Abstract
Background
Idiopathic pulmonary fibrosis (IPF) is a chronic progressive lung disease. Several risk factors such as smoking, air pollution, inhaled toxins, high body mass index and infectious agents are involved in the pathogenesis of IPF. In the present study, this meta-analysis study investigates the prevalence of viral and bacterial infections in the IPF patients and any possible association between these infections with pathogenesis of IPF.
Methods
The authors carried out this systematic literature review from different reliable databases such as PubMed, ISI Web of Science, Scopus and Google Scholar to December 2020.Keywords used were the following “Idiopathic pulmonary fibrosis”, “Infection”, “Bacterial Infection” and “Viral Infection”, alone or combined together with the Boolean operators "OR”, “AND” and “NOT” in the Title/Abstract/Keywords field. Pooled proportion and its 95% CI were used to assess the prevalence of viral and bacterial infections in the IPF patients.
Results
In this systematic review and meta-analyses, 32 studies were selected based on the exclusion/inclusion criteria. Geographical distribution of included studies was: eight studies in American people, 8; in European people, 15 in Asians, and one in Africans. The pooled prevalence for viral and bacterial infections w ere 53.72% (95% CI 38.1–69.1%) and 31.21% (95% CI 19.9–43.7%), respectively. The highest and lowest prevalence of viral infections was HSV (77.7% 95% CI 38.48–99.32%), EBV (72.02%, 95% CI 44.65–90.79%) and Influenza A (7.3%, 95% CI 2.66–42.45%), respectively. Whereas the highest and lowest prevalence in bacterial infections were related to Streptococcus sp. (99.49%, 95% CI 96.44–99.9%) and Raoultella (1.2%, 95% CI 0.2–3.08%), respectively.
Conclusions
The results of this review were confirmed that the presence of viral and bacterial infections are the risk factors in the pathogenesis of IPF. In further analyses, which have never been shown in the previous studies, we revealed the geographic variations in the association strengths and emphasized other methodological parameters (e.g., detection method). Also, our study supports the hypothesis that respiratory infection could play a key role in the pathogenesis of IP.
Similar content being viewed by others
Background
Idiopathic pulmonary fibrosis (IPF) is a chronic progressive lung disease of unknown etiology. IPF causes progressive scar tissue which gets worse over time resulting in acute dyspnea [1, 2]. Alveolar epithelial injury in IPF leads to fibroproliferation, myofibroblast differentiation and excessive collagen and extracellular matrix deposition, causing impairment of gas exchange, respiratory failure and death [3].
The prevalence of IPF is 14–27.9 and 1.25–23.4 cases per 100,000 population in the USA and Europe, respectively [4]. The attributable risk of IPF-related morbidity and mortality is associated with aging and occurs more among males than female [1, 4]. Several risk factors are involved in the IPF pathogenesis such as; smoking, high body mass index, toxins (inhaled) and infectious disease [3]. More recently, numerous studies have demonstrated the role of viruses and bacteria in the pathogenesis of IPF. It has been shown that patients with IPF have an increased bacterial load in bronchoalveolar lavage (BAL) fluid compared to healthy people or chronic obstructive pulmonary disease (COPD) patients [5,6,7,8]. Furthermore, various viruses and bacteria has been studied in the pathogenesis of IPF, including Respiratory syncytial virus (RSV), Parainfluenza virus (PIV), Rhinovirus, Coronavirus, Cytomegalovirus (CMV), Influenza virus, Strepococcus, Haemophillus and Neisseria [6, 9,10,11]. It has been reported that inflammation plays a critical role in genesis and progression of IPF in both human and murine models, indicating that viral and bacterial infections can be led to chronic infection and inflammation that maybe are the cause of IPF [12, 13].
Although several studies have been conducted to determine the prevalence of viral and bacterial infection in the IPF patients, the association between IPF pathogenesis and viral/bacterial infection remains the subject of ongoing investigation. This meta-analysis study investigates the prevalence of viral and bacterial infections in the IPF patients and any possible association between these infections with pathogenesis of IPF.
Methods
Search strategies
In this meta-analysis, a systematic search was conducted for previous studies relevant (2020) reliable databases, ISI Web of Science, PubMed, and Scopus. Literature searches were carried out by using the following keywords “Idiopathic pulmonary fibrosis”, “Infection”, “Bacterial Infection” and “Viral Infection”, alone or combined together with the Boolean operators "OR”, “AND” and “NOT” in the Title/Abstract/Keywords field. It should be noted that unpublished studies were not included, and duplicate ones were removed. Our literature searches were conducted by three reviewers independently and the search results were compared to prevent having missing data. Also, we screened citations of collected papers to identify additional eligible studies. Titles, abstracts and keywords field of all papers were screened, and unrelated studies were excluded to increase of specificity in the search results. This study was registered in PROSPRO (ID: 170736).
