Abstract
Background
Gout has been associated with weaker foot/leg muscles and altered gait patterns. There is also evidence of on-going foot pain and an increased risk of tendinopathy, with the Achilles and patella tendons most frequently affected in gout. Additionally, the inflammation associated with gout may change tissue elasticity. Ultrasound imaging utilising shear wave elastography (SWE) offers a non-invasive method of quantifying changes in tendon stiffness. SWE findings have not previously been reported in individuals with gout. We sought to determine differences in Achilles tendon stiffness in people with gout compared to controls (non-gout).
Methods
A cross sectional study comparing 24 people with gout and 26 age/sex-matched controls. Clinical and demographic data were collated, and US imaging used to determine tendon thickness, presence of gouty tophi and/or aggregates and levels of angiogenesis. Ten shear wave elastography (SWE) measures were taken along the centre of a longitudinal section of the mid-portion of each Achilles tendon. Prior to data collection, intra-observer error was good (>0.69). Data were summarised using descriptive statistics and a repeated measures ANCOVA was used to compare SWE measures between the two groups for the left and right foot separately after accounting for Body Mass Index (BMI).
Results
A small proportion of those with gout presented with intra-tendon aggregates and/or intra-tendon tophi in one or both tendons. There was no statistically significant difference in tendon thickness between groups. Neo-vascularity was present in a third of gout participants. SWE findings demonstrated significantly reduced tendon stiffness in those with gout compared to controls: right Achilles mdiff =1.04 m/s (95% CI (0.38 to 1.7) p = 0.003 and left Achilles mdiff = 0.7 m/s (95% CI 0.09 to 1.32) p = 0.025. No relationship between the presence of tophi and SWE values were detected.
Conclusion
Subjects with chronic gout show significantly reduced Achilles tendon stiffness compared to non-gout controls. From a clinical standpoint, our findings were similar to SWE measurements in subjects with Achilles tendinopathy and who did not have gout.
Similar content being viewed by others
Background
Gout occurs as a result of a metabolic inefficiency to fully metabolise purines and is considered to be the most common inflammatory arthritis [1, 2]. The resultant production of highly insoluble monosodium urate crystals located in joints and tendons causes a range of symptoms [1, 2]. Gout has an increasing prevalence both in the UK and worldwide [1,2,3,4]. While estimates vary, up to 6.8% of some populations are affected [3]. Gout is associated with acute attacks of severe joint pain and swelling, particularly affecting the foot [4]. Pain and impairment often do not entirely normalise after a gout flare-up has subsided [5], leading to general and foot-specific pain and disability, reduced ankle joint angular velocity and resulting in altered walking patterns [6,7,8]. Taken together, pain, time off work, limitations in ones’ ability to move or undertake exercise and subsequent loss of participation in social activities all reduce quality of life for those with gout [6, 9, 10].
The Achilles tendon (AT) is a common location for the deposit of monosodium urate crystals, forming subcutaneous palpable bodies known as tophi [11]. These structures are located within the tendon and will affect the structural arrangement of the tendon fibres impacting on the mechanical properties and function of the AT [12]. In gout, there are also measurable signs of inflammation in the AT (e.g. intra tendinous power Doppler signals) [13]. Inflammatory markers are generally considered to be a hallmark of rheumatic disorders such as gout. Inflammatory processes alter the tissue composition, increasing water content, and can result in changes to tissue structure and tissue stiffness (or elasticity). While the AT is the strongest tendon in the body, it is frequently injured, and the pathology of those injuries is heterogeneous [14, 15]. The mechanical properties of tendons are key to the transmission of muscle tension to the skeleton, elastic energy storage and to limiting load stress on muscles [16]. Consequently, measurement of the structural properties of the tendon is an important part of understanding the changes that may occur and affect the background pathogenesis [17]. Clinicians commonly use ultrasound imaging (US) to visualise tendon structure in AT disorders, since the technique is minimally invasive, quick and often easily available for use [18]. Shear wave elastography (SWE) is a non-invasive and dynamic method of quantifying changes in tendon elasticity, involving application of a focused ultrasound pulse to the tissue being imaged [19,20,21]. SWE is a type of US elastography that uses shear waves