9:1 of 0.106 M sodium citrate), centrifuged at 2500g and 20 °C for 10 min to obtain platelet poor plasma (PPP), snap-frozen within 60 min, and stored in small aliquots at − 80 °C until analysis. The activated partial thromboplastin time (aPTT), creatinine, eGFR, serum total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglycerides (TG), glucose and thyroid stimulating hormone (TSH) were assayed by routine laboratory techniques. Glycated hemoglobin A1c (HbA1c) was measured using immunoturbidimetry (Roche Diagnostics GmbH, Mannheim, Germany). Complete blood count including white blood cells (WBC), RBC, hemoglobin, hematocrit, red blood cell distribution width (RDW), platelet count and platelet distribution width were assayed. Fibrinogen was assessed using the Clauss method. The hsCRP was determined using immunoturbidimetry (Roche Diagnostics GmbH, Mannheim, Germany). Immunoenzymatic assay was used to determine plasminogen activator inhibitor-1 (PAI-1) antigen (American Diagnostica, Stamford, CT, USA) in citrated plasma. Plasma α2-antiplasmin (α2AP) and plasminogen were measured by chromogenic assays (STA Stachrom antiplasmin and STA Stachrom plasminogen, Diagnostica Stago, Asnieres, France). Markers of platelet activation, P-selectin (CD62P) and platelet factor-4 (PF4), were determined in citrated plasma by ELISAs (R&D Systems, Abington, UK). The interassay and intraassay coefficients of variation for all the ELISAs were < 8%.
Preparation of whole blood clots
Clotting was initiated by addition of 2 µL of activation mixture (CaCl2 [Sigma-Aldrich, St. Louis, MO, US] and human thrombin [Merck KGaA, Darmstadt, Germany] at final concentrations of 0.01 M and 1 U/mL, respectively) to 48 μL of whole blood from the antecubital vein that was prewarmed for 5 min at 37 °C. The samples were incubated at room temperature for 24 h.
Scanning electron microscopy
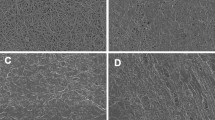
Scanning electron microscope (SEM) analysis was performed as previously described [27]. Blood clots were washed in 0.1 M NaCl for several minutes and fixed in 2.5% glutaraldehyde, dehydrated in raised ethanol concentrations and frozen in tert-Butyl alcohol for 2 h. Then, clots were dried in a vacuum and coated with gold. High-definition photographs were acquired using a scanning electron microscope (JEOL JCM-6000, Japan). We performed analysis of polyhedrocytes content and size measurement in 40 selected areas located in 3 vertical axes (from the left to the right and from the top to the bottom of the clot) with a subjective evaluation of the area covered by RBC, polyhedrocytes, or their transitional forms (Fig. 1).
A representative SEM images of a retracted whole blood clot (magnifications ×30 and ×3600) used for semiquantitative analysis of polyhedrocytes content and size measurement in 40 selected areas located in 3 vertical axes (from the left to the right and from the top to the bottom of the clot). a and b clot surface area containing fibrin cup and native red blood cells (RBCs) and eryptotic cells (marked with arrows), c and d transitional clot area composed mostly of transitional forms of polyhedrocytes and fibrin fibers, e and f clot internal area mainly composed of polyhedrocytes and small amounts of fibrin located on the edges of polyhedral RBCs
For the semiquantitative analysis, each picture was divided into 400 squares and the dominant composition (platelets, fibrin, erythrocytes, WBC) of each was assayed (Fig. 1). The visual approach consisted of a careful observation of each pictures with a subjective evaluation of the percentage of each component. Two independent investigators performed visual approach. Very low interindividual variability was found in the clot composition analysis (< 7%). The investigators performing SEM and analysis were blinded to the clinical data. Images for each clot were then assessed using ImageJ (US National Institutes of Health) to determine the area (µm2) of native RBCs and polyhedrocytes. Results were presented as means ± standard deviations (SD) of about 100 consecutive RBC areas obtained from each SEM image.
Erythrocyte compression index
Erythrocyte compression index (ECI) was determined as previously described [28]. Briefly, ECI is defined as a ratio of the mean polyhedrocyte area to the mean native RBC area expressed as a percentage. Two investigators evaluated the first 10 clots with the inter-observer and intra-observer agreement of 93% and 95%, respectively.