Inclusion and exclusion criteria
The inclusion criteria for eligible publications were defined as: all research that
-
Published: 1990 to 2020.
-
Reporting the presence of viral or/and bacterial infections [previous (colonization) and/or new infection] in IPF patients.
-
Conducting valid laboratory techniques such as: molecular technique, culture, and serology.
-
Selecting the proper sampling method including: NPS, OPS, Sputum, Serum, Blood, BAL and Lung Biopsy.
The exclusion criteria: all research that
-
Providing incomplete data or failed presented data clearly.
-
Animal models-based research.
-
Had other infectious agents.
-
Overlapping subjects, time, and place of sample collection.
Data extraction and quality assessment
Data extraction was conducted by two authors separately and independently based on author’s name, year of publication, total sample size, number of bacterial and viral infections patients, country, types of bacteria, types of viruses, types of samples and detection methods. Individual data from each included study were used in this meta-analysis. Extracted data were compared and rechecked by the first and corresponding authors. The methodological quality of the included studies was evaluated using the STROBE checklist. A maximum quality evaluation score of 32 was considered and articles with scores below 18 were excluded from this study [14].
Statistical methods
Pooled proportion and its 95% CI were used to assess the prevalence of viral and bacterial infections in the IPF patients. Generalized linear mixed and random intercept logistic regression models were used for pooling prevalence [15]. The heterogeneity of proportions between included studies was tested and quantified by using Cochran's Q test, Tau^2 and I2, respectively [16, 17]. The maximum-likelihood estimator was employed to estimate Tau^2. Logit transformation and Clopper-Pearson were used for pooled proportion and confidence interval in the individual studies. Also, continuity correction of 0.5 in studies with zero cell frequencies [18]. The pooled proportion, as an overall prevalence of viral and bacterial infections in IPF patients was derived by a random effects model because of significantly heterogeneity between the individual studies. However, influence analyses was performed by the Baujat plot which is a diagnostic plot to detect studies contributing to the heterogeneity of a meta-analysis [19]. A funnel plot was conducted to detect publication bias (logit transformed proportions against standard error). Publication bias was tested by Egger’s linear regression and Begg’s tests as it was described (P < 0.05 was considered statistically significance for publication bias) [20]. Finally, the sub-group analyses were used by types of virus and bacteria, year of publication, and country. Meta-regression was applied for assessing the effect of age on the pooled prevalence. All of statistical analyses were performed by using “metafor" and “meta” R packages.
Results
Search results and studies characteristics
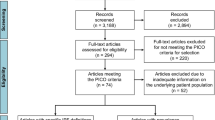
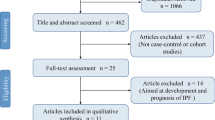
The process of research selection shown in Fig. 1 was designed according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses. In the initial search, 2813 articles were identified from ISI Web of Science, PubMed, Scopus and Google scholar databases. Based on the exclusion/inclusion criteria, 32 studies were included in the final meta-analysis. Geographical distribution of included studies was; eight studies in America, eight in Europe, 15 in Asia, and one in Africa. These studies were published from 1992 to 2020 (Table 1).
Quality assessment
Based on the results of the STROBE checklist, highest and lowest score were related to Dworniczak et al. (score = 18) and Keyvani et al. (score = 31), respectively. The mean score of STROBE tool for all of included studies was 25.8 (SD = 4.3, range = 18–31). (Table 1).
Pooled prevalence of viral and bacterial infections in the IPF patients
The total number of the IPF patients included in the study was 2203 individuals aged 26–87 years based on the results of the 32 included studies. The pooled prevalence of viral and bacterial infections in the studied patients was 57.3% (95% CI 37.91–74.75%) according to a random effects meta-analysis. The Wald test showed a significant heterogeneity of prevalence between the studies (Q statistic = 460; Wald test p-value < 0.001; I^2 = 96.5%; τ^2 = 4.69) (Fig. 2). The pooled prevalence for viral infections was 53.72% (95% CI 38.1–69.1%) according to a random effects meta-analysis. While the pooled prevalence for bacterial infections was 31.21% (95% CI 19.9–43.7%) according to a random effects meta-analysis. There was a significant difference of pooled prevalence between viral and bacterial infections (P-value < 0.001).
