Abstract
Background
Mutant prevention concentration (MPC) is an alternative pharmacodynamic parameter that has been used to measure antimicrobial activity and represents the propensities of antimicrobial agents to select resistant mutants. The concentration range between minimum inhibitory concentration (MIC) and MPC is defined as mutant selection window (MSW). The MPC and MSW parameters represent the ability of antimicrobial agents to inhibit the bacterial mutants selected. This study was conducted to determine the MIC and MPC values of four antimicrobials including ceftiofur, cefquinome, florfenicol and tilmicosin against 105 Riemerella anatipestifer isolates.
Results
The MIC50/MIC90 values of clinical isolates tested in our study for ceftiofur, cefquinome, florfenicol and tilmicosin were 0.063/0.5、0.031/0.5、1/4、1/4 μg/mL, respectively; MPC50/ MPC90 values were 4/64、8/64、4/32、16/256 μg/mL, respectively. These results provided information on the use of these compounds in treating the R. anatipestifer infection; however, additional studies are needed to demonstrate their therapeutic efficacy.
Conclusion
Based on the MSW theory, the hierarchy of these tested antimicrobial agents with respect to selecting resistant subpopulations was as follows: cefquinome > ceftiofur > tilmicosin > florfenicol. Cefquinome was the drug that presented the highest risk of selecting resistant mutant among the four antimicrobial agents.
Similar content being viewed by others
Background
Riemerella anatipestifer has been one of the most troublesome etiological agents and causes heavy loss in duck industry. It occurs worldwide, especially in Southeast Asia [1]. The existence of R. anatipestifer infection symptoms observed is characterized by fibrinous pericarditis, perihepatitis, airsacculitis, caseous salpingitis, and meningitis. To date, at least twenty-one serotypes of R. anatipestifer have been identified and little cross-immunoprotection among serotypes was reported [2]. People have been making great efforts to find new strategies to prevent or control R. anatipestifer infection, since much work has been done concentrating on the identification of factors associated with virulence [1, 3, 4] and immunogenic characterization based on outer membrane protein A in recent years [5–7]. Even so, chemotherapy is still a major approach in the treatment of R. anatipestifer infection because of the complex immunology situation currently. Due to the concern of high incidence of Riemerella anatipestifersis and increasingly severe drug resistance or reduction of susceptibility, obtaining new treatment information and promising results with antimicrobial agents seems necessary [8–10].
Ceftiofur (β-lactam), cefquinome (β-lactam), florfenicol (phenicol), and tilmicosin (macrolide) belong to three families of antimicrobial agents and were developed for exclusive use in animals. They have exhibited remarkable antibacterial effects against diverse microorganisms since being introduced, although resistance to those drugs mentioned above has also been reported [11–14]. The escalating resistance of R. anatipestifer field strains and concerns over animals as putative reservoirs for antimicrobial resistance genes force us to develop strategies to make full use of the current drugs [8, 15].
Traditionally, the in vitro activity of antimicrobial susceptibility is assessed by minimum inhibitory concentration (MIC) or minimum bactericidal concentration (MBC). The mutant prevention concentration (MPC) concept is an alternative in vitro measurement of drug susceptibility against the infecting pathogen, representing the drug concentration that prevents a population of more than 1010 colony forming unit (CFU)/mL bacteria from first mutation. Mutant selection window (MSW) is defined as the concentration range between MIC and MPC. The use of MPC and MSW is of aid in evaluating the capacity and potency of antimicrobial agents for the selection of resistant mutants [16].
Application of the MPC theory has been conducted in a variety of organisms associated with human and animals such as Escherichia coli [17], Salmonella enterica [18], Staphylococcus aureus [19], Pseudomonas aeruginosa [20] and Mannheimia haemolytica [21]. Essentially, the concept of MPC has been historically and primarily established for fluoroquinolones because of the resistance mechanisms for antimicrobial agents and is applicable to other classes under some restriction at present [22]. So far, no published data of MPC have been available for R.anatipestifer yet. Therefore, the purpose of this study was to investigate the MPC of four antimicrobials against 105 R. anatipestifer isolates from China, providing advice on rational use of these antimicrobial agents, and further compare the potencies of these antimicrobials in selecting resistant R. anatipestifer mutants.