Thrombin generation potential
Plasma thrombogenic potential was assessed using calibrated automated thrombography (CAT) (Thrombinoscope BV, Maastricht, the Netherlands) in a 96-well plate fluorometer (Ascent Reader, Thermolab systems OY, Helsinki, Finland) at 37 °C according to the manufacturer’s instructions. Eighty microliters of platelet poor plasma were diluted with 20 µL of a commercially available tissue factor (TF)-based activator (Diagnostica Stago, Asniéres, France) containing 5 pM recombinant TF, 4 micromolar phosphatidylserine/phosphatidylcholine/phosphatidylethanolamine vesicles, and 20 µL of FluCa solution (Hepes, pH 7.35, 100 nmol/L CaCl2, 60 mg/mL bovine albumin, and 2.5 mmol/L Z-Gly-Gly-Arg-amidometylcoumarin). The reference ranges of thrombin generation potential were as follows: Lag time, 1.57 ± 0.33 min; ETP, 1694 ± 373 nM × min; Peak thrombin generation, 360 ± 108 nM; ttPeak, 3.96 ± 0.71 min [29].
Fibrin clot permeability and lysis
Fibrin clot permeability was determined using a pressure-driven system [30]. Briefly, 20 mM calcium chloride and 1 U/mL human thrombin (Merck KGaA) were added to 120 µL citrated plasma. A permeation coefficient (Ks), which indicates the pore size, was calculated from the equation:
where Q is the flow rate in time t; L, the length of a fibrin gel; η, the viscosity of liquid (in poise); A, the cross-sectional area (in cm2), and Δp, a differential pressure (in dyne/cm2).
To assess efficiency of clot lysis, citrated plasma was mixed with 15 mM calcium chloride, 6 pM human tissue factor (Innovin, Siemens), 12 μM phospholipid vesicles and 60 ng/mL recombinant tPA (rtPA, Boehringer Ingelheim, Germany). The mixture was transferred to a microtiter plate and its turbidity was measured at 405 nm at 37 °C. Clot lysis time (CLT) was defined as the time from the midpoint of the clear-to-maximum-turbid transition, which represents clot formation, to the midpoint of the maximum-turbid-to-clear transition (representing the lysis of the clot). The reference ranges of fibrin clot permeability and lysis were as follows: Ks, 7.01 ± 2.06 × 10−9cm2; CLT, 100 ± 9.7 min [29, 31].
Total protein carbonyl (total PC) assessment
Oxidative modification of plasma proteins was assessed based on carbonyl content using 2–4 dinitrophenylhydrazine (DNPH), as reported by Becatti et al. [32]. DNPH reacts with PC, forming a Schiff base to produce the corresponding hydrazone, which can be analyzed spectrophotometrically. Briefly, citrated PPP (100 µL) after incubation with DNPH (400 µL) was precipitated with trichloracetic acid (TCA) and the pellet washed several times with a 1:1 mixture of ethanol/ethyl acetate. Finally, the pellet was resuspended in 500 µL guanidine hydrochloride and measured at 370 nm. PC content was calculated by using a molar extinction coefficient of 22,000 M−1 cm−1. The results, expressed as nM/mL of PC, were then normalized for protein concentration. The reference range of total PC was 1.82 ± 0.2 nmol/mg [33].
TBARS (thiobarbituric acid reactive substances) estimation
Thiobarbituric acid reactive substances levels were measured in citrated PPP using a TBARS assay kit (OXI-TEK, ENZO, USA) in accordance with the manufacturer’s instructions as previously reported [32]. Briefly, the adduct generated by reacting malondialdehyde with thiobarbituric acid after 1 h at 95 °C was measured spectrofluorimetrically, with excitation at 530 nm and emission at 550 nm. TBARS were expressed in terms of malondialdehyde equivalent (nM/mL) and then normalized for protein concentration. The reference range of TBARS was: 18.6 ± 1.16 nmol/mL [33].
Total antioxidant capacity (TAC) assay
The ORAC method (oxygen radical absorbance capacity), based on the inhibition of the peroxyl-radical-induced oxidation initiated by thermal decomposition of azo-compounds, like 2,2icazobis(2-amidinopropane) dihydrochloride (AAPH), was performed as reported by Becatti et al. [32]. Briefly, a fluorescein solution (6 nM) prepared daily from a 4 µM stock in 75 mM sodium phosphate buffer (pH 7.4), was used. Trolox (250 μM final concentration) was used as a standard. 70 μL of citrated PPP with 100 μL of fluorescein were pre-incubated for 30 min at 37 °C in each well, before rapidly adding AAPH solution (19 mM final concentration). Fluorescence was measured with excitation at 485 nm and emission at 537 nm in a Fluoroskan Ascent Microplate Fluorometer (Thermo Fisher Scientific Inc. MA, USA). Results were expressed as Trolox Equivalents (μM) and then normalized for protein concentration. The reference range of TAC assay was: 378.86 ± 20.82 nmol/mL [33].
