Abstract
Background
As an insulin-dependent disease, type 1 diabetes requires paying close attention to the glycemic control. Studies have shown that mobile health (mHealth) can improve the management of chronic diseases. However, the effectiveness of mHealth in controlling the glycemic control remains uncertain. The objective of this study was to carry out a systematic review and meta-analysis using the available literature reporting findings on mHealth interventions, which may improve the management of type 1 diabetes.
Methods
We performed a systematic literature review of all studies in the PubMed, Web of Science, and EMbase databases that used mHealth (including mobile phones) in diabetes care and reported glycated hemoglobin (HbA1c) values as a measure of glycemic control. The fixed effects model was used for this meta-analysis.
Results
This study analyzed eight studies, which involved a total of 602 participants. In the meta-analysis, the fixed effects model showed a statistically significant decrease in the mean of HbA1c in the intervention group: − 0.25 (95% confidence interval: − 0.41, − 0.09; P = 0.003, I2 = 12%). Subgroup analyses indicated that the patient’s age, the type of intervention, and the duration of the intervention influenced blood glucose control. Funnel plots showed no publication bias.
Conclusions
Mobile health interventions may be effective among patients with type 1 diabetes. A significant reduction in HbA1c levels was associated with adult age, the use of a mobile application, and the long-term duration of the intervention.
Similar content being viewed by others
Background
Type 1 diabetes mellitus (T1DM) is a metabolic disease characterized by hyperglycemia [1]. This disease, also referred to as insulin-dependent diabetes, occurs in all age groups, but especially in children and adolescents [2]. With an increasing worldwide incidence of approximately 3% to 4% a year [3]. In the United States, the number of young patients with T1DM has been predicted to increase by 23% over the next 40 years [4]. Although T1DM accounts for only 5–10% of all cases of diabetes, the prevalence of T1DM is increasing in most countries around the world [5]. The significant effect that poor glycemic control can have on the health of patients with T1DM [6], includes failure to achieve adequate levels of glycemic control ultimately results in damage to a wide range of organs, most notably the eyes, kidneys, heart, blood vessels, and nerves [7]. With the growing number of individuals with T1DM, improving diabetes care to decrease the health and economic burden caused by the disease is viewed globally as an important goal [1, 8].
Studies have shown that continuous self-health management strategies positively impact many aspects of T1DM, including preventing complications and improving both metabolic control and quality of life [9,10,11]. However, the diabetes education provided in clinics and hospitals is limited, and the availability of this education is severely restricted. The market penetration of mobile phones is extensive, and they currently meet a variety of user needs [11,12,13]. In 2015, it was reported that 88% of American teens either owned or had access to a mobile phone, compared with 45% in 2004 [14]. At present, mobile phones, apart from their recreational function, are becoming instruments of patient education and support and are also helpful for health care professionals [15, 16]. A number of studies have shown that mobile phone, telehealth and similar movements with increasing popularity as the tools to aid persons with diabetes in the managing of their condition while lowering costs [17, 18]. New mHealth technology can also improve the quality of life of diabetic patients [19, 20]. The impact of mHealth interventions on the control of HbA1c levels in type 2 diabetes is clinically significant and has been well documented [21, 22]. Toma et al. [23] investigated the effectiveness of online social networking services or mobile phone use as a management intervention for patients with diabetes, finding that social networking services interventions beneficially reduced Hemoglobin A1c (HbA1c) when compared with the non user. This finding was confirmed by sensitivity analysis; there was a significant reduction in HbA1c in the intervention group (weighted mean difference [WMD] = 0.46%; 95% confidence interval [CI]: 0.58–0.34). A number of studies have evaluated the use of mHealth in the management of patients with T1DM; however, these studies’ results have varied [24,25,26], and the effectiveness of mHealth on glycemic control remains uncertain.
Therefore, the objective of the present study was to conduct a systematic review and meta-analysis of published studies to evaluate the efficacy of interventions including mHealth compared with other interventions to control HbA1c levels in populations of children and adults.
Methods
Search strategy
Studies published in English were identified by searching PubMed, Web of Science, and EMbase. In addition, we searched within the reference lists of identified papers. We searched for studies published through June 2016 using combinations of the following search terms: “mHealth,” “mobile health,” “text-messaging,” “mobile application,” “type 1 diabetes,” “diabetes mellitus,” and “randomized controlled trial.” These search terms were connected or used alone using “and” or “or,” and the search strings were developed according to the characteristics and requirements of each database and the particular search engines employed.