Sub-group analysis and meta-regression
The highest and lowest prevalence of viral infections that was reported in patients with IPF as follows; HSV (77.7% 95% CI 38.48–99.32%), EBV (72.02%, 95% CI 44.65–90.79%) and Influenza A (7.3%, 95% CI 2.66–42.45%), respectively (Table 2). Whereas the highest and lowest of this prevalence in bacterial infections were related to Streptococcus sp. (99.49%, 95% CI 96.44–99.9%) and Raoultella (1.2%, 95% CI 0.2–3.08%), respectively. More details about heterogeneity test and publication bias is shown in Table 2. Also, lowest prevalence of viral and bacterial infections was observed in the studies that were published from 2013 to 2020 (32%, 95% CI 15–56%) and the highest of this prevalence related to the studies published between 1999 and 2006 (78%, 95% CI 50–93%). The difference of pooled prevalence between the ranges of year of publication was significant (P-value < 0.001) (Fig. 3). In sub-group analysis based on country, the highest prevalence of viral and bacterial infections was observed in United States (86.9%, 95% CI 65.7–100%) and Japan (69.9%, 95% CI 58.9–78.3%), whereas United Kingdom (5%, 95% CI 2–8.1%) and South Korea (1.5%, 95% CI 0.4–2.6%) showed the lowest prevalence. Based on the meta-regression, with increasing age of the patients, this prevalence was significantly decreased (P-value = 0.048) (Fig. 4). Sub-group analysis based on the type of detection methods showed that PCR technique detected the highest viral infections in IPF patents (76.4%, 95% CI 60.18–90.01%), while using ELISA method showed the lowest prevalence of viral infections (32%, 95% CI 16.1–51.9%). These results for types of detection methods of bacterial infections indicated the highest and lowest prevalence observed in the IPF patients related to sputum culture (60.2%, 95% CI 27.8–92.4%) and ELISA (20.2%, 95% CI 3.3–44.7%) detection methods. In addition, the highest and lowest prevalence of viral and bacterial infections were observed in the IPF patients related to serum (64.8%, 95% CI 38.7–80.9%) and lung tissues/or lung biopsy (33.7%, 95% CI 17.71–41.98%) sample types, respectively.
Publication bias and sensitivity analysis
Publication bias was statistically significant in this meta-analysis (Begg’s p-value = 0.041, Egger’s p-value = 0.046) (Fig. 5). In most of cases in Table 2, the publication bias was not significant. Furthermore, the robustness of the pooled prevalence was checked by Baujat plot as a plot to identify the studies, which overly contributing to the heterogeneity of the meta-analysis. However, the studies of Song et al. in 2011 [21] and Odashima et al. in 2020 [22] have most significant influence on the overall results (P-value < 0.001) (Fig. 6).
Discussion
IPF is a fatal progressive lung disease that there is no effective cure for this except lung transplant for end-stage IPF patients [23]. Moreover, the FDA approved drugs are associated with a number of side-effects, which compromises their tolerability in IPF patients. Although, the cause of IPF is unknown, recent studies have suggested a strong impact of viral and bacterial infections in both the initiation and progression of IPF may be through aberrant innate immunity [1, 5, 6, 9, 12, 13]. These infectious agents can play a crucial role in increased inflammation and thus in IPF pathogenesis. Viral infection, both lytic and latent, can lead to major fibrosis through increased expression of viral gene products in structural and immune cells in the lung [6, 12, 24]. More recently, a role for bacterial infection has been described in the development of a rapidly progressive clinical phenotype in IPF [5, 25, 26]. Therefore, optimum antiviral and antibacterial immunity in the lung is vital in the maintenance of lung homeostasis and health [5].
