Abstract
Background
Root-knot nematodes (RKN) are major pest of olive tree (Olea europaea ssp. europaea), especially in nurseries and high-density orchards. Soil samples were collected from main olive growing areas of Morocco, to characterize Meloidogyne species and to discuss the contribution of biotic and abiotic factors in their spatial distribution.
Results
RKN were found in 159 soil samples out of 305 from nurseries (52.1% occurrence) and in 11 out of 49 soil samples from orchards (23.2% occurrence). Biochemical and molecular characterisation (PAGE esterase and SCAR) revealed the dominance of M. javanica both in nurseries and orchards with minor presence of M. incognita only in nurseries, and M. arenaria in only one nursery. RKN were distributed on aggregated basis. Frequent presence of M. javanica in orchards might have come from nurseries. In contrast, the detection of M. incognita in nurseries alone suggests that this species could not reproduce in orchards because of either the competition with other plant-parasitic nematodes or unfit local habitats. The impact of environmental variables (climate, habitat origin and physicochemical characteristics of the substrates) on the distribution of Meloidogyne species is also discussed.
Conclusion
Olive nurseries in Morocco are not able to guarantee the safety of rooted plants. As a result, olive production systems are exposed to strong RKN invasion risks. Consequently, the use of healthy substrates in nurseries may prevent plant-parasitic nematode induction in orchards.
Similar content being viewed by others
Background
Sustainable management of key taxa depends upon understanding their distribution and behaviour towards biotic and abiotic factors. Studying the factors of RKN population dispersal can facilitate to understand their spatial structure [1]. Ecologists and conservation managers depend on spatial models to assess environmental effects on distribution of a species. These models facilitate to develop reserve selection and survey design to manage various species.
Distribution models are categorized into two groups: (1) some simulate interactive processes between environment and organisms, (2) others use pattern analysis to reveal correlation among target taxa and environmental variables. Biological (capacity of an organism to disperse and reproduce), physical (mountains or oceans) and environmental (soil texture, moisture conditions) factors hinder species dispersal [2]. These models require detailed information about organism and environment over a period of time to predict spatial and temporal patterns of an organism [3].
The production of commercial olive plantlets in the Mediterranean basin, especially in Morocco, provides a favourable environment for the development of plant pests [4]. The geographic location of Morocco, compared to other Mediterranean countries, offers specific orography and bio-climates, with endemic vegetation [5]. High mountain ranges (exceeding 4000 m in altitude) create a complex and highly compartmentalized structure, with extensive plateaux and plains. Climate of the country is deeply influenced by the Atlantic Ocean with annual rainfall of 30–2000 mm.
In Morocco, nursery substrates are often prepared with soil from cropped fields or natural environments that could be potentially infested with soil-borne pathogens such as Verticillium dahliae Kleb. (i.e., Verticillium wilt) [5] and plant-parasitic nematodes (PPN). The use of pathogen-free planting materials and non-infested soils is necessary during the early years of olive cultivation. The threat of these pathogens to olive production has also been recognised by the European Union [6].
PPN are microscopic, round and filiform worms living in soil and/or inside plant root tissues. They generally parasite the underground parts (roots, tubers, rhizomes) of the plants. They cause significant agricultural damage in the world (about 14% yield loss), that reaches over $100 billion per year [7]. Abiotic factors such as pH, soil type, organic matter content, moisture [8], and local climatic conditions affect PPN development [9]. They move only short distances and, thus their dissemination is via water [10] and wind [11]. Human activities such as the introduction of infected planting material or diffusion of infested soil with nursery practices also contribute to spreading [12]. Meloidogyne spp. are major PPN, causing a worldwide loss of about 50 billion Euros [13, 14]. Meloidogyne species like M. javanica, M. inconita, M. arenaria, M. hapla and M. lusitanica are known to infect olive trees [15, 16]. Recently M. baetica and M. spartelensis were also identified on wild olive trees in southern Spain and northern Morocco [17]. Nevertheless, little information is available about PPN host-parasite relationship between RKN and olive plantlets. RKN are known as major pest of olive trees, especially in nurseries having favourable irrigation conditions [18]. Experiments have demonstrated effect of RKN on olive plant growth and of susceptible olive cultivars [19].