Inclusion and exclusion criteria
The authors made a selection from the identified articles for this review. We defined patients with T1DM as those who had been diagnosed by a physician, and we selected interventions that lasted more than three months (the time the experimental group used the mHealth programme) [27]. Participants with T1DM were included regardless of gender, age, race, or nationality. To be included in this study, the patients had to have the reading and writing skills necessary to complete their medical histories and the questionnaires independently. Additional inclusion criteria were as follows: (1) randomized controlled trials with mHealth as an intervention (i.e., the interventions included mobile applications or text messages); (2) the inclusion of a comparison of standard therapies (i.e., receiving the standard educational approach, without mobile applications or text messages); (3) reporting HbA1c as an outcome, with values measured at both baseline and at the end of the study for each group; and (4) written in English. Studies were excluded if they (1) included patients with Severe diabetic complications(Diabetic foot, Diabetic heart disease.etc); (2) had mixed patient populations (type 1 and type 2 diabetics); (3) conducted interventions by voice via telephone; (4) covered only the use of insulin pumps, artificial pancreas, or continuous glucose monitoring equipment; (5) duplicate publications of the same data set; (6) drew upon original data that was not available; (7) were limited to pregnant women or other special populations with T1DM. All analyses for the present study were based on previous published research; thus, no ethical approval or patient consent were required.
Data extraction and quality assessment
Two reviewers independently scanned the electronic records to identify potentially eligible trials. A standard data extraction sheet was used to extract and sort these trials independently. All discrepancies were resolved in discussion with a third reviewer. We included studies that met our inclusion criteria. The variables extracted from the studies included country of origin; year of publication; number of participants; participants’ age, sex, diabetes duration, and HbA1c at baseline and at follow-up; the intervention; and the follow-up time. We reported duplicate publications of the same data set only once. For studies with more than one intervention group, we considered the most intensive intervention to be the experimental one. Following the search strategy described above, no studies were included for which the necessary data from the original study were not reported.
The risk of bias was assessed using the Cochrane Collaboration tool [28]. The studies were rated according to five predefined categories: (1) random sequence generation; (2) quality of allocation concealment; (3) quality of blinding; (4) freedom from incomplete data; and (5) freedom from selective reporting. The risk of bias in each area was scored as high, low, or unclear.
Data synthesis and analysis
We determined that the primary outcomes used the fixed effects model and weighted mean difference (WMD). Analysis was conducted using Review Manager Version 5.3 for Windows (The Cochrane Collaboration, Software Update, Oxford, UK). Heterogeneity was measured using Cochrane’s Q and the I2 index to test whether the studies were homogeneous, the p value of the Q-test and the I2 index were set at 0.10 and 50%, respectively. If P > 0.10 and I2 < 50%, the results of homogeneity were considered good, and the fixed effects model was used for analysis. Otherwise, the random effects model was used. The meta-analysis test level was set at α = 0.05. Subgroup analyses further explored the effects of different ages, types of interventions, and intervention durations on the study results. A funnel plot was used to detect publication bias.
Results
Literature screen
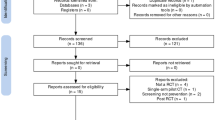
This systematic review was structured according to the Preferred Reporting Items for Systematic Review and Meta-Analyses guidelines [29]. From 5302 citations, 424 publications were identified as potentially eligible studies, and the full text of these publications was retrieved and assessed. Eight studies met the inclusion criteria [24,25,26, 30,31,32,33,34]. Details of the screening process and results are presented in Fig. 1.
Study characteristics
The studies that were included in the systematic review are listed in Table 1. All of the eight eligible studies were published from 2005 to 2016. They included a total of 638 patients, 325 of whom were randomly assigned to intervention groups; 313 were assigned to control groups. A total of 578 patients (90.5%) completed the respective intervention studies, there were 293 people in the intervention group and 285 in the control group. A total of 602 people were analyzed. And, for the six references that calculated the sample size, the power was greater than 80%. There was no statistically significant difference in the rate of loss to follow-up between the intervention group and the control group. Two studies were conducted in the United States, five in Europe, and one in Australia. The shortest study was 3 months in duration, and the longest was 12 months. Three studies had durations of 3–5 months, and five studies had durations longer than 6 months. In three studies, the intervention was only use mobile phone text messages, while the remainder used mobile phone applications.
