Abstract
Background
Percutaneous transhepatic portal embolization (PTPE) is useful for safe major hepatectomy. This study investigated the correlation between hepatic hypertrophy and hemodynamics of portal venous flow by ultrasound sonography after PTPE.
Methods
We analyzed 58 patients with PTPE, excluding those who underwent recanalization (n = 10). Using CT volumetry results 2 weeks after PTPE, the patients were stratified into a considerable hypertrophy group (CH; n = 15) with an increase rate of remnant liver volume (IR-RLV) ≥ 40% and a minimal hypertrophy group (MH; n = 33) with an IR-RLV < 40%. We investigated the hemodynamics of portal venous flow after PTPE and the favorable factors for hepatic hypertrophy.
Results
Univariate and multivariate analysis identified the indocyanine green retention rate at 15 min (ICGR15) and increase rate of portal venous flow volume (IR-pFV) at the non-embolized lobe on day 3 after PTPE as independent favorable factors of IR-RLV. Patients with IR-pFV on day 3 after PTPE ≥100% and ICGR15 ≤ 15% (n = 13) exhibited significantly increased IR-RLV compared with others (n = 35).
Conclusions
Cases with high IR-pFV on day 3 after PTPE exhibited better hepatic hypertrophy. Preserved liver function and increased portal venous flow on day 3 were important.
Similar content being viewed by others
Background
Complete resection of hepatobiliary malignant tumors is the best method to achieve long-term survival [1, 2]. Major hepatectomy is often required for complete resection, and one of the main causes of unresectability is insufficient remnant liver volume (RLV). Percutaneous transhepatic portal embolization (PTPE) is a useful method for safe major hepatectomy for hepatobiliary malignant tumors [3]. Although PTPE promotes hypertrophy in the non-embolized liver and increases the future remnant liver function and volume [4], some patients exhibit insufficient hypertrophy at the non-embolized liver with between 2.8 and 4.5% of patients unable to undergo surgery after PTPE due to insufficient hypertrophy [5, 6]. Furthermore, the degree of hypertrophy and growth rate of the remnant liver after portal vein embolization (PVE) are favorable predictors of post-hepatectomy liver failure [7]. Thus, increased hypertrophy after PTPE is a crucial factor.
Although it was previously reported that hepatic injury (fibrosis or cirrhosis) was an unfavorable factor for hepatic hypertrophy after PTPE [3], favorable factors for hypertrophy after PTPE remain elusive. In addition, the hemodynamics of portal venous flow after PTPE has been not investigated in detail. In this study, we examined the favorable factors for remnant liver hypertrophy and hemodynamics of portal venous flow volume (pFV) after PTPE at the non-embolized lobe by ultrasound sonography (US).
Methods
Patients
Between January 2004 and November 2015, 58 patients underwent PTPE for hepatectomy at the Gastroenterological Surgery I unit of Hokkaido University Hospital in Sapporo, Japan. The entire liver volume, liver resection volume, and tumor volume were calculated from contrast-enhanced computed tomography (CT) data by 3D workstations (Virtual Place Lexus; Medical Imaging Laboratory, AZE, Tokyo, Japan, and Synapse Vincent, Fujifilm Medical Co., Ltd., Tokyo, Japan). PTPE was performed in patients with an effective liver resection rate > 60% according to the formula [(liver resection volume – tumor volume) / (whole liver volume – tumor volume)] × 100 [8]. The diagnoses and surgical data of the patients are presented in Table 1. We performed bile duct drainage (mainly nasobiliary drainage) before in cases which T-bil ≥ 2.0 mg/dL. PTPE were performed for the cases which T-bil < 2.0 mg/dL.