Methods
Bacterial strains
During the period of 2008 to 2014, we randomly collected R. anatipestifer isolates from the sick ducks or geese that exhibited typical symptoms of Riemerella anatipestifersis at the animal diagnostic departments of Guangdong Province, China. The clinical cases were provided by the farm owners who volunteered to participate in the study. A total of 105 R. anatipestifer clinical strains from ducks (n = 98) and geese (n = 7) were obtained and used in this study. The bacteria were identified by colony morphology and PCR method for partial sequence of outer membrane protein A as described previously [23]. The Animal Experimentation Ethics Committee of South China Agricultural University approved all experimental procedures. In principal, MPC measurement should be performed against organisms sensitive to antimicrobial agents (by MIC testing). Because no critical susceptibility breakpoints were available for these four compounds against R. anatipestifer, the resistance breakpoints were tentatively interpreted according to the Clinical and Laboratory Standards Institute (CLSI) recommendation for E.coli or Pasteurella multocida [24].
Antimicrobial compounds
Antimicrobial agents exclusively approved for use in animals including ceftiofur, cefquinome, florfenicol, and tilmicosin were investigated in the present study. These compounds were commercially purchased from the manufactures in China. Stock solution of each antimicrobial was prepared in proper solvent according to the instructions of antimicrobial susceptibility testing for bacteria isolated from animals and stored at −20 °C. Escherichia coli ATCC 25922 and Staphylococcus aureus ATCC 29213 were used as control strains.
MIC measurements
Minimum inhibitory concentrations were determined by CLSI agar dilution methodology [24]. All studies were carried out in triplicate. Briefly, each R. anatipestifer isolate in logarithmic growth period was diluted with 0.9 % saline to achieve a 0.5 McFarland standard suspension, equal to the inoculum of 5 × 105 CFU/mL. About 5 μL suspensions were inoculated on Mueller-Hinton agar plates supplied with 5 % calf serum and containing antimicrobials (with a series of concentrations between 64 and 0.004 μg/mL). Inoculated plates were then incubated for 18 h at 37 °C in a constant temperature incubator. The MIC was recognized as the lowest antibiotic concentration showing no growth of colony morphology.
MPC testing
The measurement of MPC was performed according to a method previously described with slight modification [21]. Briefly, three or four colonies were inoculated into 3 mL R. anatipestifer broth (tryptic soy broth containing 0.5 % yeast and 5 % new calf serum) and cultured overnight. The next day, 100 μL R. anatipestifer suspensions were transferred to 100 mL of R. anatipestifer broth and shaken at the speed of 200 rpm under the temperature of 37 °C overnight. The collected cultures were concentrated by centrifugation at 4000 rpm for 20 min at 4 °C and then re-suspended in 5 mL of fresh R. anatipestifer broth to produce ≥1010 CFU/mL suspensions. Aliquots of 100 μL containing ≥1010 CFU/mL were applied to R. anatipestifer agar plates incorporating a series of antimicrobials with the concentrations ranging from 512 to 1 × MIC. Each plate was prepared freshly, stored at 4 °C and used within 7 days. Inoculated plates were then incubated as described previously and observed for five days. MPC was taken as the lowest antimicrobial concentration that allowed no R. anatipestifer isolate growth. All MPC determinations were carried out in triplicate for each isolate. Results were identical and then used for data analysis. The ratio of MPC to MIC was also calculated.
Results
MIC and MPC
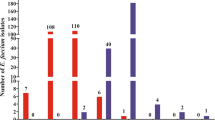
A total of 98 R. anatipestifer isolates from ducks and 7 field strains from geese were tested against ceftiofur, cefquinome, florfenicol and tilmicosin. MICs and MPCs of antimicrobials assayed are shown in Table 1. MIC50/90 and MPC50/90 values are also shown. MPC values were higher than MICs because of exposure to higher density of bacterial inoculum. Following testing, MIC values of ceftiofur ranged from ≤0.008 to 8 μg/mL, with MIC50 and MIC90 values of 0.063 μg/mL and 0.5 μg/mL respectively; cefquinome had the MIC values ranging between ≤0.008 to 16 μg/mL, with MIC50 of 0.031 μg/mL and MIC90 of 0.5 μg/mL respectively; florfenicol had the MIC values ranging from 0.125 to 16 μg/mL, with MIC50 of 1 μg/mL and MIC90 of 4 μg/mL respectively; tilmicosin had the MIC values ranging from 0.031 to 64 μg/mL, with MIC50 of 1 μg/mL and MIC90 of 4 μg/mL respectively.