RKN control is a challenging task, because: (i) their occurrence is worldwide especially under hot climate; (ii) they are highly diversified; and (iii) they exhibit various reproduction methods (mitotic and meiotic parthenogenicity and amphimixis) [20]. Therefore, in order to manage their infestation, identification of Meloidogyne species is basic requirement to understand its ecology, physiology and reproduction [21]. Conventional methods for RKN identification based on morphological traits require a great deal of skill and are often inconclusive. Polyacrylamide gel electrophoresis (PAGE) isozyme analysis is a relatively fast way to identify Meloidogyne species [22]. However, isozyme analysis can only be done with mature females embedded in roots, and not with second-stage juveniles or eggs in the soil. SCAR (Sequence Characterized Amplified Region) based molecular biomarkers are used to confirm Meloidogyne species [23].
Previous surveys revealed scarce populations of M. arenaria, M. hapla and M. spartelensis in wild olive whereas M. javanica dominated in cultivated areas of Morocco [16]. We hypothesized that its widespread distribution might be due to inductions from nurseries. Therefore, objective of this study is to test the hypothesis by: (i) characterizing the Meloidogyne species in nurseries and orchards of main olive-producing areas of Morocco; (ii) analysing their distribution in nurseries, impact of the climate, habitat and physicochemical characteristics of the substrates; and (iii) discussing their introduction from nurseries to orchards, especially in relevance to M. javanica.
Methods
Site description
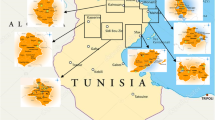
Surveys were conducted in the main olive (Olea europaea subsp. europaea) cultivated areas of Morocco (Fig. 1) ranging from the Strait of Gibraltar in the north to Agadir in the south, covering various soil types. Annual precipitation in the area ranges between 200 and 1000 mm from south to north, temperatures between 5 and 45 °C and altitudes from 200 to 1700 m [24]: (i) in the Jbala region along the west side of Rif Mountains in the north; (ii) near Taza in the Jel Plains of eastern Morocco; (iii) near Fes in the Kandar region located in northern Middle Atlas Mountains; (iv) south of Meknes in the Guerouane region; (v) near Beni Mellal in the Tadla region located on the north side of the southern Middle Atlas Mountains; (vi) near Marrakech in the Haouz region, on the north side of the High Atlas Mountains; and (vii) near Agadir in the Souss region located on the south side of the High Atlas Mountains. Overall twenty-five nurseries were selected for (i) production and the diversity of the varieties, (ii) diversity of culturing substrates, and (iii) geographic distribution (Fig. 1a; Table 1). Forty-nine orchards were selected according to traditional (100 trees/ha; no irrigation) and high-density (up to 2200 trees/ha; drip irrigation) orchards [25] (Fig. 1b; Table 1).
Soil sampling
In nurseries, olive plantlets are grown in 2–3-l plastic bags containing solid substrates from different origins (alluvial sandy soils, forest soils, loamy open-field soils) with different proportions of sand, peat fertilizer and animal manure. Five olive plantlets were sampled from each nursery for each variety. Information of variety, origin and substrates was recorded for each sample. In total, 305 olive plantlets were taken to the laboratory and maintained under greenhouse conditions.
In orchards, only soil samples were collected, as PPN spend a part of their life cycle in it. Samples were collected from upper rhizosphere of soil under the foliage. In each orchard, four trees at 10 m distance along transects, were chosen and from each tree five sub-samples were taken. Considering that the cultivation activities could homogenize the distribution of nematodes, twenty sub-samples were pooled into one (1-dm3) reference sample per orchard.
Root-knot nematode extraction and culture
From both, nursery olive plantlets and orchard soil samples, nematodes were extracted from a 250-cm3 soil aliquot according to elutriation procedure [26]. RKN were identified according to genus, counted and expressed per dm3 of fresh soil. Susceptible tomato variety (cv. Roma) was grown in 500-cm3 soil of each sample under greenhouse (12 h light at 25 °C, 12 h dark at 20 °C) to multiply the populations. Presence of RKN galls and egg masses was observed after 60 days of tomato transplantation.
Identification of Meloidogyne species
Isozyme phenotype analysis
Tomato roots were lightly washed and adult females were collected using forceps and transfer needles. 25 females and their eggs were collected per sample. Females were individually crushed in 250-µL micro-tubes containing 5 µL of Trugdill buffer with 20% sucrose (pH 8.0) [27], and stored at − 20 °C. Females of pure M. javanica population were prepared as above and used as the reference population. Micro-tubes were centrifuged (9500 rpm for 10 min) and 0.01% bromophenol-blue was added. Supernatants were transferred to 70 × 80 × 0.5 mm separating (7% bis-acrylamide, pH 8.4) and stacking (3.5% bis-acrylamide, pH 6.7) gels [22] whereas PAGE was processed in a Mini Protean II electrophoresis unit (BioRad®) at 7 °C. Each gel included two reference M. javanica females. Gels were incubated with α-naphthyl acetate and Fast Blue (37 °C for 1 h) to reveal Esterase (Est) phenotype bands. The band stain was fixed by placing gels in 10% acetic acid for several hours and sandwiched between cellophane sheets to dry for 48 h [28]. Est phenotype patterns were identified and labelled by bands (Rm) in reference to M. javanica.