PTPE
The PTPE method was previously reported [9] and is briefly described here. Most patients underwent an ipsilateral approach, but the contralateral approach was used when the ipsilateral approach was judged to be unsuitable by interventional radiologists. After the administration of local anesthesia, the intrahepatic portal vein was punctured with an 18-gauge needle (Needle for Ultrasonically Guided Puncture; Create Medic Co., Yokohama, Japan) under US guidance. A 5.5-French sheath introducer (Introducer Set; Medikit Co., Tokyo, Japan) was inserted into the portal vein with a guidewire. Direct portography was performed to evaluate the anatomy and measurement of portal venous pressure (PVP) directly. Next, selective portography was performed with a balloon occlusion catheter (Selecon MP Catheter II; Terumo Co., Tokyo, Japan). The embolic material was absolute ethanol. The method of ethanol injection was previously reported.9 After repeat embolization until the resolution of hepatic parenchymal enhancement, direct portography was performed to confirm the PTPE result. Then, we directly measured PVP at the non-embolized lobe. Finally, the 5.5-French sheath was extracted by packing the puncture tract with a gelatin sponge torpedo (Spongel; Astellas Pharma Co., Tokyo, Japan).
Assessments
The patients’ liver volumes and RLVs were semiautomatically measured by 3D workstations using contrast-enhanced CT imaging data before PTPE and 1 and 2 weeks after PTPE. pFV was measured thrice by pulsed wave Doppler US (Toshiba SSA-700A (Aplio50), Toshiba Medical Systems Co., Ltd., Tokyo, Japan, and LOGIQ P6 BT11, GE Healthcare JAPAN Co., Ltd., Tokyo, Japan) on days 1, 3, 5, and 7 after PTPE. We calculated pFV as following, pFV = portal velocity (pulsed wave Doppler US) × πr (r = radius of portal vein)2. We averaged the 3 readings on each day to calculate the pFV. We measured the pFV of the umbilical portion for right-side hepatectomy, the anterior branch for left hepatectomy, and the posterior branch for left trisegmentectomy.
Patients were stratified into a considerable hypertrophy group (CH; n = 15) and a minimal hypertrophy group (MH; n = 33) based on the increase rate of RLV (IR-RLV), i.e., ≥ 40% versus < 40%, respectively, according to the formula [(RLV at 2 weeks after PTPE – RLV before PTPE) / RLV before PTPE] × 100. We investigated the favorable factors for hepatic hypertrophy and hemodynamics of pFV after PTPE. Using the formula [(pFV after PTPE – pFV before PTPE) / pFV before PTPE] × 100, we evaluated the increase rate of pFV (IR-pFV) at the non-embolized lobe on days 1, 3, 5, and 7 after PTPE by US; the portal venous pressure (PVP) before and after PTPE; patients’ age and sex; hemoglobin, white blood cell, C-reactive protein, aspartate aminotransferase, alanine aminotransferase, platelet, cholinesterase, albumin, total bilirubin (T-bil), and A1c glycated hemoglobin levels; hepatitis B surface antigen; hepatitis C virus antibody; the indocyanine green retention rate at 15 min (ICGR15); pathological liver fibrosis; and the receptor index [uptake ratio of the liver to that of the liver plus heart at 15 min of technetium 99 m diethylenetriaminepentaacetic acid-galactosyl-human serum albumin scintigraphy (LHL15)]. Patients who had portal venous recanalization after PTPE were excluded (n = 10, 17.2%).
We performed the assessment of hypertrophy by CT at 2 weeks because most cases (86%, 50/58) underwent hepatectomy with satisfaction for our criteria at 2 weeks after PTPE.
This study was approved by the Hokkaido University Hospital Voluntary Clinical Study Committee and was performed according to Helsinki Declaration guidelines.
Statistical analysis
Values are expressed as the mean ± standard deviation. Univariate analyses were performed using Student’s t-test for continuous variables and the chi-square test for non-continuous variables. Multivariate analyses were performed using logistic regression model analyses. Pearson’s correlation coefficients were used to analyze the correlation between the IR-pFV at the non-embolized lobe by US and the IR-RLV. A P-value of < 0.05 was considered significant. Statistical analyses were performed using JMP Pro 12.0.1 for Windows (SAS Institute Inc., NC, USA).
