Abstract
Background
The risk prediction of pregnancy-associated plasma protein-A (PAPP-A) for future cardiovascular (CV) events post acute coronary syndrome (ACS) in patients with type-2 diabetes mellitus (T2DM) was investigated in comparison to other risk factors.
Methods
PAPP-A was measured at hospital admission in 320 consecutive ACS patients (136 with T2DM and 184 without). All patients were followed for 2 years for occurrence of CV death, non-fatal MI or stroke. Effect of PAPP-A on the CV event risk was estimated using Cox regression models. Receiver operating characteristics (ROC) curves were generated to demonstrate the sensitivity and specificity of PAPP-A in predicting CV events.
Results
ACS patients with T2DM had higher PAPP-A (19.29 ± 16.36 vs. 13.29 ± 13.90 ng/ml, p < 0.001) and higher rate of CV events 2 years post ACS (27.2 vs. 13.6%, p = 0.002) than those without. Higher levels of PAPP-A were significantly associated with increased risk of CV events during 2-year follow-up [HR = 2.97 for 1 SD increase in log10(PAPP-A), 95% CI 2.11–4.18, p < 0.001] in T2DM and (HR = 3.16, 95% CI 2.27–4.39, p < 0.001) in non-T2DM. Among patients with T2DM, PAPP-A showed a larger area under the curve (AUC 0.79) that was significantly more predictive than hsCRP (AUC 0.64), eGFR (AUC 0.66) and LVEF < 50% (AUC 0.52); predictive ability did not improve significantly by including those factors into the model.
Conclusions
Patients with T2DM had higher levels of PAPP-A and increased risk of CV events. Elevated PAPP-A compared to other risk factors was a stronger predictor for future CV events 2 years post ACS in patients with T2DM.
Trial registration ISRCTN10805074. Registered on 20 January 2017, retrospectively registered.
Similar content being viewed by others
Background
Patients with type-2 diabetes mellitus (T2DM) are known to suffer worse outcomes post-acute coronary syndrome (ACS) [1, 2]. Identification of factors that can improve the risk prediction of cardiovascular outcomes in T2DM remains clinically challenging [3]. Previous research has found that pregnancy-associated plasma protein-A (PAPP-A), a high molecular weight and zinc-binding metalloproteinase, is an important regulatory protein in cell proliferation and the development of atherosclerosis and can degrade the proteins that maintain the integrity of the protective fibrous cap of atherosclerotic plaques [4, 5]. PAPP-A, originally found in pregnant women, is produced by syncytiotrophoblast cells in a heterotetrameric complex (500 kDa) [6], but also by osteoblasts, fibroblasts, endothelial cells, vascular smooth muscle cells [4, 7, 8] and by monocytes and macrophages [9, 10] in a homodimer (400 kDa) with proteolytic activity [6]. Previous studies have demonstrated that PAPP-A is a potentially important biomarker of plaque instability and inflammation in patients with ACS [11, 12]. PAPP-A has been identified in vulnerable coronary plaques but not found in stable plaques [13] and higher PAPP-A levels are associated with higher 3-vessel thin-cap fibroatheroma (TCFA) burden in patients with coronary artery disease [14]. Furthermore, subjects with T2DM appeared to have higher levels of PAPP-A than age-matched controls [15, 16]. However, whether the increased levels of PAPP-A in T2DM can better predict future CV events post ACS had not been studied. Therefore, we prospectively investigated the risk prediction of serum PAPP-A concentrations for future CV events in ACS patients with and without T2DM in comparison to other risk factors.
Methods
Study population
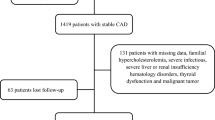
As shown in Fig. 1, a total of 420 patients with suspected ACS including ST-elevation myocardial infarction (STEMI), non-ST-elevation MI (Non-STEMI) and unstable angina (UA) were admitted to CCU and 1 of 4 cardiac wards at the Beijing Friendship Hospital Cardiovascular Center between June and November 2012. Of these 420 patients, 344 were confirmed with ACS [17, 18]. Three hundred twenty subjects completed the study and were included in the final analysis.
Informed consent was obtained from all subjects prior to any study related procedures or measurements. The study protocol and procedures received approval from the Beijing Friendship Hospital Institutional Review Board and were performed to conform to the Declaration of Helsinki.
T2DM was diagnosed based on fasting plasma glucose ≥7.0 mmol/l, or 2-h plasma glucose ≥11.1 mmol/l, or HbA1C ≥6.5%, or receiving treatment for diabetes [19].