The oxidation parameters, platelet markers, and fibrinolytic proteins were measured in a subset of patients (n = 23).
An influence of exogenous glucose on polyhedrocyte formation
To assess the direct influence of glucose concentration on polyhedrocytes formation, we added 10 mM glucose solution in 0.9% sodium chloride to blood obtained from randomly selected 10 healthy (5 men and 5 women aged 39–60 [median, 47] years; mean glucose level of 4.9 [IQR: 4.6–5.2] mM) and 7 randomly selected T2D subjects (3 men and 4 women aged 31–76 [median, 74] years; mean glucose level of 7.1 [IQR: 6.6–7.9] mM). The samples were incubated at 37 °C for 1 h. Then, the whole blood clots were prepared in duplicate and analyzed as described above by two independent investigators blinded to the clinical data.
Statistical analysis
Continuous variables were presented as mean and standard deviation or median with the first and the third quartile depending on data distribution assessed using Shapiro–Wilk test and compared using Student’s t test or Wilcoxon test. Nominal variables were described using counts and percentages and compared using χ2 test of Fisher’s Exact Test. Pearson’s or/and Spearman’s correlations were calculated when applicable for measuring the association between two continuous variables. The change in analyzed variables due to the increase in polyhedrocytes by 1% was assessed using linear regression. Several logistic regression models with production of polyhedrocytes as a dependent variable (production occurred or not occurred). The best model was then obtained stepwise backwards minimalizing Bayesian Information Criterion (BIC) with variables gender, age and level of fibrinogen locked in the model for adjustment. Association between the variables was expressed as odds ratio (OR) with corresponding 95% confidence intervals (CI). A p-value < 0.05 was considered statistically significant.
Results
Patient characteristics
The final analysis included 97 patients with a well-controlled (median HbA1c, 6.4%), long-standing (median duration, 9.3 years) T2D (Table 1) and 30 age-matched healthy controls (64.39 ± 10.12 years) not taking any medications. A majority of patients were at high risk of CAD and were treated as recommended in the current guidelines, including aspirin (Table 1).
Polyhedrocytes formation
We divided the study group based on the percentage of the polyhedrocytes content on the surface of contracted blood clots. Patients in the lowest polyhedrocyte group (below 10th percentile of the observations, n = 14) were considered as a low-polyhedrocytes group (LP), in contrast to the rest of the patients (latter 90% of observations, n = 83) who were considered as a high-polyhedrocytes group (HP). Median percentage of polyhedrocytes in blood clots in the HP group was 20% (interquartile range, IQR, 7.7–32.5%) and it was 51% lower than in healthy controls (41 [30–61.5]%, p < 0.01). There were no differences between the HP and LP groups in demographic and clinical characteristics including the medications used (Table 1). The HP group had higher glucose and fibrinogen levels (+ 15.7%, p = 0.018; + 16.6%, p = 0.004 respectively) and lower RDW (− 8.8%, p = 0.034) with no difference in leukocyte or platelet count and lipid profiles (Table 2), when compared with the LP subjects. Additionally, the HP group showed lower Ks by 21.8% (p = 0.011) and increased CLT by 6.5% (p = 0.037) when compared with the remainder (Table 3).
In the whole group polyhedrocytes content was positively correlated with glucose and TC levels (r = 0.24, p = 0.028 and r = 0.22, p = 0.044, respectively), but not with other lipid parameters. Compared with patients with glucose < 6 mmol/L, those with higher glycemia were more prone to form greater amounts of polyhedrocytes (OR = 4.81, 95% CI 1.49–16.40, p = 0.009).
Platelet markers, fibrinolytic proteins and oxidation parameters are presented in Additional file 1: Table S1. The content of polyhedrocytes was positively correlated with P-selectin (r = 0.54, p = 0.0078) and PF4 (r = 0.59, p = 0.0032), but not with platelet count, which was within the reference range for all patients. Of the oxidative stress markers, the content of polyhedrocytes was strongly associated with total PC (r = 0.67, p = 0.0004) and TBARS (r = 0.60, p = 0.0027).
Compression of erythrocytes
In the HP patients the median of the new parameter describing compression of erythrocytes, ECI was 59.4 (53.7–69.6)%. There were no associations between ECI and demographic or clinical parameters in those patients. ECI was strongly associated with total PC (r = 0.79, p = 0.036) and with fibrinogen level (r = 0.29, p = 0.030).