Results
Perioperative data of patients
Patient perioperative data are provided in Table 1. The diagnoses in our study cohort were hepatocellular carcinoma (n = 13), intrahepatic cholangiocarcinoma (n = 4), bile duct cancer (n = 34), metastatic liver cancer (n = 2), and gallbladder cancer (n = 5). The operative procedures were right hepatectomy (n = 38), extended right hepatectomy (n = 6), right trisegmentectomy (n = 3), left hepatectomy (n = 1), left trisegmentectomy (n = 4), and right hepatectomy with pancreatoduodenectomy (n = 3). Three tumors were unresectable (n = 3). The mean waiting time from PTPE to surgery was 26 days (15–96). Table 2 reports total hepatic volume, RLV, effective liver resection rate before and after PTPE. The mean RLVs before and after PTPE were 438.6 ± 106 mL and 558.8 ± 99.9 mL, respectively (P < 0.01). The median IR-RLV was 30.5%. Only one complication occurred, namely, hemobilia (1.7%). No patient exhibited transient thrombocytopenia (defined as a platelet count less than 50 × 103 cells/μL). All recanalization rates after PTPE were 17.2%. However, all cases had recanalization beyond the third portal branches. No cases exhibited recanalization at second or first portal branches. Two cases of all 10 recanalization cases needed additional PVE, that is 1 case was performed PTPE, 1 case was performed TIPE. Recanalization cases showed that the mean RLVs before and after PTPE were 304.3 ± 23 mL and 397.8 ± 53.8 mL, respectively (P < 0.01). The median IR-RLV was 26.9%. No cases exhibited post-hepatectomy liver failure. Regarding 8 cases could not achieve our criteria for hepatectomy at 2 weeks after PVE, 2 cases achieved our criteria for hepatectomy at 4 weeks after PVE, 3 cases at 5 weeks, 1 case at 6 weeks, 1 case at 7 weeks, and 1 case at 8 weeks.
Correlation between IR-RLV and high IR-pFV cases
We evaluated the IR-RLV of the cases with regard to an IR-pFV greater than 100% on days 1, 3, 5, and 7 after PTPE using ROC (receiver operating characteristics) curves to determine the cut-off values for IR-RLV. The values were 29.2% (area under the curve [AUC] = 0.5297, sensitivity = 59.1%, specificity = 57.7%), 37.3% (AUC = 0.6746, sensitivity = 52.4%, specificity = 74.1%), 42.4% (AUC = 0.6974, sensitivity = 47.8%, specificity = 96.0%), and 40.7% (AUC = 0.5661, sensitivity = 42.9%, specificity = 85.0%), respectively.
Clinicopathological characteristics in the CH and MH groups
The patients were then classified into two groups, the CH and MH groups, based on an IR-RLV cut-off value of 40% from the above results.
The clinicopathological characteristics of the CH and MH groups are provided in Table 3. The following variables were significantly different between these 2 groups: white blood cell count (4966 ± 1198/μL vs 6987 ± 2625/μL; P < 0.01), T-bil (0.98 ± 0.53 mg/dL vs 1.59 ± 0.94 mg/dL; P = 0.02), ICGR15 (10.6 ± 3.7% vs 16.1 ± 3.9%; P < 0.01), PVP after PTPE (18.2 ± 1.9 cmH2O vs 20.5 ± 3.7 cmH2O; P = 0.03), IR-pFV by US on day 3 after PTPE (156 ± 96% vs 89 ± 57%; P < 0.01), and pathological liver fibrosis [6.7% (1/15) vs 42.4% (14/33); P = 0.01].
Multivariate analysis with logistic regression revealed that ICGR15 (odds ratio 0.5836, 95% confidence interval: 0.3432–0.9922, P = 0.04) and IR-pFV by US on day 3 after PTPE (odds ratio 1.0257, 95% confidence interval: 1.0018–1.0501, P = 0.03) were independent favorable factors of increased RLV (Table 4). In addition, patients with an ICGR15 ≤ 15% and an IR-pFV on day 3 after PTPE ≥100% (favorable group, n = 13) exhibited significantly increased IR-RLV compared with the other patients (unfavorable group, n = 35) (51.6 ± 24.9% vs 23.1 ± 15.1%, P < 0.01) (Fig. 1).