Laboratory analysis
Routine laboratory tests on lipids, glucose level, Hs-CRP, hepatic and renal functions were performed using the standardized methods at the Beijing Friendship Hospital Laboratory. Cardiac troponin I (cTnI) was detected by electro-chemiluminescence technology for quantitative measurement (3rd generation TnI, Elecsys 2010, Roche, Mannheim, Germany). The lower detection limit is 0.01 µg/l with a recommended diagnostic threshold of 0.03 µg/l for MI. N-terminal B-type natriuretic peptide (NT-proBNP) was measured using electrochemiluminescence immunoassays performed on a Roche Elecsys 2010 automated platform (Roche Diagnostics, Burgess Hill, UK). Additional blood samples were collected with EDTA preparation at the time of routine laboratory tests [20] and were stored at −80 °C.
Total serum PAPP-A was measured before heparin administration and blinded to subject characteristics, laboratory results and clinical course using the ultra-sensitive enzyme-linked immunosorbent assay (ELISA) method with kits manufactured by DRG Instruments GmBH (Germany). This method with sensitivity of 0.023 ng/ml is specially designed to detect low concentrations of circulating enzyme that is associated with possible plaque rupture [21].
Echocardiograms
Echocardiography was performed routinely at hospital admission. Digital echocardiograms were analyzed on the EchoPAC workstation (Philips IE Elite). Left ventricular end-diastolic and -systolic volumes were obtained using Simpson’s method of discs in the apical 4- and 2-chamber views as recommended by the American Society of Echocardiography [22]. Left ventricular ejection fraction (LVEF) was calculated using left ventricular volumes and the formula: [(end-diastolic volume − end-systolic volume)/end-diastolic volume] × 100%.
CV outcomes
Events including CV death, nonfatal MI or stroke were identified during the initial hospitalization for ACS, and at 6 month intervals for up to 24 months per the study follow-up schedule. Each event was evaluated and confirmed with medical records. Events possibly related to ACS treatment procedures (PCI or CABG) were excluded. A composite of CV death, nonfatal MI or stroke was the pre-defined study primary endpoint.
Statistical analysis
Comparisons of clinical characteristics and laboratory measurements between subjects with and without T2DM were performed using unpaired t-tests for continuous variables and Chi square tests for categorical variables. Kaplan–Meier curves and Cox regression for the composite CV event were generated to compare the event-free survival difference between subjects with or without T2DM.
Cox regression analysis was used to determine the factors that were associated with the risk of subsequent CV events post-ACS. Four models were considered for the effect of PAPP-A on the risk of events: (1) model with PAPP-A value, (2) model with log10(PAPP-A), (3) model with linear spline for PAPP-A (with a knot at the 60th percentile, PAPPA = 13.16 ng/ml) and (4) categorical model (lower quartile ≤8.46 ng/ml, 2nd and 3rd quartiles combined >8.46 and <17.3 ng/ml and 4th quartile ≥17.4 ng/ml). Among the four candidate models log10(PAPP-A) was selected for the largest Cox & Snell pseudo R2 [23].
The effect of PAPP-A on the risk of CV events was also tested in multivariate models that adjusted for covariates including: diabetes status, age, gender, LVEF (%), HbA1C, Troponin I and hsCRP. Due to the limited number of events (37 events in T2DM subjects and 24 in non-T2DM subjects) only a single covariate was adjusted for at a time. A covariate was considered as confounding if the adjustment for the covariate changed the hazard ratio (HR) for PAPPA-A by at least 10%.
Receiver operating characteristics (ROC) curves were generated to demonstrate the ability to predict CV events. Area under the ROC curve (AUCs) was calculated for single predictor and selected multivariate models (the latter based on logistic regression). The multivariate logistic regression models used all predictors that had AUC > 0.6. To determine whether PAPP-A had a different ability to predict CV events compared to other single factors or to the multivariate model, we used DeLong’s test to compare pairs of correlated ROC curves [24]. We would like to point out that this test is perhaps unfair to PAPP-A when it is being compared to the multivariate model because PAPP-A is part of the multivariate model and DeLong’s test does not account for the number of other predictors in the multivariate model. Confidence intervals for AUC were calculated with the non-parametric bootstrap with 2000 replicates.
All tests were two tailed and p < 0.05 was considered statistically significant. All statistical analyses were performed using R version 3.1.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Clinical and laboratory characteristics
Overall, among the 320 subjects, 68% were male, mean age was 65 years (range 27–90 years), 31% were diagnosed with STEMI, 26% with non-ST elevation MI and 43% with unstable angina. Of the 320 subjects, 168 (53%) received PCI, 11 (3%) received CABG in addition to medical management, and 141 (44%) were treated only with medical therapy during hospitalization. The proportion of medical management during hospitalization and at discharge was ASA (97%), P2Y12 inhibitor (72%), statin (92%), β-blocker (75%) and angiotensin converting enzyme inhibitor/angiotensin-receptor blocker (72%). There was no significant difference in treatment during hospitalization between subjects with and without T2DM (Table 1).