Outcomes
Comparison of HbA1c levels at baseline and follow-up
There was no difference in HbA1c between the intervention and control groups at baseline, the mean of HbA1c (95% confidence interval) is (0.00 [− 0.15, 0.14]; P = 0.97). There was a significant reduction in the follow-up HbA1c in the intervention group, compared with their baseline HbA1c (− 0.54 [− 0.80, − 0.28]; P = 0.0001). In contrast, the standard-care group showed no significant difference between baseline and follow-up HbA1c (− 0.18 [− 0.45, 0.08]; P = 0.17), obtained before and after the intervention. There was a significant difference in the pooled HbA1c change from baseline between the mHealth and standard-care groups (− 0.25 [− 0.41, − 0.09]; P = 0.003) (Fig. 2). Regarding statistical heterogeneity, the variability in effect estimates (I2 = 12%, P = 0.34) showed the homogeneity of the intervention effects across the primary studies was good. Therefore, the fixed effects model was used for the primary data analysis.
Subgroup analyses of different ages, types of intervention, and intervention durations
To assess the effects of different characteristics on the intervention, we performed subgroup analyses grouped by age, type of intervention, and intervention time. The results showed that adult patients, using mobile applications for patient management, the implementation of a longer period of intervention on patients with HbA1c control is effective (Table 2).
-
1)
Age
Because T1DM is managed differently in different age groups [35], we analyzed a youth group and an adult group. We found that the change in HbA1c in the youth group was not significant (− 0.05 [− 0.43, 0.33]; P = 0.80). In the adult group, however, there was a significant change in HbA1c (− 0.29 [− 0.47, − 0.11]; P = 0.001).
-
2)
Types of interventions
For the subgroup analyses of different types of interventions, intervention methods were classified into two categories: text messages (including automated Internet text messages and short message service [SMS]) or mobile applications about diabetes self-management. The use of SMS as an intervention was not associated with a significant decrease in HbA1c (− 0.20 [− 0.73, 0.32]; P = 0.44), whereas HbA1c levels were better controlled with the use of mobile phone applications (− 0.25 [− 0.42, − 0.08]; P = 0.003).
-
3)
The duration of the interventions
We created two subgroups: interventions of less than 6 months and interventions of more 6 months. The results showed significantly decreased HbA1c levels in the group with interventions of more than 6 months (0.29 [− 0.46, − 0.11]; P = 0.001). In the group with interventions of less than 6 months HbA1c did not significantly change (− 0.01[− 0.44, 0.41]; P = 0.95).
Risk of bias
The risk of bias of the included studies is shown in Figs. 3 and 4. All of the studies were randomized and therefore at a lower risk of bias. This was because of the nature of the intervention; some of the studies could not use blinding, which influenced the confidence of the synthetic results. All of the studies were free of incomplete outcome data, and all of the trials were free of selective outcome reporting.
Publication bias
Publication bias was assessed by a funnel plot (Fig. 5). Each study was symmetrically distributed on both sides. No significant publication bias was observed.
Discussion
Maintaining a healthy lifestyle in patients with T1DM is fundamental to their health status and welfare. The main objective of the present study was to provide the most recent information on mHealth, and the findings are based on studies conducted in different countries. Among the reviewed studies, all applied randomized controlled designs, which enhanced the comparability of the outcomes. In the fixed effects model used in the meta-analysis, heterogeneity less than 50%, indicating that the results are relatively reliable. The results of this meta-analysis showed that using mHealth interventions reduced HbA1c relative to no mHelath control groups. Our results are consistent with research on the management of chronic diseases [36, 37], which has demonstrated improved medication adherence with the use of text messages or mobile applications [38]. However, it should be noted that some studies have found no significant difference in HbA1c between intervention and control groups. This may be due to the greater emphasis in developed countries on primary health care for diabetics, where a higher health consciousness might have been a confounding factor [39]. Therefore, mHealth can still improve a patient’s understanding of a disease process and self-management strategies [25]. Skrovseth [40] has demonstrated that the effective implementation of mHealth has a significant positive impact on blood glucose control. In addition, in some studies, glycosylated hemoglobin also decreased significantly in patients who did not receive the intervention (control group) [25, 32, 33]. This suggests that although routine intervention can also help patients control their HbA1C, it is less effective than the mhealth group.