IR-RLV according to the presence of favorable conditions, namely an IR-pFV on day 3 ≥ 100% and an ICGR15 ≤ 15%. Patients with an IR-pFV on 3 days after PTPE ≥100% and an ICGR15 ≤ 15% (favorable group) exhibited a significantly increased IR-RLV compared with other patients (unfavorable group) (P < 0.01)
Table 5 showed multivariate analysis for factors turned out before PTPE.
Correlation between the IR-pFV at the non-embolized lobe by US and IR-RLV.
In the CH group, the IR-pFV continued to increase on days 1 and 3 (113 ± 90% and 156 ± 96%, respectively). After day 3, the IR-pFV was largely maintained (151 ± 108% on day 5 and 160 ± 112% on day 7). Conversely, in the MH group, the IR-pFV generally remained unchanged after day 1 (91 ± 82% on day 1, 89 ± 57% on day 3, 112 ± 97% on day 5, and 110 ± 95% on day 7) (Fig. 2a). The correlation coefficient between the IR-pFV at the non-embolized lobe by US and the IR-RLV on day 3 was 0.7542 (Fig. 2b, Table 6). The correlation coefficients on days 1, 5, and 7 were 0.4613, 0.6272, and 0.5735, respectively (Table 6).
a IR-pFV by US after PTPE. In the CH group, the IR-pFV continued to increase through day 1 until day 3. After day 3, the IR-pFV generally remained unchanged until day 7. The IR-pFV generally remained unchanged after day 1 in the MH group. *P < 0.01. b Correlation between the IR-pFV at the non-embolized lobe by US on day 3 and the IR-RLV. The correlation coefficient was 0.7542
Discussion
We investigated the favorable factors for remnant liver hypertrophy and hemodynamics of portal venous flow volume after PTPE. Univariate analysis revealed that the white blood cell count, T-bil, ICGR15, PVP after PTPE, IR-pFV at the non-embolized lobe by US on day 3 after PTPE and pathological liver fibrosis were significant favorable factors for hepatic hypertrophy after PTPE. Multivariate analyses indicated that the ICGR15 and IR-pFV at the non-embolized lobe by US on day 3 after PTPE were independent favorable factors for hepatic hypertrophy after PTPE. We observed a strong positive correlation between the IR-pFV at the non-embolized lobe by US on day 3 after PTPE and the IR-RLV at 2 weeks after PTPE. In addition, we also demonstrated that the pFV after PTPE stabilized from day 3 after PTPE, even in a group of patients exhibiting increased hypertrophy. In this study, patients with an ICGR15 ≤ 15% and IR-pFV on day 3 after PTPE ≥100% were more likely to exhibit extremely favorable hepatic hypertrophy.
Rous et al. [10] found that ligation of a branch of the portal vein in rabbits resulted in marked atrophy of the parenchyma of the corresponding hepatic lobe with the lobe with uninterrupted portal flow exhibiting regenerative hypertrophy. Subsequently, Makuuchi et al. [11] stated that PVE increased the safety of major hepatectomy for hilar bile duct carcinoma. Kinoshita et al. [12] reported the utility of preoperative PVE before hepatectomy for HCC. Currently, preoperative PVE is widely used for safely performing major hepatectomy for these hepatobiliary malignant tumors [3]. PVE is classified as transileocolic portal embolization (TIPE) or PTPE according to the specific approach used. Whereas TIPE is performed via laparotomy under general anesthesia, PTPE is performed using a puncture technique with ultrasonic guidance under local anesthesia. Therefore, PTPE is more convenient and is currently more commonly used.