Molecular identification
Female egg masses of Est analyses were individually incubated in distilled water for hatching. 5–10 juveniles were taken/egg mass to extract DNA. QIAGEN DNeasy Blood & Tissue kits were used for nematode DNA extraction, and PCR-SCAR assays were carried out with specific primers: OPA-12 Fare/Rare (for M. arenaria), OPB-06 Finc/Rinc (for M. incognita), and OPA-01 Fjav/Rjav (for M. javanica) [23]. PCR amplifications were performed in 2 µL (10 ng) of template DNA, 5 µL of PCR QIAGEN kits (Multiplex-PCR), 1 µL of each SCAR primer and 2 µL of sterile water using the GeneAmpR PCR System 9700 (Applied Biosystems®). PCR amplification was carried out at: initial denaturation (95 °C for 15 min), 40 cycles denaturation (94 °C for 30 s), annealing (58 °C for 90 s), elongation (72 °C for 90 s), final extension (72 °C for 10 min). Amplified products were confirmed on 1.5% agarose gel with DNA Ladder (200–10,000 pb).
Soil and climate data recovered in nurseries
A 100-cm3 dry and sieved (2 mm) aliquot from each soil sample was used for physicochemical analyses in Soil Laboratory of the “Institut Agronomique et Vétérinaire Hassan II” (Agadir, Morocco). Soil texture analysis including clay (0–2 µm), fine (2–20 µm) and coarse (20–50 µm) silt, fine (50–200 µm) and coarse (200–2000 µm) sand was performed according to Stoke’s Law sedimentation method [29]; Carbon [to calculate organic matter (OM = 1.724 × C]), nitrogen, phosphorus and potassium content, soil pH and salinity were also evaluated.
Climatic typology of surveyed regions (Fig. 2) was characterized according to the modified Emberger diagram [30] consisting of annual rainfall and minimal average temperatures during the coldest month (MACM).
Bioclimatic diagram of the areas sampled [27]
Data analyses
Tukey’s range test was used to compare the frequency of Meloidogyne species between the regions (P value < 0.05). In order to assess the distribution of RKN in olive nurseries according to substrates, physicochemical characteristics and climate (Table 2), k + 1 multivariate method (MultiBlock Partial Least Squares (MBPLS) was followed and anlyzed in readxl, ade4 and R [31,32,33]. MBPLS regression is widely used for exploring and modelling relationships between several datasets to be predicted from several other datasets, and reveals contribution of each set in predicting response variables.
Results
Meloidogyne spp. specimens were detected in 52.1% of the nursery plants and in 23.2% of the orchard soil samples with a population range of 20–4000 individuals per dm3 of soil. All the RKN isolates reproduced on susceptible tomato plants, except one isolate detected in a traditional orchard from the Haouz region (isolate no 255).
Species characterisation
M. javanica reference population was confirmed as a J3 Est phenotype [22] with three bands (Rm of 46, 54.5 and 58.9%). Six phenotypes were detected among tested females (Fig. 3a and Table 3), labelling six different Est bands. J3 phenotype was detected widespread in both nurseries and orchards. Two phenotypes of M. javanica J2a (Rm of 46 and 58.9%) and J2b (Rm of 46 and 54.5%) were detected as being mixed with J3 in orchard isolates 260 and 252, respectively. One I1 Est phenotype, specific to M. incognita (Rm of 46%), was detected only as being mixed with J3 in low proportions of Souss and Haouz nurseries. Two a Est phenotypes specific to M. arenaria such as A2 (Rm of 53.75 and 56.25%) and A3 (Rm of 51, 53.75 and 56.25%) occurred as mixed populations in the Jabla nursery. These Est patterns were confirmed with species-specific SCAR patterns (Fig. 3b and Table 3).