Clot composition
The detailed analysis of the blood clot composition (n = 40) showed that their major components were native erythrocytes (32.6 ± 16.6%), transitional erythrocytes (28 ± 12.6%), and fibrin (24.5 ± 9.4%). Polyhedrocytes accounted for nearly 8% and platelets for 1% of clot composition. Interestingly, the percentage of polyhedrocytes was positively associated with HbA1c (r = 0.40, p = 0.024) (Fig. 1).
The influence of exogenous glucose on polyhedrocyte formation
The in vitro increase of glucose concentration from 4.9 to 14.9 mmol/L in blood obtained from healthy subjects was associated with higher content of polyhedrocytes (36 [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44] % to 47 [43–55]%; p = 0.044). Similarly in T2D subjects the increase of blood glucose concentration from 7.1 to 17.1 mmol/L was associated with increased content of polyhedrocytes (from 17 [14.5–24.2]% to 33 [25–41.9]%; p = 0.033).
Predictors of the HP content
After adjustment for age, sex and fibrinogen, multiple logistic regression analysis showed that increasing RDW was the only negative independent predictor of polyhedrocytes content in clots (OR = 0.61, 95% CI 0.39–0.92). Simple linear regression models showed that 1% increase in percentage of polyhedrocytes was significantly associated with the increase in TBARS (by 0.4%), total PC (by 0.5%), P-selectin (by 0.5%), and PF4 (by 0.3%) (Table 4).
Discussion
The present study identifies several factors that affect formation of polyhedrocytes in patients with T2D and high cardiovascular risk. Our study is the first to demonstrate that both the glycaemia and HbA1c were associated with increased polyhedrocytes formation in T2D patients, which was confirmed by in vitro experiment. Moreover, increased platelet activation, reflected by plasma concentrations of P-selectin and PF4, and protein oxidation, in particular total PC, were the major determinants of polyhedrocytes formation in blood clots of T2D patients. Our findings indicate that regulation of polyhedrocyte formation in clots is complex and dependent on factors other than RBC, platelet count and fibrinogen concentration.
Moreover, the link between the extent of oxidative stress and polyhedrocytes formation in T2D is also a novel observation. We found that compression of the erythrocytes measured as a new parameter called ECI in patients with T2D was strongly positively associated with protein oxidation. Therefore, we may speculate that disturbed clot contraction associated with oxidative stress in patients with a long-standing history of T2D and high cardiovascular risk could be one of the mechanisms behind increased atherothrombotic complication in those patients. Recently ECI was investigated in patients with venous thromboembolism (VTE) [28]. In contrast to VTE patients in whom ECI was related to age, BMI, platelet count and RDW [28] no such relationships were observed in our patients with T2D. Similarly to previous observations in patients with stroke and VTE clot contraction in T2D was associated with fibrinogen level [28, 34]. It has been shown, that compression of erythrocytes was preferably associated with platelet/fibrinogen ratio rather than the platelet or fibrinogen alone [18, 28, 35]. This may indicate the importance of major platelets effect, which can be modulated by the fibrin, and its influence on the clot contraction [18, 23].
RBCs are especially sensitive cells, therefore they are important health indicators [26]. Assessment of RBCs in terms of biophysical and shape changes such as eryptosis, may be helpful in the evaluation of hematological changes associated with inflammatory status or disease progression [26, 36,37,38]. We have observed eryptotic cells within clots made from the whole blood obtained from T2D patients (Fig. 1a, marked with arrows).
The clot’s ability to contract is a feature of each patient and could be modulated by many transient factors [21]. The major players in this process are platelets and fibrin [34]. During clot contraction, fibrinogen and fibrin fibers are required as the substrate upon which the aggregating platelets pull, and the fibrin(ogen) connecting the platelets binds to the platelet integrin αIIbβ3. Clot contraction driven by platelet contractile proteins, particularly by the actin-myosin interactions is crucial especially in the first phase of clot contraction [23, 39]. Through keeping a local tension to reestablish the fibrin-fibrin network, the platelet/fibrin ratio was found to play an important role in clot contractility [23]. Furthermore, clot retraction requires energy which is derived from glucose metabolism by platelets and in the absence of glucose, retraction will not occur [40]. In our study, we demonstrated that platelet-derived proteins, i.e. P-selectin and PF4 released from alpha granules upon activation were associated with the increased content of polyhedrocytes in blood clots. This is a novel observation. Growing evidence suggests that oxidative stress can affect platelet reactivity and intensify platelet aggregation process [41]. Therefore, it seems that the oxidative stress can also affect fibrin clot contraction by affecting the platelet reactivity. Nevertheless, platelets in T2D patients are displaying an ‘angry’ behavior. The lysosomal granules might have a significant role in T2D followed by cardiovascular complications [42]. Platelet surface receptors and platelet activation were found to be elevated in T2D, showing increased procoagulant activity and hyperreactivity [42, 43]. These coagulopathies are accompanied by augmented LPS-binding protein (LBP) levels [44].