Some reports have demonstrated that the absolute increase in the hypertrophy of the future remnant liver ranged from 28.8 to 43% [13, 14]. In this study, the value was 30.5% and thus consistent with previous reports. Regarding procedure-related complications, previous reports reported rates of 12.8 to 14.9% [15, 16]; the rate in our study was 1.7%.
Mars et al. [17] reported that urokinase-type plasminogen activator was immediately increased after partial hepatectomy. Urokinase-type plasminogen activator is induced by shear stress caused by increased blood flow [18] and activates hepatocyte growth factor [19]. Furthermore, nitric oxide (NO) is also induced by shear stress [20]. NO is produced by cytokine-inducible NO synthase (iNOS) and endothelial NO synthase (eNOS). Regeneration was inhibited after partial hepatectomy in mice with iNOS or eNOS inhibition [21, 22]. Therefore, shear stress and blood flow might influence hepatic regeneration.
Recently, it was reported that the mechanisms of hepatic hypertrophy differ between post-PTPE and post-hepatectomy [23, 24]. Some favorable and unfavorable factors have been identified for hepatic hypertrophy after PTPE, such as age, sex, body mass index, nutrition status, previous chemotherapy, and diabetes mellitus [14, 25]. Chronic liver disease and cirrhosis are unfavorable factors for hypertrophy [3]. In addition, the presence of major portal hypertension and portosystemic shunts are unfavorable factors for hypertrophy [3, 26]. Our univariate analysis also demonstrated that PVP after PTPE and pathological liver fibrosis were significant favorable factors for hepatic hypertrophy after PTPE. In general, portal pressure will increase after PVE. Chen et al. [27] reported that patients with PVP below 16 cmH2O had a lower incidence of PHLF and patients with PVP of 20 cmH2O or above showed lower incidence of grade B or C PHLF. It was reported that the 90-day mortality was associated with PVP greater than 21 mmHg by Allard et al. [28]. Modulation of PVP may be important for better outcome.
Mihara et al. [29] reported that the ICG plasma clearance rate was a significant predictive factor for hypertrophy after PTPE. Kageyama et al. [30] reported that ICGR15 > 20% and T-bil > 1.5 mg/dL were unfavorable factors for hypertrophy after PTPE. These previous findings are consistent with our results.
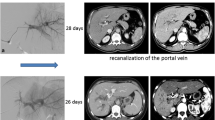
The frequency of recanalization after PTPE ranges between 7.1 and 33% [31, 32]. In this study, the recanalization rate was 17.2%. However, no cases exhibited recanalization at second or first portal branches. Some reports demonstrate that recanalization is a significant risk factor for poor hypertrophy [32, 33] possibly because pFV might be reduced at the non-embolized lobe in recanalized patients. Thus, we excluded recanalized patients.
Goto et al. [34] reported that an increase in the portal blood flow velocity after PTPE on day 1 correlated with the hypertrophy rate. Our univariate and multivariate analyses revealed that the pFV on day 3 highly correlated with increased hypertrophy after PTPE. Regarding the time course, the previous study demonstrated that the increase in the portal blood flow velocity after PTPE peaked on day 1 [34]. In contrast, we found that the increase in the portal blood flow after PTPE peaked on day 3 in the CH group and day 1 in the MH group. This discrepancy might be the key to good hypertrophy. Hayashi et al. [35] reported that the increase in the serum bile acid levels on day 3 after PTPE was a useful predictor of favorable hypertrophy. Using a portal vein ligation model in rats, Takamura et al. [24] demonstrated that the liver regeneration rate peaked between days 2 and 3 in the non-ligated lobe. Thus, we believe that the IR-pFV on day 3 after PTPE might be an important factor of hepatic hypertrophy after PTPE.
The limitation of this study is that the results may not really apply to the common population of PVE (liver metastases) because most patients were cirrhotic patients or cholestatic patients.
Conclusions
Preserved liver function, including ICGR15, and an increase in portal venous flow on day 3, i.e., portal venous flow shifted and added for the non-embolized lobe, are important for hepatic hypertrophy.
