Abstract
Background
The clinical and radiological presentation of chronic obstructive pulmonary disease (COPD) is heterogenous depending on the characterized sources of inflammation. This study aimed to evaluate COPD phenotypes associated with specific dust exposure.
Methods
This study was designed to compare the characteristics, clinical outcomes and radiological findings between two prospective COPD cohorts representing two distinguishing regions in the Republic of Korea; COPD in Dusty Area (CODA) and the Korean Obstructive Lung Disease (KOLD) cohort. A total of 733 participants (n = 186 for CODA, and n = 547 for KOLD) were included finally. A multivariate analysis to compare lung function and computed tomography (CT) measurements of both cohort studies after adjusting for age, sex, education, body mass index, smoking status, and pack-year, Charlson comorbidity index, and frequency of exacerbation were performed by entering the level of FEV1(%), biomass exposure and COPD medication into the model in stepwise.
Results
The mean wall area (MWA, %) became significantly lower in COPD patients in KOLD from urban and metropolitan area than those in CODA cohort from cement dust area (mean ± standard deviation [SD]; 70.2 ± 1.21% in CODA vs. 66.8 ± 0.88% in KOLD, p = 0.028) after including FEV1 in the model. COPD subjects in KOLD cohort had higher CT-emphysema index (EI, 6.07 ± 3.06 in CODA vs. 20.0 ± 2.21 in KOLD, p < 0.001, respectively). The difference in the EI (%) was consistently significant even after further adjustment of FEV1 (6.12 ± 2.88% in CODA vs. 17.3 ± 2.10% in KOLD, p = 0.002, respectively). However, there was no difference in the ratio of mean lung density (MLD) between the two cohorts (p = 0.077). Additional adjustment for biomass parameters and medication for COPD did not alter the statistical significance after entering into the analysis with COPD medication.
Conclusions
Higher MWA and lower EI were observed in COPD patients from the region with dust exposure. These results suggest that the imaging phenotype of COPD is influenced by specific environmental exposure.
Similar content being viewed by others
Introduction
Chronic obstructive pulmonary disease (COPD) is defined as progressive airway obstruction with parenchymal lung destruction and airway inflammation. The prevalence of COPD is increasing and COPD is predicted to become the 3rd leading cause of death worldwide by 2030 [1, 2]. The association of COPD with poor clinical outcomes including lung function decline, acute exacerbations (AE) and mortality has suggested clues for further studies to identify specific phenotypes and modifiable risk factors for COPD in individual daily life.
The clinical and radiological presentations of COPD have proposed heterogeneity. Inflammation, characterized by COPD, can differ by smoking, air pollutants, biomass, or genetic factors leading to diverse clinical or radiological findings. Heterogeneity of COPD on computed tomography (CT) findings have been broadly characterized as either; emphysema-predominant or airway-predominant type [3, 4]. Bronchial parameters such as bronchial lumen area (LA) and bronchial wall area (WA) represented bronchial wall thickness and have been shown to correlate with spirometric parameters [5,6,7]. The mean value of two segmental bronchi was defined as mean wall area (MWA) [8]. The values of airway thickness such as MWA% may be related to inflammation of airway and are associated with symptom of chronic bronchitis, acute exacerbation and bronchodilator responsiveness. Increased MWA% has strong associations with decreased lung function and increased frequency of acute exacerbations [9]. Phenotypic differences have been reported in the studies of COPD investigating the association between biomass and smoke exposure, which were suggest a small airway disease rather than an emphysematous phenotype upon cigarette smoking exposure [10,11,12]. A cluster analysis of four subgroups of smokers suggested a genetic effect with an association between the clinical characteristics of COPD and known COPD-associated genetic variants [13].
To evaluate COPD phenotypes for specific exposure, it is necessary to compare radiologic findings between patients who reside in regions representing specific dust exposure and non-residents. In this study, we investigated whether there is a difference in structural findings in CT by evaluating the characteristic of patients with COPD who lived in two functional areas; urban areas where mainly composed of metropolitan regions with residential areas, without large industrial emissions and rural areas, where large cement plants are located nearby expecting exposure to different composition of dust.