Biochemical and molecular patterns of Meloidogyne populations collected from cultivated olive soils in Morocco. a Species-specific esterase phenotypes detected (R: M. javanica reference population; Rm: relative migration; 1–6: number of specific bands according to M. javanica reference population). b Species-specific SCAR phenotypes detected
Impact of environmental factors on Meloidogyne species distribution in nurseries
Analysis of variance (Fig. 4) showed that RKN populations were more abundant in the southern regions (Souss and Haouz) as compared to the northern regions (Guerouane and Jbala). Genetic diversity of the olive trees in nurseries did not have any effect on the RKN distribution within and between nurseries (data not shown). Considering the other environmental variables (climate, physico-chemical characteristics and habitat origin of the substrates), the MBPLS analysis (Fig. 5a) clearly indicated opposite contributions of M. incognita, M. javanica and M. arenaria. It also confirmed (Fig. 5b) that M. arenaria was associated with the nursery surveyed in the Jbala region, while M. javanica and M. incognita were found in the Souss and Haouz nurseries. Nurseries surveyed in the Guerouane region and affected by high rainfall (RF, Fig. 5c) were clearly identified as being free of M. incognita (Fig. 4, Table 3). M. incognita was found to be associated with the nurseries of southern regions (Souss and Haouz), characterised by a higher MACM. Habitat origin of the substrates contributed less to the MBPLS analysis as compared to climate (Fig. 5c, second axis). However, it was clearly established that M. javanica, widespread in nearly all nurseries (96%, Table 3), was primarily associated with substrates prepared with large amounts of riverbank soils in the Haouz region and of crop soils in the Souss region. Physio-chemical soil factors did not greatly contribute to the structuring of Meloidogyne diversity.
Distribution of the Meloidogyne species in nurseries and orchards
Biochemical and molecular diagnoses confirmed the occurrence of Meloidogyne populations on cultivated olives in Morocco. M. javanica phenotype J3 was detected in 96% of the nurseries and in 92% of the orchards (Table 3), either traditionally or high-density cultivated, and was widespread throughout the main olive producing areas (Fig. 6). Two phenotypes of M. javanica J2a and J2b were detected only in traditional orchards as being mixed with J3 in the Haouz and the Souss regions, respectively (Fig. 6b). M. incognita phenotype I1 was detected only in nurseries and as mixed in low proportions with J3 (Fig. 6a, Table 3). Two M. arenaria phenotypes A2 and A3 occurred as mixed populations in the nursery of Jbala region (Fig. 6a, Table 3). M. javanica populations were more frequently abundant in the southern nurseries (up to 103 juveniles/dm3 of soil in the Souss and Haouz regions) as compared to northern nurseries (less than 102 juveniles in the Guerouane region). They were, however, less common in the Souss orchards than elsewhere (Fig. 6b). M. incognita populations flourished more in the Souss nurseries than in Haouz (with 16 and 4.5% of the total RKN populations, respectively), and the population of M. arenaria in Jbala nursery was low (< 102 juveniles/dm3 of soil).
Discussion
Main objective of this study was to understand the dispersal process of RKN from nurseries to orchards. RKN were detected in 1/2 of the nurseries and in less than 1/4 of the surveyed orchards. This corroborates with other reports which reveal only scarce detection of RKN in olive-producing areas [16]. Potential damage of these species to olive has never been properly investigated. However, Meloidogyne spp. are major pest of olive trees as high occurrence is usually noticed [34]. They induce considerable damage in nurseries and reduce olive growth in orchards [18, 19]. RKN population thresholds to olive plantlets are unknown, yet the population densities noticed during this study (up to 103 juveniles/dm3 of soil) may present a potential risk to olive plantlets, in nurseries and fields. M. incognita and M. javanica significantly reduce shoot growth of olive cultivars in nurseries, implying a potential impact of RKN [18]. RKN damage in the irrigated sandy soils of the Souss and Haouz regions (high temperature and moisture) can be even higher as many new olive plantations have been established in context of the Moroccan Green Plan [35, 36].
Meloidogyne species were characterised through biochemical (PAGE esterase phenotypes) and molecular RAPD primer (SCAR) methods [16, 21]. Est phenotype is the most instructive biochemical identification technique for Meloidogyne species [37, 38] because of its species-specificity within the Meloidogyne genus [39,40,41]. All the phenotypes have previously been reported on other crops [22, 42] including olive [15, 16]. Nevertheless, some variability within M. javanica populations in orchards and within M. arenaria populations in northern nurseries was spotted. The phenotype J2a was already reported [43] whereas other phenotype J2b was previously diagnosed on peanut [44]. Phenotypes A2 and A3 [39, 45] were apparently clustered by geographic origin. Biochemical diagnostics were further confirmed by the molecular approach with SCAR markers that demonstrated their specificity to M. incognita, M. arenaria and M. javanica [46].