Tutwiler et al. reported that higher hematocrit along with an increased RBC rigidity were associated with limited clot contractility [23]. In our study, T2D patients who formed more polyhedrocytes within blood clots had similar RBC and hematocrit, but lower RDW. Moreover after the adjustments, lower RDW was the only independent predictor of greater polyhedrocytes formation. It is possible that erythrocytes of similar size are more likely to form polyhedrocytes, while different sizes of RBCs hinder this process. Thus, increased RDW would result in poorer ability of erythrocytes to provide an impermeable seal, due to minimal interstitial space, to increase fibrinolysis resistance. Furthermore, the inflammatory process might play an important role in increased RDW values by releasing the immature RBCs which may contribute to the impaired clot contraction [45]. This issue requires further investigation.
We have shown positive association of polyhedrocytes formation with worse glycemia control. Moreover, in the vitro experiment we demonstrated, that higher glucose concentration is associated with higher polyhedrocyte content in blood clots. Erythrocytes of patients with T2D exhibit impaired deformability related to increased HbA1c and accumulation of intracellular sorbitol or to the stiffness of red blood cell membranes probably due to oxidative modification of the proteins and to an imbalance of the cholesterol/phospholipids ratio [46]. Moreover, RBCs incubated in an elevated level of glucose showed a significantly increased protein glycation and induced ATPases activity [47] and were are highly susceptible to oxidative damage as a result of the high polyunsaturated fatty acid content of their membranes and the high cellular concentration of oxygen and hemoglobin [46]. Therefore, we hypothesize that these processes may impact the rheological properties of RBCs, cause membrane damage and potentially alter the RBCs contraction, influencing blood clot shrinkage properties.
Patients who formed more polyhedrocytes in our study showed also prothrombotic fibrin clot properties, such as decreased Ks or prolonged CLT. We have previously demonstrated that prothrombotic fibrin clot phenotype and impaired fibrinolysis might be associated with prolonged T2D duration [48]. Moreover, we have previously shown that in VTE patients, greater ECI (less compression) was associated with prolonged CLT, when compared to controls [28]. Carroll et al. have also shown that clot contraction significantly facilitated clot lysis [22]. The current findings suggest that fibrin features measured in plasma clots generated in vitro that are related to T2D may also be the markers of clot compression.
Limitations
First, a limited number of patients have been included in the study, therefore subgroup comparisons should be analyzed cautiously. Second, HbA1c was measured using immunoturbidimetry, but not by high-performance liquid chromatography. Third, we did not measure the RBCs membranes rigidity or assess function of platelets such as platelet aggregation. It is unclear whether polyhedrocytes formation affects the risk of cardiovascular events or thrombosis in T2D as suggested by other studies with regard to MI [24] or stroke [34]. Finally, subjects of the present study were all Caucasians, and we did not analyze genetic subtypes of the subjects studied.
Conclusions
Increased glucose levels, enhanced oxidative stress and platelet activation were associated with increased formation of polyhedrocytes in contracted blood clots. The significance of in vivo polyhedrocytes in T2D and in other diseases remains to be established and further studies are necessary to demonstrate the role of polyhedrocytes in thromboembolic events.
Abbreviations
- T2D:
-
type 2 diabetes
- RBCs:
-
red blood cells
- eGFR:
-
estimated glomerular filtration rate
- CAD:
-
coronary artery disease
- MI:
-
myocardial infarction
- SPECT:
-
single photon emission computed tomography with Tc-99 m-MIBI
- aPTT:
-
activated partial thromboplastin time
- TC:
-
serum total cholesterol
- LDL-C:
-
low-density lipoprotein cholesterol
- HDL-C:
-
high-density lipoprotein cholesterol
- TG:
-
triglycerides
- TSH:
-
thyroid stimulating hormone
- HbA1c:
-
glycated hemoglobin A1c
- RDW:
-
red blood cell distribution width
- WBC:
-
white blood cells
- SEM:
-
scanning electron microscope
- ECI:
-
erythrocyte compression index
- CAT:
-
calibrated automated thrombography
- TF:
-
tissue factor
- Ks:
-
permeation coefficient
- rtPA:
-
recombinant tPA
- total PC:
-
total protein carbonyl
- DNPH:
-
dinitrophenylhydrazine
- TCA:
-
trichloracetic acid
- TBARS:
-
thiobarbituric acid reactive substances
- TAC:
-
total antioxidant capacity
- ORAC:
-
oxygen radical absorbance capacity
- BIC:
-
Bayesian Information Criterion
- OR:
-
odds ratio
- CI:
-
confidence interval
- LP:
-
low-polyhedrocytes group
- HP:
-
high-polyhedrocytes group
- IQR:
-
interquartile range
- CLT:
-
clot lysis time
- VTE:
-
venous thromboembolism
- PF-4:
-
platelet factor-4
References
Vazzana N, Ranalli P, Cuccurullo C, Davì G. Diabetes mellitus and thrombosis. Thromb Res. 2012;129:371–7. https://doi.org/10.1016/j.thromres.2011.11.052.