Abbreviations
- AUC:
-
Area under the curve
- CH:
-
Considerable hypertrophy
- CT:
-
Computed tomography
- eNOS:
-
Endothelial NO synthase
- ICGR15:
-
Indocyanine green retention test at 15 min
- iNOS:
-
Cytokine-inducible NO synthase
- IR-pFV:
-
Increase rate of portal venous flow volume
- IR-RLV:
-
Increase rate of remnant liver volume
- LHL15:
-
Receptor index: uptake ratio of the liver to the liver plus heart at 15 min of technetium 99 m diethylenetriaminepentaacetic acid galactosyl human serum albumin scintigraphy
- MH:
-
Minimal hypertrophy
- NO:
-
Nitric oxide
- pFV:
-
Portal venous flow volume
- PHLF:
-
Posthepatectomy liver failure
- PTPE:
-
percutaneous transhepatic portal embolization
- PVE:
-
Portal vein embolization
- PVP:
-
Portal venous pressure
- RLV:
-
Remnant liver volume
- ROC:
-
Receiver operating characteristics
- T-bil:
-
Total bilirubin
- TIPE:
-
Transileocolic portal embolization
- US:
-
Ultrasound sonography
References
Spolverato G, Vitale A, Cucchetti A, Popescu I, Marques HP, Aldrighetti L, Gamblin TC, Maithel SK, Sandroussi C, Bauer TW, Shen F, Poultsides GA, Marsh JW, Pawlik TM. Can hepatic resection provide a long-term cure for patients with intrahepatic cholangiocarcinoma? Cancer. 2015;121:3998–4006.
Shindoh J, Makuuchi M, Matsuyama Y, Mise Y, Arita J, Sakamoto Y, Hasegawa K, Kokudo N. Complete removal of the tumor-bearing portal territory decreases local tumor recurrence and improves disease-specific survival of patients with hepatocellular carcinoma. J Hepatol. 2016;64:594–600.
Farges O, Belghiti J, Kianmanesh R, Regimbeau JM, Santoro R, Vilgrain V, Denys A, Sauvanet A. Portal vein embolization before right hepatectomy: prospective clinical trial. Ann Surg. 2003;237:208–17.
Shimamura T, Nakajima Y, Une Y, Namieno T, Ogasawara K, Yamashita K, Haneda T, Nakanishi K, Kimura J, Matsushita M, Sato N, Uchino J. Efficacy and safety of preoperative percutaneous transhepatic portal embolization with absolute ethanol: a clinical study. Surgery. 1997;121:135–41.
van Lienden KP, van den Esschert JW, de Graaf W, Bipat S, Lameris JS, van Gulik TM, van Delden OM. Portal vein embolization before liver resection: a systematic review. Cardiovasc Intervent Radiol. 2013;36:25–34.
Ribero D, Abdalla EK, Madoff DC, Donadon M, Loyer EM, Vauthey JN. Portal vein embolization before major hepatectomy and its effects on regeneration, resectability and outcome. Br J Surg. 2007;94:1386–94.
Leung U, Simpson AL, Araujo RL, Gönen M, McAuliffe C, Miga MI, Parada EP, Allen PJ, D'Angelica MI, Kingham TP, DeMatteo RP, Fong Y, Jarnagin WR. Remnant growth rate after portal vein embolization is a good early predictor of post-hepatectomy liver failure. J Am Coll Surg. 2014;219:620–30.
Kamiyama T, Nakanishi K, Yokoo H, Kamachi H, Tahara M, Yamashita K, Taniguchi M, Shimamura T, Matsushita M, Todo S. Perioperative management of hepatic resection toward zero mortality and morbidity: analysis of 793 consecutive cases in a single institution. J Am Coll Surg. 2010;211:443–9.