Methods
Study design and subjects
This study was designed to compare characteristics and clinical outcomes using radiological findings between two prospective COPD cohorts representing distinguishing regions in the Republic of Korea; COPD in Dusty Area (CODA) and the Korean Obstructive Lung Disease (KOLD) cohorts. The CODA cohort was prospectively recruited from 2012 to 2015 to observe the clinical outcomes of Korean COPD patients from rural area living near cement plants [14]. This cohort was enrolled from Kangwon and Chungbuk provinces in Korea where cement plants were mostly located. The KOLD cohort, which comprised participants from 17 centers recruited from 2005 to 2013, enrolled patients at 17 centers in an urban area in Korea [15]. COPD subjects in both cohorts were recruited for medical examination which consisted of a questionnaire regarding environmental exposure, health-related symptoms and behaviors, and laboratory findings with a pulmonary function test (PFT) with post-bronchodilator use and CT. Among participants in both cohorts, individuals with COPD defined according to European Respiratory Society/American Thoracic Society (ERS/ATS) guidelines (post-bronchodilatorforced expiratory volume in 1 s (FEV1)/Forced vital capacity (FVC) < 70%) and having a history of smoking of over 10 pack-years (PY) were eligible. Finally, 733 participants (n = 186 for CODA, and n = 547 for KOLD, for each) were included.
The study design and methods were approved by the Institutional Review Board (IRB) of Kangwon National University Hospital (IRB No. KNUH 2012-06-007) and the study was conducted following the tenets of the Declaration of Helsinki.
Spirometry and biomass exposure
Spirometry tests were performed using standardized equipment by qualified technicians following the ATS/ERS guidelines [16]. Spirometry tests were repeated at least three times to achieve within- and between-maneuver acceptability criteria. After the initial spirometry testing (pre-dose spirometry), a dose of 100 μg of salbutamol was fully inhaled in one breath, and the breath was then held for 5 to 10 s before exhalation. Two separate doses (total dose 200 μg) were administered at approximately 30-s intervals. Three additional spirometry tests were performed between 10 and 15 min later for reversibility testing. Spirometric tests were done by identical ways in both cohorts. The predicted value of spirometry were calculated by Choi’ reference equation which was validated for Korean population [17].
In this study, we asked previous biomass exposure which was also known to influence the phenotype of COPD by questionnaire [10]. The question for direct exposure to charcoal and wood, which were the most common fuel for cooking and heating in Korea until 1980s regardless of where persons lived, were asked as follow; “Have you ever been exposed to wood or charcoal fuels for cooking and/or heating?”. The presence of biomass exposure was defined by more than 10 years of direct exposure history to charcoal and wood.
Computed tomography
Volumetric CT scans were taken at full inspiration and expiration, using a first-generation dual-source CT system (Somatom Definition, Siemens Healthcare, Forchheim, Germany) in CODA and 16-multidetector CT scanners produced by three different manufacturers (Somatom Sensation 16; Siemens Medical Systems, Bonn, Germany; GE Lightspeed Ultra; General Electric Healthcare, Milwaukee, WI, USA; and Philips Brilliance 16, Philips Medical Systems, Best, Netherlands) in KOLD based on their reports of both the CODA and KOLD studies [15]. Scan parameters were as follows: 120 or 140 kVp; 100–135 effective mA with dose modulation; slice thickness and reconstruction intervals of 0.6–0.8 mm. Detailed information regarding the measurement of CT findings has been previously described and an identical protocol for CT measurement were applied to participants of both cohorts [5, 6, 8]. Whole-lung images were extracted from the chest wall, mediastinum and large airway automatically, and the attenuation coefficient of each pixel was calculated. The mean of the lung density values derived from the histogram of whole lung was defined as mean lung density (MLD). From the CT data, the emphysema index (EI) was defined using the volume fraction (%) of the lung below − 950 Hounsfield Units (HU) at maximum inspiration. The ratio MLD at full expiration and inspiration was automatically calculated. The measured values are the mean of density of air in alveoli and the surrounding epithelial, capillary and extracellular matrix and small airways. The airway dimensions, wall area (WA), lumen area (LA), and WA percent (i.e., WA/(WA + LA) × 100) were measured near the origin of the two segmented bronchi (right apical and left apico-posterior) to assess airway thickness using in-house software. The mean value of two segmental bronchi was defined as mean wall area (MWA) [8]. To express the morphologic characteristics quantitatively, we used the most commonly employed approach: EI as emphysema extent, the ratio of the MLD on expiration and inspiration as air trapping extent and the percentage of the MWA (MWA %) as the extent of airway disease.