The corresponding MPC values of the four antimicrobial agents assayed against 105 R. anatipestifer isolates are also listed in Table 1. Following testing of ceftiofur, MPC values ranged from 0.125 to ≥128 μg/mL, with MPC50 and MPC90 values of 4 and 64 μg/mL respectively; for cefquinome from 0.25 to ≥128 μg/mL and 8 and 64 μg/mL respectively; for florfenicol from 1 to ≥128 μg/mL and 4 and 32 μg/mL respectively; for tilmicosin from 0.25 to ≥128 μg/mL and 16 and 256 μg/mL respectively.
Based on these MIC and MPC values, dosing to achieve the MIC or MPC values (where possible) may serve to inhibit susceptible bacterium or reduce the selection of resistant mutants. The hierarchy of potency for these tested agents based on MIC90 values was: ceftiofur = cefquinome > florfenicol = tilmicosin. The potency of antimicrobial agents tested based on MPC90 values followed the rank order: florfenicol > ceftiofur = cefquinome > tilmicosin.
Mutant selection index calculations
The ratio of MPC to MIC was defined as selection index (SI) [25]. The lower SI is, the better ability of antimicrobials to restrict the resistant mutant subpopulations. Since working with a large population of R. anatipestifer isolates is cumbersome, we calculated the mutant selection index (ratio of MPC to MIC) for each isolate so that the capacity of selecting mutant enrichment for each antimicrobial agent could be easily compared. The value distribution for each antimicrobial agent was shown in Table 2. In our investigation, MPC/MIC ratios were slightly lower for florfenicol and higher for cefquinome. The SI data indicated a better ability of florfenicol to prevent non-susceptible mutant subpopulations and a strong selective pressure of cefquinome to enrich R. anatipestifer mutants.
Pharmacokinetics/Pharmacodynamics (PK/PD) calculations
We also selected MIC90 and MPC90 as the boundaries of the mutant selective window [26]. Plasma pharmacokinetic data in ducks were available for cefquinome and florfenicol [27, 28]. In conjunction with pharmacokinetic parameters of each compound in ducks, the various PK/PD indices, including ratio of maximum plasma concentration to MIC90 (Cmax/MIC90) or MPC90 (Cmax/MPC90), ratio of area under the concentration-time curve to MIC90 (AUC/MIC90) or MPC90 (AUC/MPC90), time above MIC90 or MPC90 of the dosage interval ( %T>MIC90 or %T>MPC90), and time inside the mutant selection window of the dosage interval (%T MSW), are shown in Table 3. %T > MIC serves as an important parameter for cephalosporins and its value of cefquinome against R. anatipestifer isolates was approximately 31.67 % by integrating the pharmacokinetic values obtained from a intramuscular injection of a dose of 5 mg/kg body weight [27]. No concentration was observed to exceed the MPC90. According to the pharmacokinetic data attained with a single dose of 30 mg/kg body weight florfenicol intramuscularly [28], the values determined for Cmax/MIC90, Cmax/MPC90, AUC24/MIC90, AUC24/MPC90 were 1.62, 0.10, 18.21 h and 1.14 h for florfenicol, respectively. All the concentrations of florfenicol in plasma were lower than MPC90 and %T MSW was calculated to be approximately 21.67 %.