M. arenaria, M. incognita and M. javanica have previously been reported on olive trees in the Mediterranean basin, Asia and South America [47]. It is suggested that these species have the same geographic distribution on various hosts [23]. M. javanica and M. incognita have been reported as the most common species in the olive nurseries of Spain [18], but M. incognita was not detected in orchards. Widespread distribution of M. javanica was noticed in the southern regions (Souss and Haouz), known for RKN-susceptible vegetable production. M. javanic–M. incognita distribution in Moroccan olive nurseries is in line with their distribution in Iran [48] and Spain [18] where more than 20% of olive plantlets were infested by M. javanica alone, and 10% were co-infected by M. javanica and M. incognita.
Distribution of these RKN species in nurseries could be related to human activities and favourable environmental factors. As in orchards, intensive nursery monoculture is very susceptible to build-up of nematode populations and ultimately damaging the tree seedlings. High temperature is a favourable factor for the reproduction and multiplication of M. javanica and M. incognita populations. In fact, temperature is the key feature for their survival and fecundity [49]. High temperatures also favour hatching, mobility and root invasion of M. javanica. There are reports from southern Spain and northern Iran revealing alarming Meloidogyne population densities (28.6 and 22.3% yield loss, respectively) in nurseries [18, 48]. Besides, M. arenaria, usually found in tropical, subtropical, temperate mild regions and in glasshouses under cooler climates [50], was found in the coldest regions of northern Morocco with high annual rainfall.
In short, M. javanica and M. incognita were most probably introduced into nurseries through soil substrates from agricultural fields and riverbanks. Their widespread distribution in nurseries with highly infested substrates confirmed high fitness properties. It clearly confirms that their adaptation and reproductive success is mainly due to their mitotic parthenogenetic reproduction [20] and ability to infect various plant species [51]. M. arenaria was only found in the northern nursery (Jbala region), grown on forest soil substrates. It can be linked to previous reports about phenotype A2 on wild olives [16].
Introduction of RKN in orchards after the transplantation of rooted plantlets, seems obvious, as in case of endoparasitic nematodes. Soil nematodes move very short distances so their long-distance dissemination is only possible through human activities [52]. Consequently, their widespread distribution in cultivated olive-growing areas might have been inducted from nurseries [53]. Therefore, in case of olive production systems, the invasive species status can be attributed to M. javanica [54], which out-competes native species especially in high-density orchards [55]. However, selection processes might have occurred as no M. incognita was detected in orchards despite their occurrence in nurseries. This extinction could be explained either by non-adequate life conditions (no fitted niches) or competition during long-term cohabitation with M. javanica or with native PPN species. Species selection after invasion might influence the capacity to disperse [56] along with the physiological tolerance to the new environment [57]. Presence of M. arenaria only in one nursery supports the hypothesis of refuge conditions of the area for this species [58], especially in wild olive [59], and that could explain why M. arenaria did not disperse in a context of the low human activity.
Conclusion
To conclude, introductions of pest species through cropping practices are usually irreversible and frequently cause undesirable impacts. Therefore, we can assume that olive production systems are at greater PPN invasion risks. Sanitisation of the nursery substrates is mandatory to avoid nematode problems in new plantations.
Abbreviations
- DNA:
-
deoxyribonucleic acid
- Est:
-
esterase
- F/R:
-
forward/reverse
- ISO:
-
International Organization for Standardisation
- MACM:
-
minimal average temperatures of the coldest month
- MBPLS:
-
MultiBlock Partial Least Squares
- OM:
-
organic matter
- PAGE:
-
polyacrylamide gel electrophoresis
- PCA:
-
principal component analysis
- PCR:
-
polymerase chain reaction
- PPN:
-
plant-parasitic nematodes
- RAPD:
-
random amplification of polymorphic DNA
- RKN:
-
root-knot nematodes
- SCAR:
-
Sequence Characterized Amplified Region
References
Bowler DE, Benton TG. Causes and consequences of animal dispersal strategies: relating individual behaviour to spatial dynamics. Biol Rev. 2005;80:205–25.
Parendes LA, Jones JA. Role of light availability and dispersal in exotic plant invasion along roads and streams in the HJ Andrews experimental forest. Oregon Conser Biol. 2000;14:64–75.