Statistical analysis
The baseline characteristics of the patients were reported as mean ± standard deviation (SD). The distribution of each variable was tested by Kolmogorov–Smirnov (K–S) and Shapiro–Wilk test. Normal distribution parameters were analyzed using an independent sample t-test, and non-normal distribution data, such as the EI and hs-CRP, were analyzed using the Mann–Whitney U-test. As EI was not in normal distribution, it was analyzed by log-transformed value. Multivariate analysis of the means of clinical and CT variables were estimated using the least squares mean of the generalized linear model. Age, body mass index (BMI), and post-bronchodilator FEV1 (% of predicted) were included in the model in stepwise as these variables were associated with the extent of emphysema in smokers with COPD [18, 19]. In addition, a past- biomass exposure was also entered into multivariate analysis [10]. All statistical analyses were performed using the SAS statistical package, Version 9.2 (SAS Institute Inc., Cary, NC, USA), with p-values of less than 0.05 considered as statistically significant.
Results
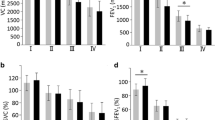
Table 1 shows the baseline characteristics of the study population in the CODA and KOLD cohort studies. Participants in CODA were older than those in the KOLD cohort (mean ± SD; 72.5 ± 7.1 vs. 67.4 ± 8.0, p < 0.001). Most of the participants are male (98.9% in CODA and 96.9% in KOLD) and former smokers (64.0% in CODA and 64.7% in KOLD, respectively) in both cohorts. Pack-years (PYs) of smoking were higher in participants from KOLD (46.4 ± 25.8 PYs) than participants from CODA (34.4 ± 24.7 PYs) despite there was no significant difference in current smoking status. Participants in the CODA cohort had more comorbidities (Charlson Comorbidity Index [CCI] ≥ 2: 18.3% in CODA vs. 6.9% in KOLD, p < 0.001)) and fewer severe COPD exacerbations requiring hospitalization over the past year (p < 0.001). FEV1 was higher in CODA. The FVC was higher in participants from the KOLD cohort. Any history of biomass exposure was higher in KOLD participants (74.6% in KOLD vs. 64.0% in CODA, p = 0.006). The CT measurements between the two cohorts were significantly different. Participants in CODA cohorts showed higher MWA (68.58 ± 4.79 vs. 66.05 ± 4.95, p < 0.001) and KOLD participants showed higher EI than those in CODA (9.28 ± 7.75 vs. 19.64 ± 14.55, p < 0.001), respectively.
Multivariate analysis for the comparison of lung function and CT measurements of both cohorts after adjusting for age, gender, educational attainment, BMI, smoking status, pack-years of their smoking among smokers, CCI, and COPD exacerbation showed higher EI in KOLD cohorts than in the CODA cohort (6.07 ± 3.06, in CODA vs. 20.0 ± 2.21 in KOLD, p < 0.001). This significant difference was consistent even after further adjustment for FEV1 (6.12 ± 2.88 vs. 17.3 ± 2.10, respectively) (Table 2). The MWA was also significantly lower in the KOLD cohort (70.2 ± 1.21 vs. 66.8 ± 0.88, p = 0.028) after including FEV1 into the model (model 2). As shown in Table 3, the MWA was found to be lower in KOLD even after including FEV1 into the model (70.3 ± 1.25 in CODA vs. 67.1 ± 0.92 in KOLD, p = 0.04 in model 2). However, there was no difference in the ratio of MLD in CT measurements between the two cohorts.