Discussion
Riemerella anatipestifer has been a problematic pathogen of commercial importance for several years and it is hard to give the correct treatment measures. One possible reason is poor cross immune protection among various serotypes; another important reason may be due to the similarity of clinical symptom between R. anatipestifer infection and E. coli infection. In recent years, R. anatipestifer strains with reduced susceptibility to antimicrobials have emerged as reported in other’s study and our previous investigation [8, 23] because of the use of antimicrobials in animals, which reflects the necessity of searching for new compounds or strategies to treat Riemerella anatipestifersis [8, 10, 23]. Maintaining the utility of antimicrobials in treating Riemerella anatipestifersis seems to be a major challenge. As proposed by other researchers, one of the strategies to tackle the resistance problem is to reduce or prevent the emergence of resistant mutants [19]. In human medicine, MPC values and MPC/MIC ratios have been determined and studied extensively in a large number of antimicrobial agents, involving many kinds of organisms [29–32]. In the current study, we first report the MPC values of four antimicrobials that were exclusively developed for animals against 105 R. anatipestifer field strains mainly isolated from South of China.
We selected several antimicrobial agents that were approved exclusively and most commonly used in animals, and also represented a wide range of antimicrobial agents. To our surprise, up to 70 % strains had high MIC values of enrofloxacin (≥2 μg/mL) and apramycin (≥64 μg/mL) (data not shown). Based on the MIC data of the six compounds, four antimicrobial agents tested in our study appeared to have excellent in vitro activities against R. anatipestifer strains, although they have different chemical structures and action mechanisms. Previously, Blondeau et al. [21] compared the MIC and MPC values of five antimicrobial agents against bovine clinical isolates of Mannheimia haemolytica. The rank order of potency that antimicrobials selected resistant mutants differed by using MIC and MPC data. Such is the case for R. anatipestifer isolates.
The concentration zone between MIC and MPC was recognized as MSW. Concentrations of antimicrobials within MSW exerted a selective pressure for accumulation of resistant strains. Ratio of MPC to MIC represented the ability of antimicrobial agents to block the resistant mutant subpopulation. MPC, MSW and MPC/MIC ratio served as a guide for the potency of antimicrobials in restricting resistant mutant selection. Based on these, a large number of references tried to address the issue of relationship between antimicrobials and bacteria. Hansen et al. [20] thought ciprofloxacin was more active than levofloxacin in selecting resistant Pseudomonas aeruginosa amplification. By determining the MPC of three quinolones against 100 clinical Streptococcus pneumococci, moxifloxacin seemed to exhibit more excellent antimutant ability than levofloxacin and gemifloxin [33]. Wang et al. [34] tested the MPC of three quinolones against Campylobacter jejuni isolated from chicken and assumed that enrofloxacin had the lowest MPC among the three tested quinolones, thus enrofloxacin represented a low selective pressure for selection of resistant subpopulations. Briales et al. [35] provided the MPC values of fluoroquinolone against E. coli isolates carrying different plasmid-mediated resistant genes qnr and harboring isogenic gyrA and parC substitutions, considering that qnr genes played a vital role in selecting one-step resistant mutants. From our results, florfenicol appeared to be a compound with excellent in vitro activity against these R. anatipestifer strains collected from South China, although some mechanism of R. anatipestifer isolates resistant to florfenicol has been described in other districts [36].
The ratio of MPC/MIC was slightly higher for ceftiofur and cefquinome among the four antimicrobials tested (Table 2). Even two cephalosporins developed for animals are only used in veterinary medicine, and they are classified as critically important antimicrobials by the WHO [37, 38], their use to treat great numbers of animals in duck industry is to be strongly discouraged because of prudent use guidelines. Similar conclusion was also obtained for the therapy of R. anatipestifer infection based on the theory of MPC and MSW. In addition, both ceftiofur and cefquinome cannot be administered orally because of poor absorption; unless used prophylactically, which would be in strong contradiction to prudent use guidelines, therefore the usefulness of ceftiofur and cefquinome may probably be reduced in duck production to a bare minimum. Although ceftiofur has successfully cured the R. anatipestifer infection in a previous report [39], it should not be the first choice considering the wider MSW and severe drug resistance situation. The MSW of cefquinome was wider than that of ceftiofur. In other words, cefquinome has weaker ability of preventing the selection of R. anatipestifer mutants than ceftiofur. Comparing MSWs and MPC/MIC ratios of the four antimicrobials, cefquinome seems to be the drug that most easily selects resistant mutants.