Pomero F, Di Minno MND, Fenoglio L, Gianni M, Ageno W, Dentali F. Is diabetes a hypercoagulable state? A critical appraisal. Acta Diabetol. 2015;52:1007–16. https://doi.org/10.1007/s00592-015-0746-8.
Liu Y, Liu H, Hao Y, Hao Z, Geng G, Han W, et al. Short-term efficacy and safety of three different antiplatelet regimens in diabetic patients treated with primary percutaneous coronary intervention: a randomised study. Kardiol Pol. 2017;75:850–8. https://doi.org/10.5603/KP.a2017.0116.
Gajos G, Konieczynska M, Zalewski J, Undas A. Low fasting glucose is associated with enhanced thrombin generation and unfavorable fibrin clot properties in type 2 diabetic patients with high cardiovascular risk. Cardiovasc Diabetol. 2015;14:44.
Bartman W, Nabrdalik K, Kwiendacz H, Sawczyn T, Tomasik A, Pierzchala K, et al. Association between carotid plaque score and microvascular complications of type 2 diabetes. Polish Arch Intern Med Arch Med Wewn. 2017;127:418–22. https://doi.org/10.20452/pamw.4024.
Konieczynska M, Fil K, Bazanek M, Undas A. Prolonged duration of type 2 diabetes is associated with increased thrombin generation, prothrombotic fibrin clot phenotype and impaired fibrinolysis. Thromb Haemost. 2013;111:685–93. https://doi.org/10.1160/TH13-07-0566.
Bochenek M, Zalewski J, Sadowski J, Undas A. Type 2 diabetes as a modifier of fibrin clot properties in patients with coronary artery disease. J Thromb Thrombolysis. 2013;35:264–70.
Dunn EJ, Philippou H, Ariens RA, Grant PJ. Molecular mechanisms involved in the resistance of fibrin to clot lysis by plasmin in subjects with type 2 diabetes mellitus. Diabetologia. 2006;49:1071–80. https://doi.org/10.1007/s00125-006-0197-4.
Collaboration ERF. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–22.
Pretorius E, Bester J, Vermeulen N, Alummoottil S, Soma P, Buys AV, et al. Poorly controlled type 2 diabetes is accompanied by significant morphological and ultrastructural changes in both erythrocytes and in thrombin-generated fibrin: implications for diagnostics. Cardiovasc Diabetol. 2015;14:30.
Borsey DQ, Prowse CV, Gray RS, Dawes J, James K, Elton RA, et al. Platelet and coagulation factors in proliferative diabetic retinopathy. J Clin Pathol. 1984;37:659–64.
Sabrkhany S, Kuijpers MJE, Verheul HMW, Griffioen AW, Oude Egbrink MGA. Platelets: an unexploited data source in biomarker research. Lancet Haematol. 2015;2:e512–3.
Cella G, Scattolo N, Vio C, Stevanato F, Lavagnini T, Padovan D, et al. Platelet factor 4 (PF4) and heparin released platelet factor 4 (HR-PF4) in diabetes mellitus. Effect of the duration of the disease. Folia Haematol (Leipzig, Ger 1928). 1986;113:646–54.
Lim HS, Blann AD, Lip GYH. Soluble CD40 ligand, soluble P-selectin, interleukin-6, and tissue factor in diabetes mellitus: relationships to cardiovascular disease and risk factor intervention. Circulation. 2004;109:2524–8.
Carr ME, Krishnaswami A, Martin EJ. Platelet contractile force (PCF) and clot elastic modulus (CEM) are elevated in diabetic patients with chest pain. Diabet Med. 2002;19:862–6.
Podolnikova NP, Yakovlev S, Yakubenko VP, Wang X, Gorkun OV, Ugarova TP. The interaction of integrin αIIbβ3 with fibrin occurs through multiple binding sites in the αIIb β-propeller domain. J Biol Chem. 2014;289:2371–83.