Sakuhara Y, Abo D, Hasegawa Y, Shimizu T, Kamiyama T, Hirano S, Fukumori D, Kawamura T, Ito YM, Tha KK, Shirato H, Terae S. Preoperative percutaneous transhepatic portal vein embolization with ethanol injection. AJR Am J Roentgenol. 2012;198:914–22.
Rous P, Larimore LD. Relation of the portal blood to liver maintenance: a demonstration of liver atrophy conditional on compensation. J Exp Med. 1920;31:609–32.
Makuuchi M, Thai BL, Takayasu K, Takayama T, Kosuge T, Gunvén P, Yamazaki S, Hasegawa H, Ozaki H. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery. 1990;107:521–7.
Kinoshita H, Sakai K, Hirohashi K, Igawa S, Yamasaki O, Kubo S. Preoperative portal vein embolization for hepatocellular carcinoma. World J Surg. 1986;10:803–8.
Azoulay D, Castaing D, Smail A, Adam R, Cailliez V, Laurent A, Lemoine A, Bismuth H. Resection of nonresectable liver metastases from colorectal cancer after percutaneous portal vein embolization. Ann Surg. 2000;231:480–6.
Kasai Y, Hatano E, Iguchi K, Seo S, Taura K, Yasuchika K, Mori A, Kaido T, Tanaka S, Shibata T, Uemoto S. Prediction of the remnant liver hypertrophyratio after preoperative portal vein embolization. Eur Surg Res. 2013;51:129–37.
Kodama Y, Shimizu T, Endo H, Miyamoto N, Miyasaka K. Complications of percutaneous transhepatic portal vein embolization. J Vasc Interv Radiol. 2002;13:1233–7.
Di Stefano DR, de Baere T, Denys A, Hakime A, Gorin G, Gillet M, Saric J, Trillaud H, Petit P, Bartoli JM, Elias D, Delpero JR. Preoperative percutaneous portal vein embolization: evaluation of adverse events in 188 patients. Radiology. 2005;234:625–30.
Mars WM, Liu ML, Kitson RP, Goldfarb RH, Gabauer MK, Michalopoulos GK. Immediate early detection of urokinase receptor after partial hepatectomy and its implications for initiation of liver regeneration. Hepatology. 1995;21:1695–701.
Sokabe T, Yamamoto K, Ohura N, Nakaztsuka H, Qin K, Obi S, Kamiya A, Ando J. Differential regulation of urokinase-type plasminogen activator expression by fluid shear stress in human coronary artery endothelial cells. Am J Physiol Heart Circ Physiol. 2004;287:H2027–34.
Mars WM, Zarnegar R, Michalopoulos GK. Activation of hepatocyte growth factor by the plasminogen activators uPA and tPA. Am J Pathol. 1993;143:949–58.
Hori N, Wiest R, Groszmann RJ. Enhanced release of nitric oxide in response to changes in flow and shear stress in the superior mesenteric arteries of portal hypertensive rats. Hepatology. 1998;28:1467–73.
Rai RM, Lee FY, Rosen A, Yang SQ, Lin HZ, Koteish A, Liew FY, Zaragoza C, Lowenstein C, Diehl AM. Impaired liver regeneration in inducible nitric oxide synthasedeficient mice. Proc Natl Acad Sci U S A. 1998;95:13829–34.
Mei Y, Thevananther S. Endothelial nitric oxide synthase is a key mediator of hepatocyte proliferation in response to partial hepatectomy in mice. Hepatology. 2011;54:1777–89.
Kogure K, Omata W, Kanzaki M, Zhang YQ, Yasuda H, Mine T, Kojima I. A single intraportal administration of follistatin accelerates liver regeneration in partially hepatectomized rats. Gastroenterology. 1995;108:1136–42.
Takamura K, Tsuchida K, Miyake H, Tashiro S, Sugino H. Activin and activin receptor expression changes in liver regeneration in rat. J Surg Res. 2005;126:3–11.
Yokoyama Y, Nagino M, Nimura Y. Mechanisms of hepatic regeneration following portal vein embolization and partial hepatectomy: a review. World J Surg. 2007;31:367–74.