MIC or MPC-based therapeutic protocols and PK/PD indices for suppressing the enrichment of resistant bacterial subpopulations have been proposed and studied in various in vivo or in vitro models extensively [40–43]. PK/PD parameters such as AUC/MIC, Cmax/MIC and T > MIC relate closely with the effect of antimicrobials. In our laboratory, pharmacodynamics on the basis of MIC values for cefquinome have been well studied in mice, yellow cattle, pigs and dogs against a series of microorganisms in recent years, involving E. coli, S. aureus, P. multocida, Klebsiella pneumoniae as well as Haemophilus parasuis [44–50]. These publications clearly described the relationship between the dosing schedules and the antimicrobial effectiveness. Also, the ability of cefquinome to restrict the selection of E. coli mutants was predicted in an in vivo model. The results demonstrated that %T > MPC of >50 % was favorable to block the resistant mutants [43]. By integrating our results with the published pharmacokinetic data of antimicrobials in ducks, serum drug concentrations of cefquinome and florfenicol may fall within the MSW and the high MPC values could hardly be attained albeit these two drugs had excellent MIC values. As cephalosporin exhibited time-dependent property, we applied this approach in our study and the predicted T > MIC was approximately 7.6 h (Table 3), which was lower than that obtained previously using P. multocida in yellow cattle [46], but slightly higher than that calculated using canine E. coli [50]. In vivo antimicrobial efficacy of cefquinome against R. anatipestifer should be further addressed. Little is known on the PK/PD relationship of florfenicol against R. anatipestifer. Until now, no killing studies of ceftiofur and tilmicosin based on MIC or MPC parameters of R. anatipestifer have been conducted in ducks. So more work based on the MSW theory should be performed for the use of antimicrobial agents in ducks.
Conclusions
This is the first study that described the MIC and MPC values of four antimicrobial agents developed exclusively for animals against R. anatipestifer isolates. Our study may shed light on the future antimicrobial therapies for treatment of R. anatipestifer infection. Further in vivo or in vitro studies are required to confirm the efficacy based on the MIC or MPC values. The mutant selection window hypothesis suggests that cefquinome is least likely to prevent the emergence of R. anatipestifer mutants among the four antimicrobials.
Abbreviations
- CFU:
-
Colony Forming Unit
- CLSI:
-
Clinical and Laboratory Standards Institute
- MBC:
-
Minimum Bactericidal Concentration
- MIC:
-
Minimum Inhibitory Concentration
- MPC:
-
Mutant Prevention Concentration
- MSW:
-
Mutant Selection Window
- PD:
-
Pharmacodynamics
- PK:
-
Pharmacokinetics
- SI:
-
Selection Index
References
Crasta KC, Chua K-L, Subramaniam S, Frey J, Loh H, Tan H-M. Identification and characterization of CAMP cohemolysin as a potential virulence factor of Riemerella anatipestifer. J Bacteriol. 2002;184(7):1932–9.
Pathanasophon P, Sawada T, Pramoolsinsap T, Tanticharoenyos T. Immunogenicity of Riemerella anatipestifer broth culture bacterin and cell-free culture filtrate in ducks. Avian Pathol. 1996;25(4):705–19.
Weng SC, Lin WH, Chang YF, Chang CF. Identification of a virulence-associated protein homolog gene and ISRa1 in a plasmid of Riemerella anatipestifer. FEMS Microbiol Lett. 1999;179(1):11–9.
Hu Q, Han X, Zhou X, Ding C, Zhu Y, Yu S. OmpA is a virulence factor of Riemerella anatipestifer. Vet Microbiol. 2011;150(3):278–83.
Huang B, Kwang J, Loh H, Frey J, Tan H-M, Chua K-L. Development of an ELISA using a recombinant 41 kDa partial protein (P45N′) for the detection of Riemerella anatipestifer infections in ducks. Vet Microbiol. 2002;88(4):339–49.
Hu Q, Ding C, Tu J, Wang X, Han X, Duan Y, et al. Immunoproteomics analysis of whole cell bacterial proteins of Riemerella anatipestifer. Vet Microbiol. 2012;157(3):428–38.