Webb DB, Wood PJ, Smith J. A guide to species selection for tropical and sub-tropical plantations. Tropical Forestry Paper No 15. Oxford: Commonw. For Inst; 1980.
Jiménez-Díaz R, Tjamos E, Cirulli M. Verticillium wilt of major tree hosts: olive. In: Hiemstra JA, Harris DC, editors. A compendium of Verticillium wilt in tree species. Wageningen, Netherlands: Pousen and Looijen; 1998. p. 13–16.
Barbero M, Bonin G, Loisel R, Quézel P. Changes and disturbances of forest ecosystems caused by human activities in the western part of the Mediterranean basin. Vegetatio. 1990;87:151–73.
OEPP/EPPO. Certification schemes. No. 7. Nursery requirements-recommended requirements for establishments participating in certification of fruit or ornamental crops. Bull OEPP/EPPO Bull. 1993;23:249–52.
Bélair G. Les nématodes, ces anguillules qui font suer les plantes… par la racine. Phytoprotection. 2005;86:65–9.
Norton DC. Ecology of plant-parasitic nematodes. New York: John Wiley; 1978. p. 128–32.
Neher DA. Ecology of plant and free-living nematodes in natural and agricultural soil. Phytopathol. 2010;48:371–94.
Villenave C, Cadet P, Planchon O, Estève M, Lapetite J-M. Transport of free-living nematodes by runoff water in a Sudano-Sahelian area. Appl Soil Ecol. 2003;23:85–91.
Orr C, Newton O. Distribution of nematodes by wind. Plant Dis Reptr. 1971;55:61–3.
Alenda C, Montarry J, Grenier E. Human influence on the dispersal and genetic structure of French Globodera tabacum populations. Infect Genet Evol. 2014;27:309–17.
Jones JT, Haegeman A, Danchin EG, Gaur HS, Helder J, Jones MG, Kikuchi T, Manzanilla-López R, Palomares-Rius JE, Wesemael WM. Top 10 plant-parasitic nematodes in molecular plant pathology. Mol Plant Pathol. 2013;14:946–61.
Nicol JM, Turner SJ, Coyne DL, Den Nijs L, Hockland S, Maafi ZT. Current nematode threats to world agriculture. In genomics and molecular genetics of plant-nematode interactions. Netherlands: Springer; 2015. p. 21–43.
Castillo P, Nico AI, Navas-Cortés JA, Landa BB, Jiménez-Díaz RM, Vovlas N. Plant-parasitic nematodes attacking olive trees and their management. Plant Dis. 2010;94:148–62.
Ali N, Tavoillot J, Chapuis E, Mateille T. Trend to explain the distribution of root-knot nematodes Meloidogyne spp. associated with olive trees in Morocco. Agric Ecosyst Environ. 2016;225:22–32.
Ali N, Tavoillot J, Mateille T, Chapuis E, Besnard G, El Bakkali A, Cantalapiedra-Navarrete C, Liébanas G, Castillo P, Palomares-Rius JE. A new root-knot nematode Meloidogyne spartelensis n. sp. (Nematoda: Meloidogynidae) in Northern Morocco. Eur J Plant Pathol. 2015;143:25–42.
Nico AI, Rapoport HF, Jiménez-Díaz RM, Castillo P. Incidence and population density of plant-parasitic nematodes associated with olive planting stocks at nurseries in southern Spain. Plant Dis. 2002;86:1075–9.
Afshar FJ, Sasanelli N, Hosseininejad S, Maafi ZT. Effects of the root-knot nematodes Meloidogyne incognita and M. javanica on olive plants growth in glasshouse conditions. Helmint. 2014;51:46–52.
Castagnone-Sereno P, Danchin EG, Perfus-Barbeoch L, Abad P. Diversity and evolution of root-knot nematodes, genus Meloidogyne: new insights from the genomic era. Annu Rev Phytopathol. 2013;51:203–20.
Adam M, Phillips M, Blok V. Molecular diagnostic key for identification of single juveniles of seven common and economically important species of root-knot nematode (Meloidogyne spp.). Plant Pathol. 2007;56:190–7.
Esbenshade P, Triantaphyllou A. Isozyme phenotypes for the identification of Meloidogyne species. J Nematol. 1990;22:10–5.
Zijlstra C, Donkers-Venne DT, Fargette M. Identification of Meloidogyne incognita, M. javanica and M. arenaria using sequence characterised amplified region (SCAR) based PCR assays. Nematology. 2000;2:847–53.