Thus far, no systematic review and meta-analysis study has been investigated in the prevalence and the role of viral and bacterial infections in IPF except an investigation by Sheng et al. They suggested viral infection as a risk factor (OR 3.48; 95% CI, 1.61–7.52), however not statistically significant relationship was detected between viral infection and exacerbation of IPF (OR 0.99; 95% CI 0.47–2.12). They also demonstrated that some viruses such as CMV, Epstein-Barr virus (EBV) and human herpesvirus 7, and 8 (HHV-7, HHV-8) were associated with IPF as the risk factors, while HHV-6 was not associated. Therefore, their results indicated that viral infections may involve in IPF pathogenesis [27]. In our study, according to a random effects meta-analysis, the pooled prevalence for viral infections was 53.72% (95% CI 38.1–69.1%) and the highest and lowest prevalence of viral infections was related to HSV (77.7% 95% CI 38.48–99.32), EBV (72.02%, 95% CI 44.65–90.79%) and Influenza A (7.3%, 95% CI 2.66–42.45), respectively (Table 2). Numerous investigations have been conducted on the role of viruses in pathogenesis of IPF, suggesting the role of viral infection in exacerbations of IPF [28]. Wootton et al. studied the detection of viral infection in IPF patients by pan-viral microarray analysis. They detected parainfluenza 1.6% (1/60), rhinovirus 3.3% (2/60) and coronavirus 1.6% (1/60) [9]. In another study, Keyvani et al. assessed forty IPF patients for viral detection and they detected RSV, parainfluenza, rhino and corona viruses in 2.5% (1/40), 7.5% (3/40), 10% (4/40), 2.5% (1/40) and 0% (0/40) of patients, respectively. Their results indicated a significant positive association between age and two viruses (rhinovirus and parainfluenza) [24]. In the current study, based on the type of detection methods of viral infections we indicated that the highest and lowest prevalence of viral infections in previously published studies on IPF were observed by PCR (83.1%, 95% CI 63.75–94.11%) and ELISA (32%, 95% CI 16.1–51.9%), respectively. Thus, it can be proposed that the type of detection method is important in the report of the prevalence of viral infections. Geographical variations might explain the inconsistent results that is present in the studies. In the present investigation, we indicated that the highest prevalence of viral and bacterial infections was in Japan (73.1%, 95% CI 69.3–76.8%) and United States (86.9%, 95% CI 65.7–100%), and the lowest prevalence was in United Kingdom (5%, 95% CI 2–8.1%) and South Korea (1.5%, 95% CI 0.4–2.6%).
The viral infection (especially respiratory viruses) may be involved in increasing of inflammation (chronic), resulting in the pathogenesis of IPF [9, 24, 29]. Due to the function of lung, it is exposed to airborne viruses and there is mounting evidence, which are provided by clinical and preclinical studies, to support a mechanistic role for pathogens of IPF [28]. Some viruses cause latent infections within the alveolar epithelium and under suitable conditions, they are reactivated. This issue can be proposed reactivation of the virus acts as a second hit to the epithelium following exposure to a first injurious insult [30]. Some animal studies have shown that viral infection lead to enhance lung fibrosis hence cofactor in the IPF development [28]. Viral infection induces stress in endoplasmic reticulum (ER) and apoptosis in epithelial cells which can implicate in the development of both IPF and the pulmonary fibrosis [31, 32]. In an investigation, have been shown that acute and chronic viral infections are different in some aspect of Immunopathogenesis [5, 9, 12]. Most of the analyzed studies (including viral infections) in our study were about persistent viruses that were probably acquired before the IPF development.
Although, numerous attempts have conducted on the role of viruses in the IPF pathogenesis, there are a few studies investigating the role of bacteria in this disease. In a recent study, it was demonstrated that 7.5% of IPF patients (BAL sample) were found to be positive in bacterial culture, while none of the controls had bacterial infection. The most common bacterial genus were Streptococcus (30%) followed by Veillonella (10.6%) and Prevotella (10.9%) [6]. In another study, Richter et al. reported 36.3% of stable IPF patients was positive BAL cultures and bacterial genus were Pseudomonas, Haemophilus and Streptococcus [33]. In our study, the pooled prevalence for bacterial infections was 31.21% (95% CI 19.9–43.7%) according to a random effects meta-analysis. In addition, the highest and lowest prevalence in bacterial infections were related to Streptococcus sp. (99.49%, 95% CI 96.44–99.9%) and Raoultella (1.2%, 95% CI 0.2–3.08%), respectively. High case fatality rate related to bacterial respiratory tract infection in IPF, indicating that bacteria are involved in driving IPF disease progression. Recently, studies using culture-independent techniques have demonstrated that increased bacterial DNA burden in IPF patients is associated with an enhanced risk of earlier mortality in IPF [6, 34, 35]. These results for types of detection methods of bacterial infections showed the highest and lowest prevalence were observed in the IPF patients related to sputum culture (60.2% 95% CI 27.8–92.4%) and ELISA (20.2% 95% CI 3.3–44.7%) detection methods. Also, as mentioned earlier our results indicated the prevalence of viral and bacterial infections was highest in Japan (73.1%, 95% CI 69.3–76.8%) and United States (86.9%, 95% CI 65.7–100%) and this prevalence was lowest in United Kingdom (5%, 95% CI 2–8.1%) and South Korea (1.5%, 95% CI 0.4–2.6%) countries. Infectious agents induce immune responses that can lead to chronic conditions and inflammatory infiltrates, both of which have shown too involved in IPF pathogenesis (23). Moreover, preclinical and clinical studies demonstrated that inflammation is probably involved in initiation and progression of IPF (9, 10).