The EI was also lower in CODA after adjusting for biomass, and COPD medication (6.24 ± 3.16 in CODA vs. 20.0 ± 2.32 in KOLD, p = 0.001, model 1 in Table 3) and was consistent with the results obtained after including FEV1 in the multivariate model (p = 0.002, model 2 in Table 3).
Discussion
In this analysis of patients with COPD (confirmed by spirometry), MWA by CT measurements was significantly higher in the CODA cohorts which represented a rural area near cement plants. These results were consistent, even after adjusting for age, gender, educational attainment, BMI, smoking habits, CCI, spirometry parameters, history of COPD exacerbation, medication they used and biomass. Patients with COPD living in rural area showed higher incidence and events of COPD exacerbation in previous studies [20, 21]. However, the reason for the mechanism was not clearly elucidated. CT findings might provide a clue for this difference in COPD characteristics between urban and rural areas.
In this study, we measured the MWA and EI by CT scan within the two cohorts. In previous studies, CT findings showed various patterns and phenotypes for several conditions and causal materials including biomass, smoking, occupational chemicals and combustion. For instance, non-smoker and female patients with COPD had less emphysema and air-trapping, as detected by HRCT, thicker basement membranes and greater endobronchial pigmentation [12]. Non-smoker COPD subjects had lower emphysema scores and airway diseases predominant patterns in CT scan than smokers, as per a recent study [22]. The biomass also showed a variation in pattern in CT scans with lower emphysema and higher air trapping [10]. Sex difference was also suggested as mechanism of COPD in CT findings in a previous study possibly by environmental, biologic and/or genetic factors [23]. In our study, patients with COPD living in rural areas had significant CT findings with higher MWA and lower EI compared with those in urban areas after adjusting for sex, smoking habit and biomass exposure, which was previously observed to have an influence on different CT findings. In a previous study, residence in a rural area was a predictor of COPD not only for smokers but also for non-smokers [21]. The characteristics of the rural area might affect COPD mechanisms other apart from the effect of tobacco (which is a representative mechanism of urban COPD). A higher EI represented in the KOLD cohort in our study could explain the characteristics.
Participants in the CODA cohort, who lived near cement plants and were exposed to cement dust, had increased wall thickening on chest CT. In a previous study, exposure to cement dust by living or working near cement plants was associated with a higher prevalence and hospitalization of patients with COPD and who also experienced more respiratory symptoms [24, 25]. Recent studies support the fact that specific cement dust effects structural changes in CT findings. First of all, the cement dust-exposed subjects with normal spirometry demonstrated airway narrowing in the lower lobes, wall thickening at all segmental airways, a different bifurcation angle at the central airways, and a loss of airway wall elasticity at the lower lobes compared to the non-dust-exposed subjects [26]. Furthermore, dust exposure to cement was significantly associated with bronchial alterations in segmental scales rather than parenchymal changes such as emphysematous changes. As the particles from cement dust range in sizes from 0.5 to 5 um, they are usually deposited on the segmental airway [27, 28]. In the case of cigarette-smoke particles, the rate of deposition was greater in the parenchymal region than in the segmental region due to small size of the particles ranging from 0.01 to 1 um [29, 30].. Kim et al. studied the relationship between different characteristics of particles and CT findings among persons with normal spirometry in a previous study [26]. As subjects with normal spirometry results, airway structural alteration by cement dust also influenced patients with COPD who showed airway limitation in spirometry results and had a smoking intensity of more than 10PY.
Structural changes represented by wall thickness in CT have been reported in studies of other types of dust. Individuals with low FVC who were occupationally exposed to the World Trade Center disaster showed higher WA% representing proximal airway inflammation. However, there was no difference in the measurement of distal airway and emphysema compared to persons with normal spirometry [31]. The carbon black packer also showed a higher WA and lower LA on CT as reported by Xue et al. [32]. However, the shared mechanism between dust exposure and small airway thickening has not been clearly established. We added evidence that small airway wall thickening may explain the pathophysiology induced by dust exposure.