As shown in Table 1, 136 subjects with T2DM, compared to 184 without, were significantly more likely to be female (39 vs. 27%, p = 0.021), to have hyperlipidemia (61 vs. 49%, p = 0.042) and lower LVEF (56 ± 12 vs. 60 ± 12%, p = 0.001). Subjects with T2DM had significantly higher PAPP-A concentrations (19.3 ± 16.4 vs. 13.3 ± 13.9 ng/ml, p < 0.001) and hsCRP levels (8.4 ± 5.9 vs. 6.5 ± 5.7 ng/ml, p < 0.001) than those without. As expected, subjects with T2DM showed significantly higher levels of fasting blood glucose (8.2 ± 3.5 vs. 5.5 ± 1.7 mmol/l, p < 0.001) and HbA1C (7.6 ± 1.8 vs. 5.7 ± 0.5%, p < 0.001). The T2DM group also had higher triglycerides (2.13 ± 1.37 vs. 1.65 ± 1.04 mmol/l, p < 0.001), but no significant difference was seen in the levels of total cholesterol, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), aspartate aminotransferase (AST), alanine aminotransferase (ALT), creatinine, CK-MB, cTnI, NT-proBNP or eGFR.
CV outcomes
During the 24 months of follow-up, 4.4, 9.0 and 7.8% were confirmed for CV death, nonfatal MI and stroke, respectively. Overall, there were 62 patients (37 with T2DM and 25 without T2DM) with a CV event. The overall 2-year event free survival rate for the composite CV outcome was 80.9% (95% CI 76.7, 85.4). As shown in Fig. 2, the 2-year event free survival rate in subjects with T2DM was 72.8% (95% CI 65.7–80.7), which was, not surprisingly, significantly lower than that in subjects without T2DM 86.9% (95% CI 82.2–92.0) (p = 0.002, Cox regression).
Unadjusted and adjusted association between PAPP-A and CV outcomes
The univariate Cox regression analysis identified that elevated PAPP-A was significantly associated with increased occurrence of CV events in all subjects and subjects with and without T2DM as shown in Table 2. HR per 1 SD increase in log10(PAPP-A) was 2.97 (95% CI 2.11–4.18) in subjects with T2DM, 3.16 (95% CI 2.27–4.39) in those without T2DM and 3.10 (95% CI 2.48–3.87) in all subjects, all p < 0.001, respectively. In addition, older age, hypertension, lower LVEF, higher HbA1C, lower eGFR, higher cTnT and higher hsCRP were also significantly associated with increased risk of CV events in the univariate analysis.
After the adjustment for age, gender, LVEF, HbA1C, cTnI or hsCRP (a single adjustment variable at a time), the effect of PAPP-A on CV events in all subjects and subjects with and without T2DM changed by <10% suggesting that these factors do not confound the relationship between PAPP-A and the CV event risk (Table 3).
Predictive effects of PAPP-A on CV outcomes
Areas under the ROC curves (AUC) for PAPP-A, age, LVEF, eGFR, hsCRP and the combination of all these predictors (from a multivariate logistic regression including all predictors) are shown in Fig. 3 and Table 4. Specifically, among subjects with T2DM (Fig. 3a), PAPP-A showed AUC of 0.79 (95% CI 0.70–0.87) which was significantly larger than LVEF (AUC 0.52; 95% CI 0.40–0.64; p < 0.001), eGFR (AUC 0.66; 95% CI 0.56–0.77; p = 0.03) and hsCRP (AUC 0.64; 95% CI 0.52–0.76; p = 0.01). Similar results were also seen in those without T2DM (Fig. 3b) and in all subjects (Fig. 3c).
Predictors of cardiovascular outcome 2 years post ACS. Receiver operating characteristics (ROC) curves for PAPP-A and other factors predicting cardiovascular outcome 2 years post ACS in a patients with T2DM; b patients without T2DM; and c all patients. Specifically, in ACS patients with T2DM, area under the ROC curve (AUC) was 0.79 for PAPP-A in brown color line, 0.69 for age in purple, 0.52 for LVEF in gold, 0.66 for GFR in blue, 0.64 for hsCRP in red, and 0.83 for all 5 factors combined in black
Furthermore, as shown in Table 4, when PAPP-A was combined with other factors, the AUC improved to 0.83 (95% CI 0.75–0.91) in subjects with T2DM, 0.83 (95% CI 0.77–0.89) in all subjects, and 0.80 (95% CI 0.70–0.90) in those without T2DM. None of these improvements in AUC was statistically significant compared to PAPP-A alone.