Cytokine patterns in patients with IPF may shed light on the predominant cell types pivotal to various stages of the disease. Overexpression of Th2 Cytokines including IL-4, IL-5, and IL-13 in cellular cultures from patients with IPF has been previously reported [13]. A series of cytokines (MIP-1α/CCL3), MCP-1/CCL2, and IL-8) connected to neutrophil, monocytes, and lymphocyte chemotaxis and activation are increased significantly in tissue or fluid from the lungs of IPF patients [13, 24]. IL-1α and IL-1β are widely expressed cytokines by alveolar macrophages of IPF patients. This expression lead to induce a pro-fibrotic phenotype through the synthesis of platelet-derived growth factor and procollagen types I and III [36]. Tumor necrosis factor alpha (TNF-α), which is produced by epithelial cells, endothelial cells, lymphocytes and macrophages, upregulates various pathways and factors those involved in inflammation such as the IL-1, IL-6, growth factor beta (TGF-β), C-X-C motif chemokine ligand 8, stimulation of cell–cell adhesion and transendothelial migration [37]. The overexpression of TGF-β results in modulation of extracellular matrix (ECM) productions. This modulation is due to the effects of different factors including fibronectin, proteoglycans, collagens I, III, IV, V and the inhibition of modifying ECM enzymes such as plasminogen and metalloproteinase [38].
All researches that reported the viral and bacterial infection in IPF patients were included in this meta-analysis. The sample size in some researches was small. Furthermore, the viral and bacterial infection rates were variable due to Variety of geographical locations, viral/bacterial detection techniques and the sites of biological samples or type of samples. Different methods led to alterations in sensitivity and specificity. Given these challenges, larger-scale samples are needed in the future to draw conclusions about causal relationship between IPF and viral-bacterial infections.
Our study had several limitations; first, the small sample size, relative wide confidence intervals and study were conducted in a single center. Second, the pathogen types were specific to the study area. Thus, our results are probably not applicable to other patient populations. The association between viral infection and acute exacerbation of IPF requires further investigation.
Conclusion
The current study provides the overall viral and bacterial infection prevalence in IPF patients and information about circulating types of viruses and bacterial worldwide. The presence of viral and bacterial infections is a risk factor in the pathogenesis of IPF. We revealed the geographic variations in the association strengths and emphasized other methodological parameters (e.g., detection method) in further analyses that have never been shown in the previous studies. Also, our study supports the hypothesis that respiratory infection could play a key role in the pathogenesis of IPF.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its supplementary information files.
Abbreviations
- IPF:
-
Idiopathic pulmonary fibrosis
- BAL:
-
Bronchoalveolar lavage
- COPD:
-
Chronic obstructive pulmonary disease
- RSV:
-
Respiratory syncytial virus
- PIV:
-
Parainfluenza virus
- CMV:
-
Cytomegalovirus
- EBV:
-
Epstein-Barr virus
- ER:
-
Endoplasmic reticulum
- TNF-a:
-
Tumor necrosis factor alpha
- TGF-β:
-
Transforming growth factor beta
References
Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier J-F, Flaherty KR, Lasky JA. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824.
Lederer DJ, Martinez FJ. Idiopathic pulmonary fibrosis. N Engl J Med. 2018;378:1811–23.
Borchers AT, Chang C, Keen CL, Gershwin ME. Idiopathic pulmonary fibrosis—an epidemiological and pathological review. Clin Rev Allergy Immunol. 2011;40:117–34.
Nalysnyk L, Cid-Ruzafa J, Rotella P, Esser D. Incidence and prevalence of idiopathic pulmonary fibrosis: review of the literature. Eur Respir Rev. 2012;21:355–61.
Moore BB, Moore TA. Viruses in idiopathic pulmonary fibrosis. Etiology and exacerbation. Ann Am Thorac Soc. 2015;12:S186-92.
Molyneaux PL, Cox MJ, Willis-Owen SA, Mallia P, Russell KE, Russell A-M, Murphy E, Johnston SL, Schwartz DA, Wells AU. The role of bacteria in the pathogenesis and progression of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2014;190:906–13.
Moghoofei M, Azimzadeh Jamalkandi S, Moein M, Salimian J, Ahmadi A. Bacterial infections in acute exacerbation of chronic obstructive pulmonary disease: a systematic review and meta-analysis. Infection. 2020;48:19–35.
Jafarinejad H, Moghoofei M, Mostafaei S, Salimian J, Jamalkandi SA, Ahmadi A. Worldwide prevalence of viral infection in AECOPD patients: a meta-analysis. Microb Pathog. 2017;113:190–6.