Chu C-Y, Liu C-H, Liou J-J, Lee J-W, Cheng L-T. Development of a subunit vaccine containing recombinant Riemerella anatipestifer outer membrane protein A and CpG ODN adjuvant. Vaccine. 2015;33(1):92–9.
Zhong CY, Cheng AC, Wang MS, Zhu DK, Luo QH, De Zhong C, et al. Antibiotic susceptibility of Riemerella anatipestifer field isolates. Avian Dis. 2009;53(4):601–7.
Zheng F, Lin G, Zhou J, Cao X, Gong X, Wang G, et al. Discovery and characterization of gene cassettes-containing integrons in clinical strains of Riemerella anatipestifer. Vet Microbiol. 2012;156(3):434–8.
Yang F-F, Sun Y-N, Li J-X, Wang H, Zhao M-J, Su J, et al. Detection of aminoglycoside resistance genes in Riemerella anatipestifer isolated from ducks. Vet Microbiol. 2012;158(3):451–2.
Ma J, Zeng Z, Chen Z, Xu X, Wang X, Deng Y, et al. High prevalence of plasmid-mediated quinolone resistance determinants qnr, aac (6′)-Ib-cr, and qepA among ceftiofur-resistant Enterobacteriaceae isolates from companion and food-producing animals. Antimicrob Agents Chemother.2009;53(2):519–24.
Corti S, Sicher D, Regli W, Stephan R. Current data on antibiotic resistance of the most important bovine mastitis pathogens in Switzerland. Schweiz Arch Tierheilkd. 2003;145(12):571–5.
White DG, Hudson C, Maurer JJ, Ayers S, Zhao S, Lee MD, et al. Characterization of chloramphenicol and florfenicol resistance in Escherichia coli associated with bovine diarrhea. J Clin Microbiol. 2000;38(12):4593–8.
Ioana V, Herman V, Pascu C, Bogdan F, Csuka E, Biksi I. Antimicrobial susceptibility of some Actinobacillus pleuropneumoniae strains. Bulletin of University of Agricultural Sciences and Veterinary Medicine Cluj-Napoca. Vet Med. 2011;2(68):300-2.
Bates J, Jordens JZ, Griffiths DT. Farm animals as a putative reservoir for vancomycin-resistant enterococcal infection in man. J Antimicrob Chemother. 1994;34(4):507–14.
Dong Y, Zhao X, Domagala J, Drlica K. Effect of fluoroquinolone concentration on selection of resistant mutants of Mycobacterium bovis BCG and Staphylococcus aureus. Antimicrob Agents Chemother. 1999;43(7):1756–8.
Linde H-J, Lehn N. Mutant prevention concentration of nalidixic acid, ciprofloxacin, clinafloxacin, levofloxacin, norfloxacin, ofloxacin, sparfloxacin or trovafloxacin for Escherichia coli under different growth conditions. Antimicrob Agents Chemother. 2004;53(2):252–7.
Kehrenberg C, de Jong A, Friederichs S, Cloeckaert A, Schwarz S. Molecular mechanisms of decreased susceptibility to fluoroquinolones in avian Salmonella serovars and their mutants selected during the determination of mutant prevention concentrations. J Antimicrob Chemother.2007;59(5):886–92.
Metzler K, Hansen G, Hedlin P, Harding E, Drlica K, Blondeau J. Comparison of minimal inhibitory and mutant prevention drug concentrations of 4 fluoroquinolones against clinical isolates of methicillin-susceptible and-resistant Staphylococcus aureus. Int J Antimicrob Agents. 2004;24(2):161–7.
Hansen GT, Zhao X, Drlica K, Blondeau JM. Mutant prevention concentration for ciprofloxacin and levofloxacin with Pseudomonas aeruginosa. Int J Antimicrob Agents. 2006;27(2):120–4.
Blondeau J, Borsos S, Blondeau L, Blondeau B, Hesje C. Comparative minimum inhibitory and mutant prevention drug concentrations of enrofloxacin, ceftiofur, florfenicol, tilmicosin and tulathromycin against bovine clinical isolates of Mannheimia haemolytica. Vet Microbiol. 2012;160(1):85–90.