Subgroup analyses found that the impact of mHealth differed for teenage and adult patients with T1DM. mhealth interventions had a significant positive impact among adults, whereas the effects of these interventions were subtler for younger people. This is consistent with the results of several previous systematic reviews [41]. The management of children with T1DM is complicated by multiple factors that must be taken into consideration, such as growth, activity, diet, insulin enhancement, and psychological factors [42]. Additionally, as children mature into adolescents and then adults, changes in growth hormone secretion result in insulin resistance [43, 44]. Furthermore, although the patients who were enrolled in our study were required to be able to read and write, compared with adults, adolescents’ self-management ability is poor, and their understanding of health education is not clear enough [39]. Poor compliance may affect glycemic control [45]. Although HbA1c were not significantly different following the intervention in the adolescent group, both quality of life and treatment compliance were greatly improved [25, 26]. Others have found that teenagers are prone to accept this form of education [46]. This also suggests that mhealth treatment has potential but needs to be refined by teenagers and can be cost-effective as a means of intervention. In-depth study is required to determine an effective self-management model for minors.
Our subgroup analyses found that using mobile applications was more effective than using text messages. Text message interventions were associated with lower costs and increased ease of operation, providing a wide range of intervention opportunities [47]. However, in contrast to the mobile application, text messaging lacks two-way communication. Text messaging also has a simpler intervention content and a lower rate of patient feedback. Studies have shown that the use of mobile applications by diabetic patients is increasing, indicating that patients with diabetes are interested in using these methods to improve their self-management [48]. Compared with other methods, mobile applications can provide better education and more timely feedback [49]. Some mobile applications also support the real-time monitoring of blood glucose [48]. The future direction of mhealth should strive to facilitate the expansion of effective health management practices among the general population. Therefore, studies should further explore the use of mhealth to improve the level of patient self-management the most effective method.
The duration of the intervention may be an important factor in determining whether mHealth is effective. Our subgroup analysis showed that the decrease in HbA1c was significant in groups exposed to longer continuous intervention time. This indicates that longer intervention time periods lead to better glycemic control. Comparing groups exposed to interventions of different durations, Kirwan et al. [24] found that blood glucose control in patients with 9 months of intervention was better than in those with 3 months. This is likely because diabetes is a chronic disease with a slow process of control. HbA1c reacts to blood glucose levels for nearly 2 months. Therefore, short-term interventions may not result in significant changes in HbA1c levels. In summary, we need to pay attention to the duration of the intervention.
Limitations
Despite the positive findings of this study, a number of limitations should be considered when interpreting the results: First, although the findings from the reviewed studies showed the use of mobile phones to be promising for improving T1DM management, some of these studies had small sample sizes. Especially in the subgroup analyses, small numbers may be lead to false positive results. Therefore, future studies with large sample sizes are needed to determine whether the increased patient–provider communication with mHealth has a significant impact on clinical outcomes and public health. Second, it is possible that our search for relevant literature for inclusion in the current review paper may have overlooked some publications. If so, this could cause selection bias. Further studies should be conducted to confirm the present findings. Third, the studies included in this review had different characteristics; these factors can lead to heterogeneity and influence the reliability of the results. Fourth, Glycosylated hemoglobin (HbA1c) is a gold standard to measure blood glucose control, which can effectively reflect the past blood glucose control in patients with diabetes mellitus. Part of the literature included in this study had a relatively short intervention time, which may have an impact on the results.
Conclusions
In summary, this study found that the use of mobile health for patients with T1DM positively impacted disease management and improved HbA1c levels in certain subgroups. However, because of the limitations inherent in both the quantity and quality of the included studies, high quality, multicenter, randomized controlled trials with large sample sizes are needed to substantiate these results.
Abbreviations
- CI:
-
Confidence interval
- HbA1c:
-
Hemoglobin A1c
- mHealth:
-
Mobile health
- SMS:
-
Short message service
- T1DM:
-
Type 1 diabetes mellitus
- WMD:
-
Weighted mean difference
References
Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. 2014;383(9911):69–82.