Macfarlane RG. A simple method for measuring clot-retraction. Lancet. 1939;233:1199–201.
Cines DB, Lebedeva T, Nagaswami C, Hayes V, Massefski W, Litvinov RI, et al. Clot contraction: compression of erythrocytes into tightly packed polyhedra and redistribution of platelets and fibrin. Blood. 2014;123:1596–603. https://doi.org/10.1182/blood-2013-08-523860.
Soma P, Pretorius E. Interplay between ultrastructural findings and atherothrombotic complications in type 2 diabetes mellitus. Cardiovasc Diabetol. 2015;14:96.
Tutwiler V, Peshkova AD, Andrianova IA, Khasanova DR, Weisel JW, Litvinov RI. Contraction of blood clots is impaired in acute ischemic stroke. Arterioscler Thromb Vasc Biol. 2016. https://doi.org/10.1161/ATVBAHA.116.308622/-/DC1.
Ząbczyk M, Undas A. Plasma fibrin clot structure and thromboembolism: clinical implications. Polish Arch Intern Med. 2017;127:873–81. https://doi.org/10.20452/pamw.4165.
Carroll RC, Gerrard JM, Gilliam JM. Clot retraction facilitates clot lysis. Blood. 1981;57:44–8.
Tutwiler V, Litvinov RI, Lozhkin AP, Peshkova AD, Lebedeva T, Ataullakhanov FI, et al. Kinetics and mechanics of clot contraction are governed by the molecular and cellular composition of the blood. Blood. 2016;127:149–59. https://doi.org/10.1182/blood-2015-05-647560.
Zalewski J, Bogaert J, Sadowski M, Woznicka O, Doulaptsis K, Ntoumpanaki M, et al. Plasma fibrin clot phenotype independently affects intracoronary thrombus ultrastructure in patients with acute myocardial infarction. Thromb Haemost. 2015;113:1258–69. https://doi.org/10.1160/TH14-09-0801.
Zabczyk M, Sadowski M, Zalewski J, Undas A. Polyhedrocytes in intracoronary thrombi from patients with ST-elevation myocardial infarction. Int J Cardiol. 2015;179:186–7. https://doi.org/10.1016/j.ijcard.2014.10.004.
Pretorius E, Oore-ofe O, Mbotwe S, Bester J. Erythrocytes and their role as health indicator: using structure in a patient-orientated precision medicine approach. Blood Rev. 2016;30:263–74.
Silvain J, Collet JP, Nagaswami C, Beygui F, Edmondson KE, Bellemain-Appaix A, et al. Composition of coronary thrombus in acute myocardial infarction. J Am Coll Cardiol. 2011;57:1359–67. https://doi.org/10.1016/j.jacc.2010.09.077.
Zabczyk M, Natorska J, Undas A. Erythrocyte compression index is impaired in patients with residual vein obstruction. J Thromb Thrombolysis. 2018;46:31. https://doi.org/10.1007/s11239-018-1650-1.
Kremers RMW, Peters TC, Wagenvoord RJ, Hemker HC. The balance of pro-and anticoagulant processes underlying thrombin generation. J Thromb Haemost. 2015;13:437–47.
Mills JD, Ariëns RAS, Mansfield MW, Grant PJ. Altered fibrin clot structure in the healthy relatives of patients with premature coronary artery disease. Circulation. 2002;106:1938–42. https://doi.org/10.1161/01.CIR.0000033221.73082.06.
Pieters M, Philippou H, Undas A, De Lange Z, Rijken DC, Mutch NJ, et al. An international study on the feasibility of a standardized combined plasma clot turbidity and lysis assay: communication from the SSC of the ISTH. J Thromb Haemost. 2018;16:1007–12.
Becatti M, Marcucci R, Bruschi G, Taddei N, Bani D, Gori AM, et al. Oxidative modification of fibrinogen is associated with altered function and structure in the subacute phase of myocardial infarction. Arterioscler Thromb Vasc Biol. 2014;34:1355–61. https://doi.org/10.1161/ATVBAHA.114.303785.
Siudut J, Natorska J, Zabczyk M, Zajac D, Seweryn K, Rąpała-Kozik M, et al. Impaired plasminogen binding in patients with venous thromboembolism: association with protein carbonylation. Thromb Res. 2018;163:12–8.
Tutwiler V, Peshkova AD, Andrianova IA, Khasanova DR, Weisel JW, Litvinov RI. Contraction of blood clots is impaired in acute ischemic stroke. Arterioscler Thromb Vasc Biol. 2017;37:271–9. https://doi.org/10.1161/ATVBAHA.116.308622.