Wootton SC, Kim DS, Kondoh Y, Chen E, Lee JS, Song JW, Huh JW, Taniguchi H, Chiu C, Boushey H. Viral infection in acute exacerbation of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;183:1698–702.
Yonemaru M, Kasuga I, Kusumoto H, Kunisawa A, Kiyokawa H, Kuwabara S, Ichinose Y, Toyama K. Elevation of antibodies to cytomegalovirus and other herpes viruses in pulmonary fibrosis. Eur Respir J. 1997;10:2040–5.
Moghoofei M, Monavari SH, Mostafaei S, Hadifar S, Ghasemi A, Babaei F, Kavosi H, Tavakoli A, Javanmard D, Esghaei M. Prevalence of influenza A infection in the Middle-East: a systematic review and meta-analysis. Clin Respir J. 2018;12:1787–801.
Homer RJ, Elias JA, Lee CG, Herzog E. Modern concepts on the role of inflammation in pulmonary fibrosis. Arch Pathol Lab Med. 2011;135:780–8.
Bringardner BD, Baran CP, Eubank TD, Marsh CB. The role of inflammation in the pathogenesis of idiopathic pulmonary fibrosis. Antioxid Redox Signal. 2008;10:287–302.
Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147:573–7.
Stijnen T, Hamza TH, Özdemir P. Random effects meta-analysis of event outcome in the framework of the generalized linear mixed model with applications in sparse data. Stat Med. 2010;29:3046–67.
Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods. 2006;11:193.
Monavari SHR, Hadifar S, Mostafaei S, Miri A, Keshavarz M, Babaei F, Moghoofei M. Epidemiology of rotavirus in the Iranian children: a systematic review and meta-analysis. J Glob Infect Dis. 2017;9:66.
Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36:1–48.
Baujat B, Mahé C, Pignon JP, Hill C. A graphical method for exploring heterogeneity in meta-analyses: application to a meta-analysis of 65 trials. Stat Med. 2002;21:2641–52.
Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34.
Song JW, Hong S-B, Lim C-M, Koh Y, Kim DS. Acute exacerbation of idiopathic pulmonary fibrosis: incidence, risk factors and outcome. Eur Respir J. 2011;37:356–63.
Odashima K, Kagiyama N, Kanauchi T, Ishiguro T, Takayanagi N. Incidence and etiology of chronic pulmonary infections in patients with idiopathic pulmonary fibrosis. PLoS ONE. 2020;15:e0230746.
Maher T, Wells A, Laurent G. Idiopathic pulmonary fibrosis: multiple causes and multiple mechanisms? Eur Respir J. 2007;30:835–9.
Keyvani H, Moghoofei M, Bokharaei-Salim F, Mostafaei S, Mousavi S-AJ, Monavari SH, Esghaei M. Prevalence of respiratory viruses in Iranian patients with idiopathic pulmonary fibrosis. J Med Microbiol. 2017;66:1602–6.
Molyneaux PL, Cox MJ, Wells AU, Kim HC, Ji W, Cookson WO, Moffatt MF, Kim DS, Maher TM. Changes in the respiratory microbiome during acute exacerbations of idiopathic pulmonary fibrosis. Respir Res. 2017;18:29.
Molyneaux PL, Maher TM. Respiratory microbiome in IPF: cause, effect, or biomarker? Lancet Respir Med. 2014;2:511–3.
Sheng G, Chen P, Wei Y, Yue H, Chu J, Zhao J, Wang Y, Zhang W, Zhang H-L. Viral infection increases the risk of idiopathic pulmonary fibrosis: a meta-analysis. Chest. 2019;157:1175–87.
Molyneaux PL, Maher TM. The role of infection in the pathogenesis of idiopathic pulmonary fibrosis. Eur Respir Rev. 2013;22:376–81.
Desai O, Winkler J, Minasyan M, Herzog EL. The role of immune and inflammatory cells in idiopathic pulmonary fibrosis. Front Med. 2018;5:43.
Kropski JA, Lawson WE, Blackwell TS. Right place, right time: the evolving role of herpesvirus infection as a “second hit” in idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2012;302:L441–4.
Isler JA, Skalet AH, Alwine JC. Human cytomegalovirus infection activates and regulates the unfolded protein response. J Virol. 2005;79:6890–9.
Lawson WE, Crossno PF, Polosukhin VV, Roldan J, Cheng D-S, Lane KB, Blackwell TR, Xu C, Markin C, Ware LB. Endoplasmic reticulum stress in alveolar epithelial cells is prominent in IPF: association with altered surfactant protein processing and herpesvirus infection. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1119–26.