Smith HJ, Nichol KA, Hoban DJ, et al. Stretching the mutant prevention concentration (MPC) beyond its limits. J Antimicrob Chemother. 2003;51(6):1323–5.
Sun N, Liu J-H, Yang F, Lin D-C, Li G-H, Chen Z-L, et al. Molecular characterization of the antimicrobial resistance of Riemerella anatipestifer isolated from ducks. Vet Microbiol. 2012;158(3):376–83.
Clinical Laboratory Standards Institute (CLSI). Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals; Approved Standard-Fourth Edition. CLSI document VET01-A4 and VET01-S2. Wayne: CLSI; 2013.
Zhao X, Drlica K. Restricting the selection of antibiotic-resistant mutant bacteria: measurement and potential use of the mutant selection window. J Infect Dis. 2002;185(4):561–5.
Zhao X, Drlica K. Restricting the selection of antibiotic-resistant mutants: a general strategy derived from fluoroquinolone studies. Clin Infect Dis. 2001;33:S147–56.
Yuan L, Sun J, Wang R, Sun L, Zhu L, Luo X, et al. Pharmacokinetics and bioavailability of cefquinome in healthy ducks. Am J Vet Res. 2011;72(1):122–6.
El-Banna H. Pharmacokinetics of florfenicol in normal and Pasteurella-infected Muscovy ducks. Br Poultry Sci. 1998;39(4):492–6.
Quinn B, Hussain S, Malik M, Drlica K, Zhao X. Daptomycin inoculum effects and mutant prevention concentration with Staphylococcus aureus. J Antimicrob Chemother. 2007;60(6):1380–3.
Marcusson LL, Olofsson SK, Lindgren PK, Cars O, Hughes D. Mutant prevention concentrations of ciprofloxacin for urinary tract infection isolates of Escherichia coli. J Antimicrob Chemother. 2005;55(6):938–43.
Rodriguez J, Cebrian L, Lopez M, Ruiz M, Jimenez I, Royo G. Mutant prevention concentration: comparison of fluoroquinolones and linezolid with Mycobacterium tuberculosis. J Antimicrob Chemother.2004;53(3):441–4.
Credito K, Kosowska-Shick K, Appelbaum PC. Mutant prevention concentrations of four carbapenems against gram-negative rods. Antimicrob Agents Chemother. 2010;54(6):2692–5.
Credito K, Kosowska-Shick K, McGhee P, Pankuch GA, Appelbaum PC. Comparative study of the mutant prevention concentrations of moxifloxacin, levofloxacin, and gemifloxacin against pneumococci. Antimicrob Agents Chemother. 2010;54(2):673–7.
Wang L, Yuanshu Z, Yuhan Z, Yingxia L. Mutant prevention concentrations of fluoroquinolones against Campylobacter jejuni isolated from chicken. Vet Microbiol. 2010;144(3):409–14.
Briales A, Rodriguez-Martinez J, Velasco C, de Alba PD, Domínguez-Herrera J, Pachón J, et al. In vitro effect of qnrA1, qnrB1, and qnrS1 genes on fluoroquinolone activity against isogenic Escherichia coli isolates with mutations in gyrA and parC. Antimicrob Agents Chemother.2011;55(3):1266–9.
Chen Y-P, Lee S-H, Chou C-H, Tsai H-J. Detection of florfenicol resistance genes in Riemerella anatipestifer isolated from ducks and geese. Vet Microbiol. 2012;154(3):325–31.
Zonca A, Gallo M, Locatelli C, Carli S, Moroni P, Villa R, et al. Cefquinome sulfate behavior after intramammary administration in healthy and infected cows. J Dairy Sci. 2011;94(7):3455–61.
Cavaco L, Abatih E, Aarestrup FM, Guardabassi L. Selection and persistence of CTX-M-producing Escherichia coli in the intestinal flora of pigs treated with amoxicillin, ceftiofur, or cefquinome. Antimicrob Agents Chemother. 2008;52(10):3612–6.
Chang C-F, Lin W-H, Yeh T-M, Chiang T-S, Chang Y-F. Antimicrobial susceptibility of Riemerella anatipestifer isolated from ducks and the efficacy of ceftiofur treatment. J Vet Diagn Invest. 2003;15(1):26–9.