Discussion
Recurrent CV events post ACS in T2DM
Recurrent cardiovascular events in post ACS patients treated with current standard medical therapy and revascularization interventions (PCI or CABG) remain an important clinical issue [25, 26]. A multinational study by the GRACE investigators [27] showed that CV death occurred in 4.1%, re-infarction in 4.4%, and stroke in 1.3% during 2 years of follow-up in 22,937 ACS patients from 57 sites. Our study found 4.4% CV death, 9.0% nonfatal MI and 7.8% stroke during 24 months of post ACS. The higher recurrent event rates in our study could be associated with a higher percentage of patients with T2DM (43%) compared to 25% in GRACE since other patient characteristics and ACS treatment were similar between our study and GRACE [27]. Indeed, we found a higher composite endpoint (CV death, nonfatal MI, stroke) rate during 24 months post ACS in the patients with T2DM than those without (27 vs. 13%), which is consistent with the accumulated evidence that diabetes increases the risk of recurrent cardiovascular events post ACS [28,29,30].
PAPP-A forms and levels among populations
There are 2 forms of PAPP-A. During pregnancy, PAPP-A produced by placental syncytiotrophoblast cells is a heterotetrameric complex (500 kDa) covalently linked with eosinophil major basic protein [6]. This form is proteolytically inactive. The distribution of PAPP-A was investigated in pregnant women and found that it varies by ethnicity, gestational age, maternal weight and smoking status [31,32,33,34,35]. The clinical investigations of the complex relationship among PAPP-A levels during pregnancy, gestational diabetes (GDM) and cardiovascular disease risk are evolving [36,37,38,39]. Low levels of PAPP-A during the first trimester are associated with fetal Down’s syndrome [40] and other adverse events [37, 41,42,43]. A recent study [39] among Chinese women showed that PAPP-A levels in the first trimester were not predictive of development of GDM which is highly prevalent (approximately 19%) in China [44]. History of GDM in the 102 women (100 post-menopause) enrolled in our study was not collected.
In contrast, the other form of PAPP-A secreted by vascular cells [4, 7,8,9,10] is a homodimer (400 kDa) not covalently linked with eosinophil major basic protein. This form has proteolytic activity [6] and is considered to play an important role in cardiovascular disease [45]. In general, the concentration of PAPP-A is found to be very low in adult males and non-pregnant females [16]. In addition to the association of elevated PAPP-A levels with atherosclerotic vascular disease [11,12,13,14], recently, PAPP-A was found to be a useful biomarker for cardiovascular dysfunction, inflammatory state and malnutrition in chronic kidney disease (CKD) patients undergoing hemodialysis [46]. Additionally, PAPP-A may be involved in the pathogenesis of retinal vein occlusion (RVD) [47] and the development of chronic obstructive pulmonary disease (COPD) [48].
PAPP-A was reported to be higher in T2DM than healthy controls in 2 studies [15, 16], appeared to be negatively associated with HbA1C levels [49], and its expression in glomeruli was associated with diabetic nephropathy [50]. Our study found significantly higher concentrations of PAPP-A in ACS patients with T2DM than those without (19 vs. 13 ng/ml, p < 0.001). These results represent the increased inflammatory state in ACS and T2DM, particularly in ACS combined with T2DM [51] and is supported by previous investigations [9, 10, 13, 14]. These investigations have demonstrated that PAPP-A was produced and released by monocytes/macrophages, was found in ruptured and eroded plaques and co-localized with vascular smooth muscle cells and activated macrophages, and was associated with higher 3-vessel TCFA burden as assessed by virtual histology (VH)-intravascular ultrasound (IVUS) [14] and carotid plaque instability by Doppler ultrasonography in patients with acute ischemic stroke [52].
Prediction of PAPP-A for recurrent CV events post ACS in T2DM
Previously, the MERLIN–TIMI-36 study investigators reported that PAPP-A was independently associated with recurrent cardiovascular events in patients with non-STEMI ACS [53]. Our study found that PAPP-A was a significantly stronger predictor of 2-year recurrent events post ACS in all subjects and in T2DM. A larger AUC (0.79) was observed in comparison to other identified risk predictors (Table 2) including age (AUC 0.69), LVEF (AUC 0.52), eGFR (AUC 0.66) and hsCRP (AUC 0.64). Wlazeł and colleagues [54] examined the clinical value of PAPP-A in predicting future events post ACS and suggested that a panel of 2–3 biomarkers (PAPP-A—hsCRP, PAPP-A—FBG, PAPP-A—hsCRP—FBG) can improve the risk prediction. In our study, when other risk predictors were combined with PAPP-A, the AUC increased from 0.79 to 0.83 in T2DM, which was not a statistically significant improvement compared to the model with PAPP-A alone (Fig. 3; Table 4). However, the independent risk prediction of PAPP-A for future cardiovascular events post ACS in patients with T2MD needs to be confirmed in larger prospective population studies. Furthermore, other investigators suggested that the free PAPP-A was a stronger risk predictor of recurrent CV events 1 year post-ACS when compared to total PAPP-A [55] as measured in our study. Future studies are also needed to confirm whether there is different clinical value between the free PAPP-A and total PAPP-A.