Litvinov RI, Weisel JW. Role of red blood cells in haemostasis and thrombosis. ISBT Sci Ser. 2017;12:176–83.
Kempe-Teufel DS, Bissinger R, Qadri SM, Wagner R, Peter A, Lang F. Cellular markers of eryptosis are altered in type 2 diabetes. Clin Chem Lab Med. 2018;56:e177–80.
Lang F, Lang E, Föller M. Physiology and pathophysiology of eryptosis. Transfus Med Hemotherapy. 2012;39:308–14.
Bissinger R, Bhuyan AAM, Qadri SM, Lang F. Oxidative stress, eryptosis and anemia: a pivotal mechanistic nexus in systemic diseases. FEBS J. 2018. https://doi.org/10.1111/febs.14606.
Kasahara K, Kaneda M, Miki T, Iida K, Sekino-Suzuki N, Kawashima I, et al. Clot retraction is mediated by factor XIII-dependent fibrin-αIIbβ3-myosin axis in platelet sphingomyelin-rich membrane rafts. Blood. 2013;122:3340 LP–8 LP.
Warshaw AL, Laster L, Shulman NR. The stimulation by thrombin of glucose oxidation in human platelets. J Clin Invest. 1966;45:1923–34.
Santilli F, Simeone P, Liani R, Davì G. Platelets and diabetes mellitus. Prostaglandins Other Lipid Mediat. 2015;120:28–39.
Soma P, Swanepoel AC, Du Plooy JN, Mqoco T, Pretorius E. Flow cytometric analysis of platelets type 2 diabetes mellitus reveals ‘angry’platelets. Cardiovasc Diabetol. 2016;15:52.
Miao X, Zhang W, Huang Z, Li N. Unaltered angiogenesis-regulating activities of platelets in mild type 2 diabetes mellitus despite a marked platelet hyperreactivity. PLoS ONE. 2016;11:e0162405.
Pretorius E, Page MJ, Engelbrecht L, Ellis GC, Kell DB. Substantial fibrin amyloidogenesis in type 2 diabetes assessed using amyloid-selective fluorescent stains. Cardiovasc Diabetol. 2017;16:141.
Lippi G, Targher G, Montagnana M, Salvagno GL, Zoppini G, Guidi GC. Relation between red blood cell distribution width and inflammatory biomarkers in a large cohort of unselected outpatients. Arch Pathol Lab Med. 2009;133:628–32.
Szablewski L, Sulima A. The structural and functional changes of blood cells and molecular components in diabetes mellitus. Biol Chem. 2017;398:411–23.
Kumar R, Kumar AN, Ahmed S. Changes in erythrocyte membrane in type-2 diabetes mellitus with and without dyslipidemia. J Diabetes Metab. 2011;2:2.
Konieczynska M, Fil K, Bazanek M, Undas A. Prolonged duration of type 2 diabetes is associated with increased thrombin generation, prothrombotic fibrin clot phenotype and impaired fibrinolysis. Thromb Haemost. 2014;112:685–93.
Authors’ contributions
GG and AS contributed to the work by acquisition of data, analysis and interpretation of data, as well as writing and revision of the manuscript. AS, RGW, MZ, JS and PR contributed to the work by acquisition and analysis of data. MZ, JN and JS contributed to the work by performing most of laboratory testing and revision of the manuscript. KPM performed most statistical analysis. AU made substantial contributions to conception and design of the study, analysis and interpretation of data, revision of the manuscript and final approval of the version to be published. All authors read and approved the final manuscript.
Acknowledgements
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The study complied with the principles of the Good Clinical Practice International Conference on Harmonization rules and was approved by the Jagiellonian University Ethics Committee-No: KBET/190/B/12. Each study participant provided written informed consent.
Funding
The study was supported by research grants: K/ZDS/007982 from the Jagiellonian University Medical College (to G.G.), K/ZDS/007181 from the Jagiellonian University Medical College (to P.R.) and K/ZDS/007177 from the Jagiellonian University Medical College (to A.U.).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Additional file
Additional file 1: Table S1.
Platelet markers, fibrinolytic proteins and oxidation parameters in a subset of the study group T2D patients (n=23).
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Gajos, G., Siniarski, A., Natorska, J. et al. Polyhedrocytes in blood clots of type 2 diabetic patients with high cardiovascular risk: association with glycemia, oxidative stress and platelet activation. Cardiovasc Diabetol 17, 146 (2018). https://doi.org/10.1186/s12933-018-0789-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-018-0789-6