Gale EAM. Type 1 diabetes in the young: the harvest of sorrow goes on. Diabetologia. 2005;48(8):1435–8.
Patterson CC, Gyürüs E, Rosenbauer J, Cinek O, Neu A, Schober E, Parslow RC, Joner G, Svensson J, Castell C. Trends in childhood type 1 diabetes incidence in Europe during 1989–2008: evidence of non-uniformity over time in rates of increase. Diabetologia. 2012;55(8):2142–7.
Giuseppina I, Boyle JP, Thompson TJ, Doug C, Dana D, Hamman RF, Lawrence JM, Liese AD, Liu LL, Mayer-Davis EJ: Projections of type 1 and type 2 diabetes burden in the U.S. population aged <20 years through 2050: dynamic modeling of incidence, mortality, and population growth. Diabetes Care 2012, 35(12):págs. 2515–2520.
Gong C, Meng X, Jiang Y, Wang X, Cui H, Chen X. Trends in childhood type 1 diabetes mellitus incidence in Beijing from 1995 to 2010: A retrospective multicenter study based on hospitalization data. Diabetes Technol Ther. 2015;17(3):159-65.
Rita Angélica GD, Nayely GN, Niels WR, Carlos Alberto AS. Epidemiology of type 1 diabetes in Latin America. Curr Diabetes Rev. 2014;10(2):75–85.
Herrath MGV, Korsgren O, Atkinson MA. Factors impeding the discovery of an intervention-based treatment for type 1 diabetes. Clin Exp Immunol. 2016;183(1):1-7.
Nathan DM, Margaret B, Patricia C, Saul G, Rose GK, Lachin JM, Gayle L, Bernard Z. Diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: advances and contributions. Diabetes. 2013;62(12):3976–86.
Cafazzo JA, Casselman M, Hamming N, Katzman DK, Palmert MR. Design of an mHealth app for the self-management of adolescent type 1 diabetes: A Pilot study. J Med Internet Res. 2012;14(3):e70.
Munt R, Hutton A. Type 1 diabetes mellitus (T1DM) self management in hospital; is it possible? A literature review. Contemp Nurse. 2012;40(2):179–93.
Deacon AJ, Edirippulige S. Using mobile technology to motivate adolescents with type 1 diabetes mellitus: A systematic review of recent literature. J Telemed Telecare. 2015;21(8):431.
Montori VM, Helgemoe PK, Guyatt GH, Dean DS, Leung TW, Smith SA, Kudva YC. Telecare for patients with type 1 diabetes and inadequate glycemic control: a randomized controlled trial and meta-analysis. Diabetes Care. 2004;27(5):1088.
Balkhi AM, Reid AM, Westen SC, Olsen B, Janicke DM, Geffken GR. Telehealth interventions to reduce management complications in type 1 diabetes:A review. World J Diabetes. 2015;6(3):371–9.
Lenhart A. Teens, Social Media & Technology Overview 2015; 2015.
Edmonds M, Bauer M, Osborn S, Lutfiyya H, Mahon J, Doig G, Grundy P, Gittens C, Molenkamp G, Fenlon D. Using the vista 350 telephone to communicate the results of home monitoring of diabetes mellitus to a central database and to provide feedback. Int J Med Inform. 1998;51(2–3):117–25.
Piette JD, Weinberger M, Mcphee SJ, Mah CA, Kraemer FB, Crapo LM. Do automated calls with nurse follow-up improve self-care and glycemic control among vulnerable patients with diabetes? Am J Med. 2000;108(1):20–7.
Costa BM, Fitzgerald KJ, Jones KM, Trisha Dunning AM: Effectiveness of IT-based diabetes management interventions: a review of the literature. BMC Fam Pract 2009, 10(4):72–72.
Biermann E, Dietrich W, Rihl J, Standl E. Are there time and cost savings by using telemanagement for patients on intensified insulin therapy? A randomised, controlled trial. Comput Methods Programs Biomed. 2002;69(2):137–46.
Courtney Rees L, Harris LT, Tung L, Jan F, James T, Diane B, James H, Hirsch IB, Goldberg HI, Ralston JD. Qualitative evaluation of a Mobile phone and web-based collaborative care intervention for patients with type 2 diabetes. Diabetes Technol Ther. 2011;13(5):563–9.