Limitations and future directions
Our study was performed in a very high-risk population all with ACS and >40% with T2DM, plus 40% of subjects with multiple risk factors including hypertension, hyperlipidemia and smoking. The sensitivity and specificity of PAPP-A as a strong predictor for CV events could be influenced by the selected high-risk population. In addition, this study was performed at a single center. Although a recent report by Conover and colleagues showed that inhibition of PAPP-A proteolytic activity by monoclonal antibody reduced atherosclerotic plaque progression in the apolipoprotein E knock-out mice [56] and a previous study showed that high dose of atorvastatin was associated with PAPP-A reduction in patients with ACS [57], whether reduction of PAPP-A and by which therapeutic options can improve clinical outcomes remain unanswered.
Finally, the assay methods need to be standardized technically and the optimal cut-off levels for risk stratification need to be defined clinically. Future research will be necessary to address these questions and to investigate the biological role and therapeutic implications of PAPP-A.
In conclusion, PAPP-A, a novel plaque inflammatory marker, was significantly higher in ACS patients with T2DM than those without. It was found to be a stronger predictor for increased risk of future CV events 2 years post-ACS in patients with T2DM when compared to hsCRP and other factors.
References
Woodward M, Zhang X, Barzi F. The effects of diabetes on the risks of major cardiovascular diseases and death in the Asia-Pacific region. Diabetes Care. 2003;26:360–6.
Emerging Risk Factors Collaboration, Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, Ingelsson E, Lawlor DA, Selvin E, Stampfer M, Stehouwer CD, Lewington S, Pennells L, Thompson A, Sattar N, White IR, Ray KK, Danesh J. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–22.
Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA. 2002;287:2570–81.
Lawrence JB, Oxvig C, Overgaard MT, Sottrup-Jensen L, Gleich GJ, Hays LG, Yates JR 3rd, Conover CA. The insulin-like growth factor (IGF)-dependent IGF binding protein-4 protease secreted by human fibroblasts is pregnancy-associated plasma protein-A. Proc Natl Acad Sci USA. 1999;96:3149–53.
Laursen LS, Overgaard MT, Nielsen CG, Boldt HB, Hopmann KH, Conover CA, Sottrup-Jensen L, Giudice LC, Oxvig C. Substrate specificity of the metalloproteinase pregnancy-associated plasma protein-A (PAPP-A) assessed by mutagenesis and analysis of synthetic peptides: substrate residues distant from the scissile bond are critical for proteolysis. Biochem J. 2002;367:31–40.
Overgaard MT, Sorensen ES, Stachowiak D, Boldt HB, Kristensen L, Sottrup-Jensen L, Oxvig C. Complex of pregnancy-associated plasma protein-A and the proform of eosinophil major basic protein. Disulfide structure and carbohydrate attachment. J Biol Chem. 2003;278(4):2106–17.
Ortiz CO, Chen BK, Bale LK, Overgaard MT, Oxvig C, Conover CA. Transforming growth factor-β regulation of the insulin-like growth factor binding protein-4 protease system in cultured human osteoblasts. J Bone Miner Res. 2003;18:1066–72.
Conover CA, Harrington SC, Bale LK. Differential regulation of pregnancy associated plasma protein-A in human coronary artery endothelial cells and smooth muscle cells. Growth Horm IGF Res. 2008;18:213–20.
Li WP, Li HW, Gu FS. CRP and TNF-α induce PAPP-A expression in human peripheral blood mononuclear cells. Mediators Inflamm. 2012;2012:697832.
Sangiorni G, Maurielo A, Bonanno E, Oxvig C, Conover CA, Christiansen M, Trimarchi S, Rampoldi V, Holmes DR Jr, Schwartz RS, Spagnoli LG. Pregnancy associated plasma protein A is markedly expressed by monocyte-macrophage cells in vulnerable and ruptured carotid atherosclerotic plaques: a link between inflammation and cerebrovascular events. J Am Coll Cardiol. 2006;47:2201–11.
Iversen KK, Dalsgaard M, Teisner AS, Schoos M, Teisner B, Nielsen H, Clemmensen P, Grande P. Usefulness of pregnancy-associated plasma protein a in patients with acute coronary syndrome. Am J Cardiol. 2009;104:1465–71.
Iversen KK, Dalsgaard M, Teisner AS, Schoos M, Teisner B, Nielsen H, Grande P, Clemmensen P. Pregnancy-associated plasma protein-A, a marker for outcome in patients suspected for acute coronary syndrome. Clin Biochem. 2010;43:851–7.