Occupational factors according to regional distinction have also influenced COPD incidence or exacerbations in previous studies. Agricultural workers who are often exposed to dust and vapors that can cause or aggravate underlying lung diseases are the most prevalent rural occupation [33, 34]. It has also been reported that agricultural work affects COPD development and aggravation of their symptoms [34,35,36]. Focusing on rural regions, however, the results for the effects of occupation on COPD have been inconsistent. One study found an increased risk of COPD exacerbation through a multivariate analysis, but another revealed no significant association in the adjusted model [20, 21]. Subjects in CODA cohort where about 70% of participants worked in cement factories or in farm [37] had a pathophysiology characterized by small airway thickening rather than parenchymal changes. Further long-term consequences regarding cement dust exposure on the COPD phenotype should be investigated.
Biomass exposure is another cause of COPD development which had been studied in non-smoker and female subjects [38, 39]. Biomass-related COPD had a greater reduction in mid-expiratory volume and less pronounced markers of emphysema than smoking-exposed COPD in previous studies [10, 12]. Biomass exposure is associated with greater intimal proliferation, basement membrane thickening, fewer neutrophil and greater numbers of lymphocytes and macrophages in lavage fluid. This was consistent with autopsy findings, wherein more fibrosis was found in the small airway walls and greater pigment deposition occurred within the lung parenchyma [12, 40]. In our study, subjects in both cohorts had experienced more than 10-PY of smoking and the direct exposure to charcoal and wood even though there were difference between the two groups. We found that the results were consistent after adjusting for smoking and biomass exposure. We could assume that these CT findings could be distinct characteristics of patients with COPD living in rural regions near cement plants, rather than cigarette smoking or biomass. These findings were also consistent with the fact that the incidence of COPD in rural areas has increased among both smokers and non-smokers [21].
To our knowledge, this study is the first to provide a direct comparison of the phenotype of CT findings between patients with COPD from rural and urban areas. This study could explain the different clinical characteristics of patients with COPD from a rural area. Despite the strengths of this study, there were several limitations. First, this study had a cross-sectional design, so there was no chronologic information for changes in these results due to the nature of this study design. The findings of longitudinal CT images of further studies should be followed to confirm the characteristics revealed in this study. Second, we compared two distinguished cohorts representing patients with COPD living in rural and urban areas which might have less comparability while exploring the effect of various exposures. We could not distinguish occupational exposure from regional exposure of individuals who lived within the same district. Despite this limitation, we included almost all possible confounders mediating the COPD phenotype by stepwise multivariable analysis. In addition, the intensity, duration and type of biomass which are well-known COPD phenotype factors between the two cohorts, were not compared in this study. However, as far as we included household biomass exposure, we found consistent results. Further studies regarding quantitative data of individual environmental, occupational or biomass exposure are still needed concerning their effect on CT findings, or their biological effects on COPD. Third, quantitative analysis in COPD is influenced by the CT scanner and scan parameters such as reconstruction algorithm, radiation dose, section thickness [41]. Scan parameters used in our study were designed to minimize interpatient differences, but CT scanners vary among institutions. A study showed Interscanner variability by measuring mean lung density in a humanoid phantom [42]. Therefore, EI and MLD may have affected by the difference between CT scanners in the present study.
Conclusions
Higher MWA and lower EI were observed in the dust exposure region. These results suggest that the imaging phenotype of COPD is influenced by environmental exposure.
Availability of data and materials
The datasets used for the current study are available from the corresponding author on reasonable request.