S L, SM K, H S, HJ L, J WY, SH Y, SY K, SY Y, HS J, KS P, et al. Improved glycemic control without hypoglycemia in elderly diabetic patients using the ubiquitous healthcare service, a new medical information system. Diabetes Care. 2011;34(2):308–13.
Huang Z, Tao H, Meng Q, Jing L. Management of endocrine disease. Effects of telecare intervention on glycemic control in type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Eur J Endocrinol. 2015;172(3):R93–101.
Mohsen S, Ghader G, Koenig HG. Health education via mobile text messaging for glycemic control in adults with type 2 diabetes: a systematic review and meta-analysis. Primary Care Diabetes. 2014;8(4):275–85.
Toma T, Athanasiou T, Harling L, Darzi A, Ashrafian H. Online social networking services in the management of patients with diabetes mellitus: systematic review and meta-analysis of randomised controlled trials. Diabetes Res Clin Prac. 2014;106(2):200–11.
Morwenna K, Corneel V, Andrew F, Duncan MJ. Diabetes self-management smartphone application for adults with type 1 diabetes: Randomized controlled trial. J Med Internet Res. 2013;15(11):197–204.
Berndt RD, Takenga C, Preik P, Kuehn S, Berndt L, Mayer H, Kaps A, Schiel R. Impact of information technology on the therapy of type-1 diabetes: a case study of children and adolescents in Germany. J Personalized Med. 2014;4(2):200–17.
Yi H, Faulkner MS, Fritz H, Fadoju D, Muir A, Abowd GD, Head L, Arriaga RI, Pilot Randomized A. Trial of text-messaging for symptom awareness and diabetes knowledge in adolescents with type 1 diabetes. J Pediatr Nurs. 2015;30(6):850–61.
Inada M, Oishi M, Nishikawa M, Kurata S, Imura H. Clinical evaluation of measuring glycosylated hemoglobin levels for assessing the long-term blood glucose control in diabetics. Endocrinologia Japonica. 1980;27(4):411–5.
Higgins Jpt GS. Cochrane handbook for systematic reviews of interventions version 510. Naunyn-Schmiedebergs Archiv für experimentelle Pathologie und Pharmakologie. 2011;5(2):S38.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339(10):e1–e34.
Hanauer DA, Wentzell K, Laffel N, Laffel LM. Computerized automated reminder diabetes system (CARDS): e-mail and SMS cell phone text messaging reminders to support diabetes management. Diabetes Technol Ther. 2009;11(2):99–106.
Charpentier G, Benhamou PY, Dardari D, Clergeot A, Franc S, Schaepelynck-Belicar P, Catargi B, Melki V, Chaillous L, Farret A. The Diabeo software enabling individualized insulin dose adjustments combined with telemedicine support improves HbA1c in poorly controlled type 1 diabetic patients: a 6-month, randomized, open-label, parallel-group, multicenter trial (TeleDiab 1 study). Diabetes Care. 2011;34(3):533–9.
Rossi MCE, Nicolucci A, Bartolo PD, Bruttomesso D, Girelli A, Ampudia FJ, Kerr D, Ceriello A, Mayor CDLQ, Pellegrini F. Diabetes interactive diary: a new telemedicine system enabling flexible diet and insulin therapy while improving quality of life: an open-label, international, multicenter, randomized study. Diabetes care. 2010;33(1):109–15.
Maria Chiara R, Antonio N, Giuseppe L, Fabio P, Paolo DB, Valerio M, Roberto A, Giacomo V. Impact of the “diabetes interactive diary” telemedicine system on metabolic control, risk of hypoglycemia, and quality of life: A Randomized clinical trial in type 1 diabetes. Diabetes Technol Ther. 2013;15(8):670–9.
Franklin VL, Waller A, ., Pagliari C, ., Greene SA: A randomized controlled trial of sweet talk, a text-messaging system to support young people with diabetes. Diabet Med 2006, 23(12):1332–1338.
Winocour PH. Care of adolescents and young adults with diabetes - much more than transitional care: a personal view. Clin Med. 2014;14(3):274–8.