Bayes-Genis A, Conover CA, Overgaard MT, Bailey KR, Christiansen M, Holmes DR Jr, Virmani R, Oxvig C, Schwartz RS. Pregnancy associated plasma protein A as a marker of acute coronary syndromes. N Engl J Med. 2001;345:1022–9.
Wu XF, Yang M, Qu AJ, Mintz GS, Yang Y, Shang YP, Gao H, Zhang YC, Ge CJ, Wang LY, Wang L, Pu J. Level of pregnancy-associated plasma protein-A correlates with coronary thin-cap fibroatheroma burden in patients with coronary artery disease: novel findings from 3-vessel virtual histology intravascular ultrasound assessment. Medicine. 2016;95(3):e2563.
Aso Y, Okumura K, Wakabayashi S, Takebayashi K, Taki S, Inukai T. Elevated pregnancy-associated plasma protein-a in sera from type 2 diabetic patients with hypercholesterolemia: associations with carotid atherosclerosis and toe-brachial index. J Clin Endocrinol Metab. 2004;89:5713–7.
Heidari B, Fotouhi A, Sharifi F, Mohammad K, Pajouhi M, Paydary K, Fakhrzadeh H. Elevated serum levels of pregnancy-associated plasma protein-A in type 2 diabetics compared to healthy controls: associations with subclinical atherosclerosis parameters. Acta Med Iran. 2015;53(7):395–402.
Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE Jr, Chavey WE 2nd, Fesmire FM, Hochman JS, Levin TN, Lincoff AM, Peterson ED, Theroux P, Wenger NK, Wright RS, Jneid H, Ettinger SM, Ganiats TG, Philippides GJ, Jacobs AK, Halperin JL, Albert NM, Creager MA, DeMets D, Guyton RA, Kushner FG, Ohman EM, Stevenson W, Yancy CW. 2012 ACCF/AHA focused update incorporated into the ACCF/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:e179–347.
Hoffmann U, Truong QA, Schoenfeld DA, Chou ET, Woodard PK, Nagurney JT, Pope JH, Hauser TH, White CS, Weiner SG, Kalanjian S, Mullins ME, Mikati I, Peacock WF, Zakroysky P, Hayden D, Goehler A, Lee H, Gazelle GS, Wiviott SD, Fleg JL, Udelson JE, ROMICAT-II Investigators. Coronary CT angiography versus standard evaluation in acute chest pain. N Engl J Med. 2012;367:299–308.
American Diabetes Association. Executive summary: standards of medical care in diabetes-2012. Diabetes Care. 2012;35(Suppl 1):S4–10.
Baldus S, Rudolph V, Roiss M, Ito WD, Rudolph TK, Eiserich JP, Sydow K, Lau D, Szöcs K, Klinke A, Kubala L, Berglund L, Schrepfer S, Deuse T, Haddad M, Risius T, Klemm H, Reichenspurner HC, Meinertz T, Heitzer T. Heparins increase endothelial nitric oxide bioavailability by liberating vessel-immobilized myeloperoxidase. Circulation. 2006;113:1871–8.
Furenes EB, Arnesen H, Solheim S, Grøgaard HK, Hoffmann P, Seljeflot I. The profile of circulating metalloproteinases after PCI in patients with acute myocardial infarction or stable angina. Thromb Res. 2009;124:560–4.
Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ, Chamber Quantification Writing Group, American Society of Echocardiography’s Guidelines and Standards Committee, European Association of Echocardiography. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group. J Am Soc Echocardiogr. 2005;18:1440–63.
Cox DR, Snell EJ. The analysis of binary data. 2nd ed. London: Chapman and Hall; 1989.
Delong ER, Delong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45.
Pilgrim T, Vranckx P, Valgimigli M, Stefanini GG, Piccolo R, Rat J, Rothenbühler M, Stortecky S, Räber L, Blöchlinger S, Hunziker L, Silber S, Jüni P, Serruys PW, Windecker S. Risk and timing of recurrent ischemic events among patients with stable ischemic heart disease, non-ST-segment elevation acute coronary syndrome, and ST-segment elevation myocardial infarction. Am Heart J. 2016;175:56–65.
Eisen A, Giugliano RP, Braunwald E. Updates on acute coronary syndrome: a review. JAMA Cardiol. 2016;1:718–30.
Alnasser SM, Huang W, Gore JM, Steg PG, Eagle KA, Anderson FA Jr, Fox KA, Gurfinkel E, Brieger D, Klein W, van de Werf F, Avezum Á, Montalescot G, Gulba DC, Budaj A, Lopez-Sendon J, Granger CB, Kennelly BM, Goldberg RJ, Fleming E, Goodman SG, GRACE Investigators. Late consequences of acute coronary syndromes: global registry of acute coronary events (GRACE) follow-up. Am J Med. 2015;128:766–75.