Olofsson SK, Marcusson LL, Lindgren PK, Hughes D, Cars O. Selection of ciprofloxacin resistance in Escherichia coli in an in vitro kinetic model: relation between drug exposure and mutant prevention concentration. J Antimicrob Chemother. 2006;57(6):1116–21.
Firsov AA, Smirnova MV, Lubenko IY, Vostrov SN, Portnoy YA, Zinner SH. Testing the mutant selection window hypothesis with Staphylococcus aureus exposed to daptomycin and vancomycin in an in vitro dynamic model. J Antimicrob Chemother. 2006;58(6):1185–92.
Gebru E, Damte D, Choi M-J, Lee S-J, Kim Y-H, Park SC. Mutant prevention concentration and phenotypic and molecular basis of fluoroquinolone resistance in clinical isolates and in vitro-selected mutants of Escherichia coli from dogs. Vet Microbiol. 2012;154(3):384–94.
Zhang B, Gu X, Li Y, Li X, Gu M, Zhang N, et al. In vivo evaluation of mutant selection window of cefquinome against Escherichia coli in piglet tissue-cage model. BMC Vet Res. 2014;10(1):297-304.
Zhang B, Lu X, Gu X, Li X, Gu M, Zhang N, et al. Pharmacokinetics and ex vivo pharmacodynamics of cefquinome in porcine serum and tissue cage fluids. Vet J. 2014;199(3):399–405.
Zhang B, Gu X, Li X, Gu M, Zhang N, Shen X, et al. Pharmacokinetics and ex-vivo pharmacodynamics of cefquinome against Klebsiella pneumonia in healthy dogs. J Vet Pharmacol Ther. 2014;37(4):367–73.
Shan Q, Yang F, Wang J, Ding H, He L, Zeng Z. Pharmacokinetic/pharmacodynamic relationship of cefquinome against Pasteurella multocida in a tissue-cage model in yellow cattle. J Vet Pharmacol Ther. 2014;37(2):178–85.
Shan Q, Liang C, Wang J, Li J, Zeng Z. In vivo activity of cefquinome against Escherichia coli in the thighs of neutropenic mice. Antimicrob Agents Chemother. 2014;58(10):5943–6.
Wang J, Shan Q, Ding H, Liang C, Zeng Z. Pharmacodynamics of cefquinome in a neutropenic mouse thigh model of Staphylococcus aureus infection. Antimicrob Agents Chemother. 2014;58(6):3008–12.
Xiao X, Sun J, Chen Y, Huang R-J, Huang T, Qiao GG, et al. In vitro dynamic pharmacokinetic/pharmacodynamic (PK/PD) modeling and PK/PD cutoff of cefquinome against Haemophilus parasuis. BMC Vet Res. 2015;11(1):33-9.
Zhou Y, Zhao D, Yu Y, Yang X, Shi W, Peng Y, et al. Pharmacokinetics, bioavailability and PK/PD relationship of cefquinome for Escherichia coli in Beagle dogs. J Vet Pharmacol Ther. 2015. doi:10.1111/jvp.12225.
Acknowledgements
We thank Dr. Rong Xiang for the technical assistance of identifying isolates.
Funding
This work was financially supported by the Program for Changjiang Scholars and Innovative Research Team in University of Ministry of Education of China (Grant No.IRT13063) and National Natural Science Foundation of China (Grant No.31072169).
Availability of data and materials
All the data supporting our findings is contained within the manuscript.
Authors’ contributions
ZLZ conceived and designed the experiments. YFL, YNZ, XM, WL and JXZ performed all the experiments. YFL wrote the first draft of the manuscript. HZD revised the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The Ethics Committee of South China Agricultural University approved the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Li, Y., Zhang, Y., Ding, H. et al. In vitro susceptibility of four antimicrobials against Riemerella anatipestifer isolates: a comparison of minimum inhibitory concentrations and mutant prevention concentrations for ceftiofur, cefquinome, florfenicol, and tilmicosin. BMC Vet Res 12, 250 (2016). https://doi.org/10.1186/s12917-016-0796-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12917-016-0796-3