Abbreviations
- COPD:
-
Chronic obstructive lung disease
- AE:
-
Acute exacerbation
- CT:
-
Computed tomography
- CODA:
-
COPD in dusty area
- KOLD:
-
Korean obstructive lung disease
- PFT:
-
Pulmonary function test
- MLD:
-
Mean lung density
- WA:
-
Wall area
- LA:
-
Lumen area
- WA%:
-
Wall area percent
- MWA %:
-
Mean wall area percent
- EI:
-
CT-emphysema index
References
Chapman KR, Mannino DM, Soriano JB, Vermeire PA, Buist AS, Thun MJ, Connell C, Jemal A, Lee TA, Miravitlles M, et al. Epidemiology and costs of chronic obstructive pulmonary disease. Eur Respir J. 2006;27:188–207.
Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet. 2007;370:765–73.
Han MK, Agusti A, Calverley PM, Celli BR, Criner G, Curtis JL, Fabbri LM, Goldin JG, Jones PW, Macnee W, et al. Chronic obstructive pulmonary disease phenotypes: the future of COPD. Am J Respir Crit Care Med. 2010;182:598–604.
Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, Barnes PJ, Fabbri LM, Martinez FJ, Nishimura M, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–65.
Nakano Y, Muro S, Sakai H, Hirai T, Chin K, Tsukino M, Nishimura K, Itoh H, Pare PD, Hogg JC, Mishima M. Computed tomographic measurements of airway dimensions and emphysema in smokers. Correlation with lung function. Am J Respir Crit Care Med. 2000;162:1102–8.
Hasegawa M, Nasuhara Y, Onodera Y, Makita H, Nagai K, Fuke S, Ito Y, Betsuyaku T, Nishimura M. Airflow limitation and airway dimensions in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173:1309–15.
Montaudon M, Berger P, Lederlin M, Marthan R, Tunon-de-Lara JM, Laurent F. Bronchial morphometry in smokers: comparison with healthy subjects by using 3D CT. Eur Radiol. 2009;19:1328–34.
Lee YK, Oh YM, Lee JH, Kim EK, Lee JH, Kim N, Seo JB, Lee SD, Group KS. Quantitative assessment of emphysema, air trapping, and airway thickening on computed tomography. Lung. 2008;186:157–65.
Bhatt SP, Washko GR, Hoffman EA, Newell JD Jr, Bodduluri S, Diaz AA, Galban CJ, Silverman EK, San José Estépar R, Lynch DA. Imaging advances in chronic obstructive pulmonary disease. Insights from the genetic epidemiology of chronic obstructive pulmonary disease (COPDGene) study. Am J Respir Crit Care Med. 2019;199:286–301.
Camp PG, Ramirez-Venegas A, Sansores RH, Alva LF, McDougall JE, Sin DD, Pare PD, Muller NL, Silva CI, Rojas CE, Coxson HO. COPD phenotypes in biomass smoke- versus tobacco smoke-exposed Mexican women. Eur Respir J. 2014;43:725–34.
Fernandes L, Gulati N, Fernandes Y, Mesquita AM, Sardessai M, Lammers JJ, Mohamed Hoesein FA, Ten Hacken NHT, van den Berge M, Galban CJ, Siddiqui S. Small airway imaging phenotypes in biomass- and tobacco smoke-exposed patients with COPD. ERJ Open Res. 2017. https://doi.org/10.1183/23120541.00124-2016.
Zhao D, Zhou Y, Jiang C, Zhao Z, He F, Ran P. Small airway disease: a different phenotype of early stage COPD associated with biomass smoke exposure. Respirology. 2018;23:198–205.
Castaldi PJ, Dy J, Ross J, Chang Y, Washko GR, Curran-Everett D, Williams A, Lynch DA, Make BJ, Crapo JD, et al. Cluster analysis in the COPDGene study identifies subtypes of smokers with distinct patterns of airway disease and emphysema. Thorax. 2014;69:415–22.
Han Y, Heo Y, Hong Y, Kwon SO, Kim WJ. Correlation between physical activity and lung function in dusty areas: results from the chronic obstructive pulmonary disease in dusty areas (CODA) cohort. Tuberc Respir Dis (Seoul). 2019;82:311–8.
Park TS, Lee JS, Seo JB, Hong Y, Yoo JW, Kang BJ, Lee SW, Oh YM, Lee SD, Group KS. study design and outcomes of korean obstructive lung disease (KOLD) cohort study. Tuberc Respir Dis (Seoul). 2014;76:169–74.
Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–38.
Choi HS, Park YB, Yoon HK, Lim SY, Kim TH, Park JH, Lee WY, Park SJ, Lee SW, Kim WJ, et al. Validation of previous spirometric reference equations and new equations. J Korean Med Sci. 2019;34:e304.
Camp PG, Coxson HO, Levy RD, Pillai SG, Anderson W, Vestbo J, Kennedy SM, Silverman EK, Lomas DA, Pare PD. Sex differences in emphysema and airway disease in smokers. Chest. 2009;136:1480–8.
Gu S, Li R, Leader JK, Zheng B, Bon J, Gur D, Sciurba F, Jin C, Pu J. Obesity and extent of emphysema depicted at CT. Clin Radiol. 2015;70:e14-19.
Burkes RM, Gassett AJ, Ceppe AS, Anderson W, O'Neal WK, Woodruff PG, Krishnan JA, Barr RG, Han MK, Martinez FJ, et al. Rural residence and COPD exacerbations: analysis of the SPIROMICS Cohort. Ann Am Thorac Soc. 2018;15:808–16.
Raju S, Keet CA, Paulin LM, Matsui EC, Peng RD, Hansel NN, McCormack MC. Rural residence and poverty are independent risk factors for chronic obstructive pulmonary disease in the United States. Am J Respir Crit Care Med. 2019;199:961–9.
Salvi SS, Brashier BB, Londhe J, Pyasi K, Vincent V, Kajale SS, Tambe S, Mandani K, Nair A, Mak SM, et al. Phenotypic comparison between smoking and non-smoking chronic obstructive pulmonary disease. Respir Res. 2020;21:50.
Hong Y, Ji W, An S, Han SS, Lee SJ, Kim WJ. Sex differences of COPD phenotypes in nonsmoking patients. Int J Chron Obstruct Pulmon Dis. 2016;11:1657–62.
Molgaard EF, Hannerz H, Tuchsen F, Brauer C, Kirkeskov L. Chronic lower respiratory diseases among demolition and cement workers: a population-based register study. BMJ Open. 2013. https://doi.org/10.1136/bmjopen-2012-001938.
Mbelambela EP, Eitoku M, Muchanga SMJ, Villanueva AF, Hirota R, Pulphus TY, Sokolo GJ, Yasumitsu-Lovell K, Komori K, Suganuma N. Prevalence of chronic obstructive pulmonary disease (COPD) among Congolese cement workers exposed to cement dust, in Kongo Central Province. Environ Sci Pollut Res Int. 2018;25:35074–83.
Kim T, Cho HB, Kim WJ, Lee CH, Chae KJ, Choi SH, Lee KE, Bak SH, Kwon SO, Jin GY, et al. Quantitative CT-based structural alterations of segmental airways in cement dust-exposed subjects. Respir Res. 2020;21:133.
Fell AK, Notø H, Skogstad M, Nordby KC, Eduard W, Svendsen MV, Ovstebø R, Trøseid AM, Kongerud J. A cross-shift study of lung function, exhaled nitric oxide and inflammatory markers in blood in Norwegian cement production workers. Occup Environ Med. 2011;68:799–805.
Usmani OS, Biddiscombe MF, Barnes PJ. Regional lung deposition and bronchodilator response as a function of beta2-agonist particle size. Am J Respir Crit Care Med. 2005;172:1497–504.
Ashraf H, Lo P, Shaker SB, de Bruijne M, Dirksen A, Tønnesen P, Dahlbäck M, Pedersen JH. Short-term effect of changes in smoking behaviour on emphysema quantification by CT. Thorax. 2011;66:55–60.
Mohamed Hoesein FA, de Hoop B, Zanen P, Gietema H, Kruitwagen CL, van Ginneken B, Isgum I, Mol C, van Klaveren RJ, Dijkstra AE, et al. CT-quantified emphysema in male heavy smokers: association with lung function decline. Thorax. 2011;66:782–7.