Farkouh ME, Aneja A, Reeder GS, Smars PA, Lennon RJ, Wiste HJ, Traverse K, Razzouk L, Basu A, Holmes DR Jr, Mathew V. Usefulness of diabetes mellitus to predict long-term outcomes in patients with unstable angina pectoris. Am J Cardiol. 2009;104:492–7.
Marso SP, Safley DM, House JA, Tessendorf T, Reid KJ, Spertus JA. Suspected acute coronary syndrome patients with diabetes and normal troponin-I levels are at risk for early and late death: identification of a new high-risk acute coronary syndrome population. Diabetes Care. 2006;29:1931–2.
Donahoe SM, Stewart GC, McCabe CH, Mohanavelu S, Murphy SA, Cannon CP, Antman EM. Diabetes and mortality following acute coronary syndromes. JAMA. 2007;2987:765–75.
Spencer K, Ong CY, Liao AW, Nicolaides KH. The influence of ethnic origin on first trimester biochemical markers of chromosomal abnormalities. Prenat Diagn. 2000;20:491–4.
Cowans NJ, Spencer K. Effect of gestational age on first trimester maternal serum prenatal screening correction factors for ethnicity and IVF conception. Prenat Diagn. 2013;33:56–60.
Browne JL, Klipstein-Grobusch K, Koster MP, Ramamoorthy D, Antwi E, Belmouden I, Franx A, Grobbee DE, Schielen PC. Pregnancy associated plasma protein-A and placental growth factor in a sub-Saharan African population: a nested cross-sectional study. PLoS ONE. 2016;11(8):e0159592.
Tul N, Pusenjak S, Osredkar J, Spencer K, Novak-Antolic Z. Predicting complications of pregnancy with first-trimester maternal serum free-βhCG, PAPP-A and inhibin-A. Prenat Diagn. 2003;23:990–6.
Kulaksizoglu S, Kulaksizoglu M, Kebapcilar AG, Torun AN, Ozcimen E, Turkoglu S. Can first-trimester screening program detect women at high risk for gestational diabetes mellitus? Gynecol Endocrinol. 2013;29:137–40.
Lekva T, Michelsen AE, Bollerslev J, Norwitz ER, Aukrust P, Henriksen T, Ueland T. Low circulating pentraxin 3 levels in pregnancy is associated with gestational diabetes and increased apoB/apoA ratio: a 5-year follow-up study. Cardiovasc Diabetol. 2016;15:23.
Pummara P, Tongsong T, Wanapirak C, Sirichotiyakul S, Luewan S. Association of first-trimester pregnancy-associated plasma protein A levels and idiopathic preterm delivery: a population-based screening study. Taiwan J Obstet Gynecol. 2016;55(1):72–5.
Goueslard K, Cottenet J, Mariet AS, Giroud M, Cottin Y, Petit JM, Quantin C. Early cardiovascular events in women with a history of gestational diabetes mellitus. Cardiovasc Diabetol. 2016;15:15.
Cheuk QK, Lo TK, Wong SF, Lee CP. Association between pregnancy-associated plasma protein-A levels in the first trimester and gestational diabetes mellitus in Chinese women. Hong Kong Med J. 2016;22(1):30–8.
Wald NJ, Watt HC, Hackshaw AK. Integrated screening for Down’s syndrome on the basis of tests performed during the first and second trimesters. N Engl J Med. 1999;341(7):461–7.
Brizot ML, Hyett JA, McKie AT, Bersinger NA, Farzaneh F, Nicolaides KH. Early-pregnancy origins of low birth weight. Nature. 2002;417(6892):916.
Poon LC, Maiz N, Valencia C, Plasencia W, Nicolaides KH. Firsttrimester maternal serum pregnancy-associated plasma protein-A and pre-eclampsia. Ultrasound Obstet Gynecol. 2009;33(1):23–33.
Smith GC, Stenhouse EJ, Crossley JA, Aitken DA, Cameron AD, Connor JM. Early pregnancy levels of pregnancy-associated plasma protein a and the risk of intrauterine growth restriction, premature birth, preeclampsia, and stillbirth. J Clin Endocrinol Metab. 2002;87(4):1762–7.
Wei Y, Yang H, Zhu W, Yang H, Li H, Yan J, Zhang C. International Association of Diabetes and Pregnancy Study Group criteria is suitable for gestational diabetes mellitus diagnosis: further evidence from China. Chin Med J (Engl). 2014;127(20):3553–6.
Ziviello F, Conte S, Cimmino G, Sasso FC, Trimarco B, Cirillo P. Pregnancy-associated plasma protein-A and its role in cardiovascular disease. Biology, experimental/clinical evidences and potential therapeutic approaches. Curr Vasc Pharmacol. 2016. doi:10.2174/1570161114666161230112126.