Bruix J, Castells A, Bosch J, Feu F, Fuster J, Garcia-Pagan JC, Visa J, Bru C, Rodés J. Surgical resection of hepatocellular carcinoma in cirrhotic patients: prognostic value of preoperative portal pressure. Gastroenterology. 1996;111:1018–22.
Chen X, Zhai J, Cai X, Zhang Y, Wei L, Shi L, Wu D, Shen F, Lau WY, Wu M. Severity of portal hypertension and prediction of postoperative liver failure after liver resection in patients with child-Pugh grade a cirrhosis. Br J Surg. 2012;99:1701–10.
Allard MA, Adam R, Bucur PO, Termos S, Cunha AS, Bismuth H, Castaing D, Vibert E. Posthepatectomy portal vein pressure predicts liver failure and mortality after major liver resection on noncirrhotic liver. Ann Surg. 2013;258:822–9.
Mihara K, Sugiura T, Okamura Y, Kanemoto H, Mizuno T, Moriguchi M, Aramaki T, Uesaka K. A predictive factor of insufficient liver regeneration after preoperative portal vein embolization. Eur Surg Res. 2013;51:118–28.
Kageyama Y, Kokudo T, Amikura K, Miyazaki Y, Takahashi A, Sakamoto H. Impaired liver function attenuates liver regeneration and hypertrophy after portal vein embolization. World J Hepatol. 2016;8:1200–4.
van Lienden KP, Hoekstra LT, Bennink RJ, van Gulik TM. Intrahepatic left to right portoportal venous collateral vascular formation in patients undergoing right portal vein ligation. Cardiovasc Intervent Radiol. 2013;36:1572–9.
Malinowski M, Stary V, Lock JF, Schulz A, Jara M, Seehofer D, Gebauer B, Denecke T, Geisel D, Neuhaus P, Stockmann M. Factors influencing hypertrophy of the left lateral liver lobe after portal vein embolization. Langenbeck's Arch Surg. 2015;400:237–46.
Wilms C, Mueller L, Lenk C, Wittkugel O, Helmke K, Krupski-Berdien G, Rogiers X, Broering DC. Comparative study of portal vein embolization versus portal vein ligation for induction of hypertrophy of the future liver remnant using a mini-pig model. Ann Surg. 2008;247:825–34.
Goto Y, Nagino M, Nimura Y. Doppler estimation of portal blood flow after percutaneous transhepatic portal vein embolization. Ann Surg. 1998;228:209–13.
Hayashi H, Beppu T, Sugita H, Horino K, Komori H, Masuda T, Okabe H, Takamori H, Baba H. Increase in the serum bile acid level predicts the effective hypertrophy of the nonembolized hepatic lobe after right portal vein embolization. World J Surg. 2009;33:1933–40.
Acknowledgments
The authors would like to thank the staff of Gastroenterological Surgery I, Hokkaido University Graduate School of Medicine for their kind cooperation.
Funding
This study was not supported by any funding.
Availability of data and materials
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
SS and TK conceived the study concept and design, were involved with patient care and drafted the manuscript and literature review. HY, TO, KW, AN, TK, HK, and AT were involved with the formation of the study concept and design, patient care and drafting of the manuscript and literature review. DA and YS performed portal embolization on the patients and were contributors to the manuscript. All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures in studies involving human participants were performed in accordance with the ethical standards of the Hokkaido University Hospital.
This retrospective study was approved by the Hokkaido University Hospital Voluntary Clinical Study Committee with study number #016–0091. Voluntary written consent was obtained from all patients.
Consent for publication
Not applicable. Individual identifying data were not included in this manuscript.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Shimada, S., Kamiyama, T., Yokoo, H. et al. Hepatic hypertrophy and hemodynamics of portal venous flow after percutaneous transhepatic portal embolization. BMC Surg 19, 23 (2019). https://doi.org/10.1186/s12893-019-0486-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12893-019-0486-8