Weber J, Reeves AP, Doucette JT, Jeon Y, Sood A, San Jose Estepar R, Celedon JC, de la Hoz RE. Quantitative CT evidence of airway inflammation in WTC workers and volunteers with low FVC spirometric pattern. Lung. 2020;198:555–63.
Cao X, Lin L, Sood A, Ma Q, Zhang X, Liu Y, Liu H, Li Y, Wang T, Tang J, et al. Small airway wall thickening assessed by computerized tomography is associated with low lung function in Chinese carbon black packers. Toxicol Sci. 2020. https://doi.org/10.1093/toxsci/kfaa134.
Bailey KL, Meza JL, Smith LM, Von Essen SG, Romberger DJ. Agricultural exposures in patients with COPD in health systems serving rural areas. J Agromed. 2007;12:71–6.
Nordgren TM, Bailey KL. Pulmonary health effects of agriculture. Curr Opin Pulm Med. 2016;22:144–9.
Alif SM, Dharmage SC, Benke G, Dennekamp M, Burgess JA, Perret JL, Lodge CJ, Morrison S, Johns DP, Giles GG, et al. Occupational exposure to pesticides are associated with fixed airflow obstruction in middle-age. Thorax. 2017;72:990–7.
Paulin LM, Diette GB, Blanc PD, Putcha N, Eisner MD, Kanner RE, Belli AJ, Christenson S, Tashkin DP, Han M, et al. Occupational exposures are associated with worse morbidity in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;191:557–65.
Kwon SO, Hong SH, Han YJ, Bak SH, Kim J, Lee MK, London SJ, Kim WJ, Kim SY. Long-term exposure to PM10 and NO2 in relation to lung function and imaging phenotypes in a COPD cohort. Respir Res. 2020;21:247.
Ramirez-Venegas A, Sansores RH, Perez-Padilla R, Regalado J, Velazquez A, Sanchez C, Mayar ME. Survival of patients with chronic obstructive pulmonary disease due to biomass smoke and tobacco. Am J Respir Crit Care Med. 2006;173:393–7.
Eisner MD, Anthonisen N, Coultas D, Kuenzli N, Perez-Padilla R, Postma D, Romieu I, Silverman EK, Balmes JR, Committee on Nonsmoking Copd E, Occupational Health A. An official American Thoracic Society public policy statement: novel risk factors and the global burden of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;182:693–718.
Rivera RM, Cosio MG, Ghezzo H, Salazar M, Perez-Padilla R. Comparison of lung morphology in COPD secondary to cigarette and biomass smoke. Int J Tuberc Lung Dis. 2008;12:972–7.
Mets OM, de Jong PA, van Ginneken B, Gietema HA, Lammers JW. Quantitative computed tomography in COPD: possibilities and limitations. Lung. 2012;190:133–45.
Kemerink GJ, Lamers RJ, Thelissen GR, van Engelshoven JM. Scanner conformity in CT densitometry of the lungs. Radiology. 1995;197:749–52.
Acknowledgements
None.
Funding
This study is not supported by any other source.
Author information
Authors and Affiliations
Contributions
WJK planned this study, had access to the data and took responsibility for the integrity of the data and data analysis. JK and WJK contributed to study concept and design, analysis, and preparation of the manuscript. WJK, BK, SHB and Y-MO contributed to data collection and preparation of manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Participants from two different prospective COPD cohort studies in Korea were enrolled: COPD in Dusty Area (CODA) Registry (KCT0000552) and Korean Obstructive Lung Disease Cohort (KOLD). The study design and methods were approved by the Institutional Review Board and informed consent was obtained from each patient of all cohort studies.
Consent for publication
Not required due to study design.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kim, J., Kim, B., Bak, S.H. et al. A comparative study of chest CT findings regarding the effects of regional dust exposure on patients with COPD living in urban areas and rural areas near cement plants. Respir Res 22, 43 (2021). https://doi.org/10.1186/s12931-021-01649-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12931-021-01649-4