Mazur-Laskowska M, Bała-Błądzińska A, Zegartowska P, Dumnicka P, Ząbek-Adamska A, Kapusta M, Maleszka A, Maziarz B, Kuźniewski M, Kuśnierz-Cabala B. Serum pregnancy-associated plasma protein A correlates with inflammation and malnutrition in patients treated with maintenance hemodialysis. Folia Med Cracov. 2015;55(3):37–47.
Yilmaz T, Yilmaz A, Gunay M, Ocal MC, Ozveren M. Increased pregnancy-associated plasma protein A in retinal vein occlusion. Eur Rev Med Pharmacol Sci. 2016;20(11):2189–93.
Talay F, Tosun M, Yaşar ZA, Kar Kurt Ö, Karği A, Öztürk S, Özlü MF, Alçelik A. Evaluation of pregnancy-associated plasma protein-A levels in patients with chronic obstructive pulmonary disease and associations with disease severity. Inflammation. 2016;39(3):1130–3.
Pellitero S, Reverter JL, Pizarro E, Pastor MC, Granada ML, Tàssies D, Reverter JC, Salinas I, Sanmartí A. Pregnancy-associated plasma protein-a levels are related to glycemic control but not to lipid profile or hemostatic parameters in type 2 diabetes. Diabetes Care. 2007;30(12):3083–5.
Mader JR, Resch ZT, McLean GR, Mikkelsen JH, Oxvig C, Marler RJ, Conover CA. Mice deficient in PAPP-A show resistance to the development of diabetic nephropathy. J Endocrinol. 2013;219(1):51–8.
Heo JM, Park JH, Kim JH, You SH, Kim JS, Ahn CM, Hong SJ, Shin KH, Lim DS. Comparison of inflammatory markers between diabetic and nondiabetic ST segment elevation myocardial infarction. J Cardiol. 2012;60:204–9.
Wang YJ, Gong ZQ, Bi XM, Li YL. Correlation of plasma soluble cluster of differentiation 40 ligand, alpha fetoprotein A, and pregnancy-associated plasma protein A with carotid plaque in patients with ischemic stroke. Genet Mol Res. 2015;14(3):8091–9.
Bonaca MP, Scirica BM, Sabatine MS, Jarolim P, Murphy SA, Chamberlin JS, Rhodes DW, Southwick PC, Braunwald E, Morrow DA. Prospective evaluation of pregnancy-associated plasma protein-a and outcomes in patients with acute coronary syndromes. J Am Coll Cardiol. 2012;60:332–8.
Wlazeł RN, Rysz J, Paradowski M. Examination of serum pregnancy-associated plasma protein A clinical value in acute coronary syndrome prediction and monitoring. Arch Med Sci. 2013;9:14–20.
Lund J, Wittfooth S, Qin QP, Ilva T, Porela P, Pulkki K, Pettersson K, Voipio-Pulkki LM. Free vs total pregnancy associated plasma protein A (PAPP-A) as a predictor of 1-year outcome in patients presenting with non-ST-elevation acute coronary syndrome. Clin Chem. 2010;56:1158–65.
Conover CA, Bale LK, Oxvig C. Targeted inhibition of pregnancy-associated plasma protein-A activity reduces atherosclerotic plaque burden in mice. Cardiovasc Transl Res. 2016;9(1):77–9.
Miedema MD, Conover CA, MacDonald H, Harrington SC, Oberg D, Wilson D, Henry TD, Schwartz RS. Pregnancy-associated plasma protein-A elevation in patients with acute coronary syndrome and subsequent. atorvastatin therapy. Am J Cardiol. 2008;101(1):35–9.
Authors’ contributions
WPL provided funding support, designed and performed study, and wrote manuscript. MN performed statistical analysis. FSG reviewed and edited manuscript. DI contributed discussion and edited manuscript. ZJS participated in study data collection. XW participated in study data collection. HWL designed study and reviewed manuscript. XQZ designed study, data analysis and manuscript development. All authors read and approved the final manuscript.
Acknowledgements
Not applicable.
Competing interests
All authors declare that they have no competing interests.
Availability of data and materials
All study data will be available on request.
Consent for publication
All authors consented to the anonymous publication of the study data.
Ethics approval and consent to participate
The study protocol and procedures received approval from the Beijing Friendship Hospital Institutional Review Board and were performed to conform to the Declaration of Helsinki. Informed consent was obtained from all subjects prior to any study related procedures or measurements.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No 81300161) and the High-level Technical Talents Foundation of Beijing Health System (Grant No 2013-3-060).
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Li, WP., Neradilek, M.B., Gu, FS. et al. Pregnancy-associated plasma protein-A is a stronger predictor for adverse cardiovascular outcomes after acute coronary syndrome in type-2 diabetes mellitus. Cardiovasc Diabetol 16, 45 (2017). https://doi.org/10.1186/s12933-017-0526-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-017-0526-6