Ouazzani N, Lumaret R, Villemur P. Genetic variation in the olive tree (Olea europaea L.) cultivated in Morocco. Euphytica. 1996;91:9–20.
Therios IN. Olives. Wallingford: CABI; 2009.
ISO 23611-4. Soil quality—Sampling of soil invertebrates—Part 4: Sampling, extraction and identification of soil-inhabiting nematodes. Paris: AFNOR; 2007.
Trudgill D, Carpenter J. Disk electrophoresis of proteins of Heterodera species and pathotypes of Heterodera rostochiensis. Ann Appl Biol. 1971;69:35–41.
Carneiro RMG, Almeida MRA. Técnica de eletroforese usada na estudo de enzimas dos nematóides de galhas para identificação de espécies. Nematol Bras. 2001;25:35–44.
Hedges J, Oades J. Comparative organic geochemistries of soils and marine sediments. Org Geochem. 1997;27:319–61.
Stewart P. Un nouveau climagramme pour l’Algérie et son application au barrage vert. Bull Soc Hist Nat Afrique du Nord. 1975;59:22–36.
Bougeard S, Qannari EM, Lupo C, Hanafi M. From multiblock partial least squares to multiblock redundancy analysis. Continuum approach. Informatica. 2011;22:11–26.
Wickham H. readxl: Read Excel Files. R package version 0.1.1. https://CRAN.R-project.org/package=readxl/.2016.
R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0. http://www.R-project.org 2011.
Hashim Z. Distribution, pathogenicity and control of nematodes associated with olive. Rev Nématol. 1982;5:169–81.
El Mouhtadi I, Agouzzal M, Guy F. L’olivier au Maroc. OCL. 2014;21(2):D203.
Koenning S, Walters S, Barker K. Impact of soil texture on the reproductive and damage potentials of Rotylenchulus reniformis and Meloidogyne incognita on cotton. J Nematol. 1996;28:527–36.
Janati A, Berge JB, Triantaphyllou AC, Dalmasso A. Nouvelles données sur l’utilisation des isoestérases pour l’identification des Meloidogyne. Nematology. 1982;5:147–54.
Dalmasso A, Bergé J. Molecular polymorphism and phylogenetic relationship in some Meloidogyne spp.: application to the taxonomy of Meloidogyne. J Nematol. 1978;10:323–32.
Esbenshade P, Triantaphyllou A. Use of enzyme phenotypes for identification of Meloidogyne species. J Nematol. 1985;17:6–20.
Carneiro RM, Tigano MS, Randig O, Almeida MRA, Sarah JL. Identification and genetic diversity of Meloidogyne spp. (Tylenchida: Meloidogynidae) on coffee from Brazil, Central America and Hawaii. Nematology. 2004;6:287–98.
Flores-Romero P, Navas A. Enhancing taxonomic resolution: distribution dependent genetic diversity in populations of Meloidogyne. Nematology. 2005;7:517–30.
Quénéhervé P, Godefroid M, Mège P, Marie-Luce S. Diversity of Meloidogyne spp. parasitizing plants in Martinique Island, French West Indies. Nematropica. 2011;41:191–9.
Cofcewicz ET, Carneiro RM, Castagnone-Sereno P, Quénéhervé P. Enzyme phenotypes and genetic diversity of root-knot nematodes parasitising Musa in Brazil. Nematology. 2004;6:85–95.
Tomaszewski E, Khalil M, El-Deeb A, Powers TO, Starr J. Meloidogyne javanica parasitic on peanut. J Nematol. 1994;26:436–41.
Carneiro RM, Dos Santos MF, Almeida MRA, Mota FC, Gomes ACM, Tigano MS. Diversity of Meloidogyne arenaria using morphological, cytological and molecular approaches. Nematology. 2008;10:819–34.
Jepson SB. Identification of root-knot nematodes (Meloidogyne species). Wallingford: CAB International; 1987.
Ali N, Chapuis E, Tavoillot J, Mateille T. Plant-parasitic nematodes associated with olive tree (Olea europaea L.) with a focus on the Mediterranean Basin: A review. C R Biol. 2014;337:423–42.
Sanei S, Okhovvat S. Incidence of plant-parasitic nematodes associated with olive planting stocks at nurseries in northern Iran. Int J Appl. 2011;1:79–82.
Evans AA, Perry RN. Survival Mechanisms. In: Perry RN, Moens M, Starr J, editors. Root-knot Nematodes. London: CABI; 2009. p. 201–22.