Richter AG, Stockley RA, Harper L, Thickett DR. Pulmonary infection in Wegener granulomatosis and idiopathic pulmonary fibrosis. Thorax. 2009;64:692–7.
Hilty M, Burke C, Pedro H, Cardenas P, Bush A, Bossley C, Davies J, Ervine A, Poulter L, Pachter L. Disordered microbial communities in asthmatic airways. PLoS ONE. 2010;5:e8578.
Huang YJ, Kim E, Cox MJ, Brodie EL, Brown R, Wiener-Kronish JP, Lynch SV. A persistent and diverse airway microbiota present during chronic obstructive pulmonary disease exacerbations. OMICS. 2010;14:9–59.
Goldring M, Birkhead J, Sandell L, Kimura T, Krane S. Interleukin 1 suppresses expression of cartilage-specific types II and IX collagens and increases types I and III collagens in human chondrocytes. J Clin Investig. 1988;82:2026–37.
Zhang K, Gharaee-Kermani M, McGarry B, Remick D, Phan SH. TNF-alpha-mediated lung cytokine networking and eosinophil recruitment in pulmonary fibrosis. J Immunol. 1997;158:954–9.
Agostini C, Gurrieri C. Chemokine/cytokine cocktail in idiopathic pulmonary fibrosis. Proc Am Thorac Soc. 2006;3:357–63.
Ueda T, Ohta K, Suzuki N, Yamaguchi M, Hirai K, Horiuchi T, Watanabe J, Miyamoto T, Ito K. Idiopathic pulmonary fibrosis and high prevalence of serum antibodies to hepatitis C virus1• 2. Am Rev Respir Dis. 1992;1:268–268.
Meliconi R, Andreone P, Fasano L, Galli S, Pacilli A, Miniero R, Fabbri M, Solforosi L, Bernardi M. Incidence of hepatitis C virus infection in Italian patients with idiopathic pulmonary fibrosis. Thorax. 1996;51:315–7.
Kuwano K, Nomoto Y, Kunitake R, Hagimoto N, Matsuba T, Nakanishi Y, Hara N. Detection of adenovirus E1A DNA in pulmonary fibrosis using nested polymerase chain reaction. Eur Respir J. 1997;10:1445–9.
Stewart JP, Egan JJ, Ross AJ, Kelly BG, Lok SS, Hasleton PS, Woodcock AA. The detection of Epstein-Barr virus DNA in lung tissue from patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1999;159:1336–41.
Tsukamoto K, Hayakawa H, Sato A, Chida K, Nakamura H, Miura K. Involvement of Epstein-Barr virus latent membrane protein 1 in disease progression in patients with idiopathic pulmonary fibrosis. Thorax. 2000;55:958–61.
Tang Y-W, Johnson J, Cruz-Gervis R, Graham B, Brigham K, Oates J, Loyd J, Stecenko A. Increased detection of herpesvirus DNA in idiopathic pulmonary fibrosis. Chest. 2001;120:S74–5.
Lok S, Stewart J, Kelly B, Hasleton P, Egan J. Epstein-Barr virus and wild p53 in idiopathic pulmonary fibrosis. Respir Med. 2001;95:787–91.
Kelly BG, Lok SS, Hasleton PS, Egan JJ, Stewart JP. A rearranged form of Epstein-Barr virus DNA is associated with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2002;166:510–3.
Magro CM, Allen J, Pope-Harman A, Waldman WJ, Moh P, Rothrauff S, Ross P Jr. The role of microvascular injury in the evolution of idiopathic pulmonary fibrosis. Am J Clin Pathol. 2003;119:556–67.
Tang Y-W, Johnson JE, Browning PJ, Cruz-Gervis RA, Davis A, Graham BS, Brigham KL, Oates JA Jr, Loyd JE, Stecenko AA. Herpesvirus DNA is consistently detected in lungs of patients with idiopathic pulmonary fibrosis. J Clin Microbiol. 2003;41:2633–40.
Dworniczak S, Ziora D, Kapral M, Mazurek U, Niepsuj G, Rauer R, Wilczok T, Kozielski J. Human cytomegalovirus DNA level in patients with idiopathic pulmonary fibrosis. J Physiol Pharmacol. 2004;55:67–75.
Miyake Y, Sasaki S, Yokoyama T, Chida K, Azuma A, Suda T, Kudoh S, Sakamoto N, Okamoto K, Kobashi G. Case-control study of medical history and idiopathic pulmonary fibrosis in Japan. Respirology. 2005;10:504–9.