Fischer HH, Moore SL, Ginosar D, Davidson AJ, Rice-Peterson CM, Durfee MJ, Mackenzie TD, Estacio RO, Steele AW. Care by cell phone: text messaging for chronic disease management. Am J Manag Care. 2012;18(2):e42–7.
Huang JS, Laura T, Trevor T, Lindsay D, Mark P, Michael G, Norman GJ, L Kay B. Preparing adolescents with chronic disease for transition to adult care: a technology program. Pediatrics. 2014;133(6):e1639–46.
Fischer MA, Jones JB, Wright E, Van Loan RP, Xie J, Gallagher L, Wurst AM, Shrank WH. A Randomized telephone intervention trial to reduce primary medication nonadherence. J Managed Care Specialty Pharmacy. 2015;21(2):124–31.
Guo J, Whittemore R, Grey M, Wang J, Zhou ZG, He GP. Diabetes self-management, depressive symptoms, quality of life and metabolic control in youth with type 1 diabetes in China. J Clin Nurs. 2012;22(1–2):69–79.
Skrøvseth SO, E Å, Godtliebsen F, Joakimsen RM. Data-driven personalized feedback to patients with type 1 diabetes: A Randomized trial. Diabetes Technol Ther. 2015;17(7).
Herbert L, Owen V, Pascarella L, Streisand R. Text message interventions for children and adolescents with type 1 diabetes: A systematic review. Diabetes Technol Ther. 2013;15(5):362–70.
Harris MA, Hood KK, Mulvaney SA. Pumpers, Skypers, surfers and texters: technology to improve the management of diabetes in teenagers. Diabetes Obes Metab. 2012;14(11):967–72.
Borus JS, Lori L. Adherence challenges in the management of type 1 diabetes in adolescents: prevention and intervention. Curr Opin Pediatr. 2010;22(4):405–11.
Rausch JR, Hood KK, Alan D, Jennifer SP, Rohan JM, Grafton R, Lawrence D, Dennis D: Changes in treatment adherence and glycemic control during the transition to adolescence in type 1 diabetes. Diabetes Care 2012, 35(6):págs. 1219–1224.
Greening L, Stoppelbein L, Konishi C, Jordan SS, Moll G. Child routines and youths' adherence to treatment for type 1 diabetes. J Pediatr Psychol. 2007;32(4):437–47.
Tasker AP, Gibson L, Franklin V, Gregor P, Greene S. What is the frequency of symptomatic mild hypoglycemia in type 1 diabetes in the young?: assessment by novel mobile phone technology and computer-based interviewing. Pediatr Diabetes. 2007;8(1):15–20.
Déglise C, Suggs LS, Odermatt P. SMS for disease control in developing countries: a systematic review of mobile health applications. J Telemedicine Telecare. 2012;18(5):273–81.
Brzan PP, Rotman E, Pajnkihar M, Klanjsek P. Mobile applications for control and self Management of Diabetes: A systematic review. J Med Syst. 2016;40(9):1–10.
Goyal S, ., Cafazzo JA: Mobile phone health apps for diabetes management: current evidence and future developments. Qjm Monthly Journal of the Association of Physicians 2013, 106(12):1067–1069.
Acknowledgments
We thank Jennifer Barrett, Ph.D, from Liwen Bianji, Edanz Editing China (https://www.liwenbianji.cn/), for editing the English text of a draft of this manuscript.
Funding
The study was supported by Inner Mongolia Science and Technology Major Plan Program (Intelligent Health Monitoring and Modern Medical Information System Development Based on Internet of Things Technology).and the Commission of Health and Family Planning of Inner Mongolia Autonomous Region fund project (201302050).
Availability of data and materials
We declare that the data supporting the conclusions of this article are fully described within the article.
Author information
Authors and Affiliations
Contributions
MLD and PYW design of the work. XMW and WS wrote the paper. MMX, HQZ, YFJ and SHY contributed to the discussion and edited the manuscript. DYL, RQW and LNH did the work of data collecting and data was analyzed by WS and JD. All authors approved the final version to be submitted for consideration for publication.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Wang, X., Shu, W., Du, J. et al. Mobile health in the management of type 1 diabetes: a systematic review and meta-analysis. BMC Endocr Disord 19, 21 (2019). https://doi.org/10.1186/s12902-019-0347-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12902-019-0347-6