Hunt DJ, Handoo ZA. Taxonomy, identification and principal species. In: Perry RN, Moens M, Starr J, editors. Root-knot nematodes. London: CABI; 2009. p. 55–88.
Chitwood DJ, Perry RN. Reproduction, physiology and biochemistry. In: Perry RN, Moens M, Starr J, editors. Root-knot nematodes. London: CABI; 2009. p. 182–200.
Robinet C, Roques A, Pan H, Fang G, Ye J, Zhang Y, Sun J. Role of human-mediated dispersal in the spread of the pinewood nematode in China. PLoS ONE. 2009;4:e4646.
Dias PC. Sources and sinks in population biology. Trends Ecol Evol. 1996;11:326–30.
Singh S, Hodda M, Ash G, Banks N. Plant-parasitic nematodes as invasive species: characteristics, uncertainty and biosecurity implications. Ann Appl Biol. 2013;163:323–50.
Palomares-Rius JE, Castillo P, Montes-Borrego M, Navas-Cortés JA, Landa BB. Soil properties and olive cultivar determine the structure and diversity of plant-parasitic nematode communities infesting olive orchards soils in southern Spain. PLoS ONE. 2015;10:e0116890.
Thouvenot L, Puech C, Martinez L, Haury J, Thiébaut G. Strategies of the invasive macrophyte Ludwigia grandiflora in its introduced range: competition, facilitation or coexistence with native and exotic species? Aqua Bot. 2013;107:8–16.
Novo M, Cunha L, Maceda-Veiga A, Talavera J, Hodson ME, Spurgeon D, Bruford M, Morgan A, Kille P. Multiple introductions and environmental factors affecting the establishment of invasive species on a volcanic island. Soil Biol Biochem. 2015;85:89–100.
Médail F, Diadema K. Glacial refugia influence plant diversity patterns in the Mediterranean Basin. J Biogeogr. 2009;36:1333–45.
Besnard G, Khadari B, Navascués M, Fernández-Mazuecos M, El Bakkali A, Arrigo N, Baali-Cherif D, de Caraffa VB-B, Santoni S, Vargas P. The complex history of the olive tree: from late quaternary diversification of Mediterranean lineages to primary domestication in the northern Levant. Proc R Soc B Lond Biol Sci. 2013;280:2012–833.
Authors’ contributions
HB, AEM and TM coordinated research; MAH, NA and TM designed the sampling device; MAH, NA, JT, AEM, AEO and TM acquired the field data; MAH, NA, JT and TM processed the nematode extraction from soils; MAH, NA and JT carried out the biochemical and molecular characterisation of the root-knot nematode species; MAH, NA, JT, OFG and TM analysed the data; all of the authors drafted the manuscript. All the authors read and approved the final manuscript.
Acknowledgements
Authors are grateful to Dr. Mohamed ATER (Univ. Abdelmalek Essaâdi, Tétouan, Morocco) and Dr. Abdelmajid MOUKHLI (INRA, CRRA, Marrakech, Morocco) for their technical assistance in surveys, to Dr. Moulay Cherif HARROUNI (Soil Laboratory, Institut Agronomique et Vétérinaire Hassan II, Agadir, Morocco) for soil physico-chemical analyses, and to Dr. Laurent FOLCHER (Nematology Unit, Plant Health Laboratory, ANSES, Le-Rheu, France) who provided the reference Meloidogyne javanica population.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets used during and/or analysed during the current study are available from the corresponding author upon reasonable request.
Consent to publish
Not applicable.
Ethics approval and consent to participate
The collection of samples was done with permission of the olive plantlet producers.
Funding
Research was funded by PhD grant from the Institut de Recherche pour le Développement (IRD, Marseille, France) (data analyses and writing the manuscript). It was also funded by the BIONEMAR project (PHC-Toubkal action 054/SVS/13): Development of fungal bionematicides for organic agriculture in Morocco (logistic support, sampling, nematode extraction), and by the PESTOLIVE project (ARIMNet action KBBE 219262): Contribution of olive history for the management of soil-borne parasites in the Mediterranean Basin (RKN culture and biochemical/molecular typing).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Aït Hamza, M., Ali, N., Tavoillot, J. et al. Diversity of root-knot nematodes in Moroccan olive nurseries and orchards: does Meloidogyne javanica disperse according to invasion processes?. BMC Ecol 17, 41 (2017). https://doi.org/10.1186/s12898-017-0153-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12898-017-0153-9