Bando M, Takahashi M, Ohno S, Hosono T, Hironaka M, Okamoto H, Sugiyama Y. Torque teno virus DNA titre elevated in idiopathic pulmonary fibrosis with primary lung cancer. Respirology. 2008;13:263–9.
Pozharskaya V, Torres-Gonzalez E, Rojas M, Gal A, Amin M, Dollard S, Roman J, Stecenko AA, Mora AL. Twist: a regulator of epithelial-mesenchymal transition in lung fibrosis. PLoS ONE. 2009;4:e7559.
Lasithiotaki I, Antoniou KM, Vlahava V-M, Karagiannis K, Spandidos DA, Siafakas NM, Sourvinos G. Detection of herpes simplex virus type-1 in patients with fibrotic lung diseases. PLoS ONE. 2011;6:e27800.
Pulkkinen V, Salmenkivi K, Kinnula VL, Sutinen E, Halme M, Hodgson U, Lehto J, Jääskeläinen A, Piiparinen H, Kere J. A novel screening method detects herpesviral DNA in the idiopathic pulmonary fibrosis lung. Ann Med. 2012;44:178–86.
Calabrese F, Kipar A, Lunardi F, Balestro E, Perissinotto E, Rossi E, Nannini N, Marulli G, Stewart JP, Rea F. Herpes virus infection is associated with vascular remodeling and pulmonary hypertension in idiopathic pulmonary fibrosis. PLoS ONE. 2013;8:e55715.
dos Santos G, Parra E, Stegun F, Cirqueira C, Capelozzi V. Immunohistochemical detection of virus through its nuclear cytopathic effect in idiopathic interstitial pneumonia other than acute exacerbation. Braz J Med Biol Res. 2013;46:985–92.
Folcik VA, Garofalo M, Coleman J, Donegan JJ, Rabbani E, Suster S, Nuovo A, Magro CM, Di Leva G, Nuovo GJ. Idiopathic pulmonary fibrosis is strongly associated with productive infection by herpesvirus saimiri. Mod Pathol. 2014;27:851–62.
Bando M, Nakayama M, Takahashi M, Hosono T, Mato N, Yamasawa H, Okamoto H, Sugiyama Y. Serum torque teno virus DNA titer in idiopathic pulmonary fibrosis patients with acute respiratory worsening. Intern Med. 2015;54:1015–9.
Ushiki A, Yamazaki Y, Hama M, Yasuo M, Hanaoka M, Kubo K. Viral infections in patients with an acute exacerbation of idiopathic interstitial pneumonia. Respir Investig. 2014;52:65–70.
Rabea AEM, Zidan M, Daabis R, El Sayed P, Samir S. Prevalence of chronic hepatitis C virus (HCV) infection in patients with idiopathic pulmonary fibrosis. Egypt J Chest Dis Tuberc. 2015;64:907–13.
Saraya T, Kimura H, Kurai D, Tamura M, Ogawa Y, Mikura S, Sada M, Oda M, Watanabe T, Ohkuma K. Clinical significance of respiratory virus detection in patients with acute exacerbation of interstitial lung diseases. Respir Med. 2018;136:88–92.
Weng D, Chen X-Q, Qiu H, Zhang Y, Li Q-H, Zhao M-M, Wu Q, Chen T, Hu Y, Wang L-S. The role of infection in acute exacerbation of idiopathic pulmonary fibrosis. Mediat Inflamm. 2019;2019:1–10.
Le Hingrat Q, Ghanem M, Cazes A, Visseaux B, Collin G, Descamps D, Charpentier C, Crestani B. No association between human herpesvirus or herpesvirus saimiri and idiopathic pulmonary fibrosis. ERJ Open Res. 2020. https://doi.org/10.1183/23120541.00243-2020.
Jafarian AH, Roshan NM, Ayatollahi H, Omidi AA, Ghaznavi M, Gharib M. Epstein-Barr virus and human herpesvirus 8 in idiopathic pulmonary fibrosis. Iran J Pathol. 2020;15:30.
Acknowledgements
None to disclose.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Conception and design: MM and SM; Search strategy: SH and AB. Study selection: SH and AB. Data extraction: SH, AB and MEFA. Data synthesis and analysis: SM, PR and BB. Data interpretation: SM, BS, MD and MM. Manuscript drafting: SM, MD, and MM. Manuscript revision and editing: JSN, BMH, MD and MM. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflicting interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mostafaei, S., Sayad, B., Azar, M.E.F. et al. The role of viral and bacterial infections in the pathogenesis of IPF: a systematic review and meta-analysis. Respir Res 22, 53 (2021). https://doi.org/10.1186/s12931-021-01650-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12931-021-01650-x