generated by a remote radiation force of focused ultrasonic beams to assess tissue elasticity. It measures the velocity of shear waves (in metres per second) moving through tissue, and displays these in a quantitative manner via a real-time elastogram, in which elasticity is portrayed as a colour-coded representation over a B-mode image. In SWE images stiffer tissues are red, while softer tissues are blue [22,23,24]. Importantly in ultrasonography, previous work indicates SWE is more independent of user skill than other modes of US imaging [25]. A systematic review of SWE as a diagnostic tool indicated good correlation with conventional ultrasound results, MRI and clinical examination [26]. More recently, SWE per se has received attention with regard to conditions involving tendons. Compared to other methods commonly used, it has been shown to be a simple way to measure tendon stiffness and identify pathology with good precision and increased diagnostic accuracy [27]. A review of imaging modalities in gout has highlighted the value of ultrasound for monitoring monosodium urate deposition and damage associated with inflammation, but SWE was not reported upon [28]. We aimed to utilise SWE to identify differences in Achilles tendon stiffness in people with gout compared to controls (non-gout). We hypothesised that stiffness of the AT would be reduced by the inflammatory processes and the consequences of monosodium urate crystal deposits affecting the structure and function of the AT.
Methods
Participants and setting
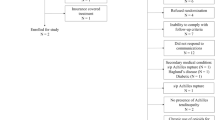
The terminology used for gout used in the paper follows the G-CAN consensus statements [29]. In this cross sectional, case-controlled study, participants with gout were recruited from an out patient podiatry clinic in East Sussex. Ethical approval was granted by the University of Brighton (17–009) and Office for Research Ethics Committees Northern Ireland (ORECNI) (232339). The inclusion criteria (Table 1) were those diagnosed with gout (aged 25–80) by their GP with no self-reported Achilles tendinosis. Most patients with gout are managed in the primary care setting and the diagnosis for gout is usually clinical with identification of monosodium urate crystals and/or serum urate measurements not generally undertaken [3]. Consequently, the clinical presentation of gout was checked according to the 2015 ACR/EULAR classification criteria [30], ensuring the clinical presentation met the clinical criteria. Non-gout participants were simultaneously recruited from the same patient population, typically with the same co-morbidities as those with gout, and met the same exclusion criteria. Inclusion/exclusion criteria for both groups are detailed in Table 1.
A clinician identified potential participants over an 18-month period (July 2018–December 2019) during their normal podiatry appointment, based on presenting complaint and/or medical history. If agreeable, potential participants were provided with an information pack and contacted after 48 h by the chief investigator (SO) to answer questions and arrange an appointment if they wished to opt in to the study. Data were collected during a single session outside of participants’ normal appointment time. Informed, written consent was obtained prior to data collection. We sought to recruit 25 people in each group. As SWE has not been carried out in those with gout previously, a formal power calculation was not possible. However, research focusing on ultrasound imaging of the AT alone in those with gout used a similar number of participants and found significant differences between populations [13].
Data collection
Baseline demographic details were captured together with comorbidities and key potential confounding factors including BMI and self-reported levels of exercise using the validated relative physical activity question [31].
Ultrasound scanning techniques
All measurements were taken with a Siemens ACUSON S2000™ Helix Evolution Ultrasound system (Siemens Medical Solutions, Malvern, PA, USA) ultrasound imaging system, utilising a pre-programmed AT setting. The AT is a favoured site for assessment as it meets the parameters required for SWE, while avoiding common variables that lead to limitations with US imaging [32]. The AT was selected as it offers:
-
Close proximity target tissue (AT) to the transducer (within 3 cm)
-
Largely homogeneous tissue
-
No structures present to dampen shear stress measurement (e.g. large vessels)
-
Distance between tissue boundaries
-
Ease of identifying anatomical location
Previously compression elastography ultrasound measurements have been limited by poor reproducibility [33]. Therefore a standardised protocol for SWE ultrasound assessment was adopted, based on validated studies where Inter-observer reliability varied between κ0.87–1 [13] and ICC between operators was 0.80 [34].
Conventional ultrasound technique
All US images were captured by an experienced operator (SO) with 7 years ultrasound imaging experience. A 40 mm 14 L5 linear probe was used to visualise the AT length from the insertion of the AT over the calcaneus to the lowest fibres of the gastrocnemius. Scanning for pathological features of gout were undertaken in transverse and longitudinal axis. All SWE measures were taken in a longitudinal axis between 40 mm and 80 mm proximal to the insertion of the AT, capturing the mid-portion of the tendon where tendon pathology is most frequently encountered [35, 36]. For SWE measures a 40 mm 9LT linear probe was utilised. To maintain standardisation, the following uniform US settings to optimise imaging were used:
-
Greyscale US settings 15 MHz probe, 15 dB, DR60
-
Elastography US settings 9Mhz probe, 6 dB, DR65
(where: MHz = probe frequency, dB = decibels, DR = dynamic range (gain))
Throughout, participants were invited to lie prone with their feet relaxed freely overhanging an examination couch in line with previous validated protocols [34].
Intra-observer reliability US image interpretation
To assess intra-observer agreement of US characteristics, reliability was determined using (ICC (2)) with measures captured from three control subjects using 10 images on two separate occasions. The anonymous images were used to calculate reliability. Greyscale US measurements (tendon thickness) varied between 3.25–3.63 mm and SWE measures 9.27–10. Intra-observer error (ICC (2)) values were consistently between 0.69 and 0.707. Intra-observer error values of 0 to 0.2 are considered poor, 0.2 to 0.4 fair, 0.4 to 0.6 moderate, 0.6 to 0.8 good and 0.8 to 1.0 excellent [37]. We determined the mean of three measurements could used to determine tendon thickness without loss of reliability. Previously validated protocols [13] demonstrated almost perfect inter-rater reliability for those pathologies unique to gout (e.g. tophi) together with good intra-observer reliability. Therefore, subsequent US images and SWE measures were assessed by a single operator (SO) in real time.
Tendon structure & damage
AT thickness was measured at the thickest part of the mid-portion in a longitudinal axis. A partial tendon tear was defined as a focal discontinuity [38], whilst a complete tendon rupture was defined as a complete loss of tendon substance [39]. Tendon grading was based on a semi-quantitative system proposed by Archambault et al. [40] detailed in Table 2 and recorded bilaterally.
Features of urate deposition
Deposits of monosodium urate (MSU) or ‘tophi’; are frequently seen in gout and strongly reflect US waves. As a consequence, the presence of tophi can easily be confirmed [41]. US characteristics for tophi and aggregates were pre-determined in accordance with standardized definitions; the Outcome Measures in Rheumatology (OMERACT) group [42]. Tophus are circumscribed, inhomogeneous, hyperechoic accumulations, whereas ‘aggregates’ are heterogeneous and hyperechoic foci of uric acid deposits that maintain a high degree of reflectivity. The number of gouty tophi and/or aggregates in the AT was recorded bilaterally.
Features of inflammation
Tendon vascularity was assessed over the length of the tendon and defined as the presence of the power Doppler signal. A semi-quantitative scale (0–4) previously reported by Ohberg et al. [43] was used to quantify the level of neo angiogenesis present bilaterally.
Shear wave elastography (SWE)
Following conventional ultrasound, the system was placed in the virtual touch IQ (VTIQ) mode. Ten real-time SWE measures between 0.5–10 m/s were taken along the centre of a longitudinal section of the mid-portion of the AT bilaterally (Fig. 1). If SWE readings > 10 m/s are present the machine reads ‘HIGH’ and the highest value of 10 m/s was recorded.
Data analysis
Data were entered into SPSS and checked for accuracy, adherence to normal distribution and assumptions for inferential analysis (Fig. 2) [44]. Baseline clinical and demographic characteristics were summarised using descriptive statistics: count (n), mean, standard deviation (sd), range and percentages (%). Between-group (gout vs non-gout) comparisons were made for each characteristic, using Chi square and Student’s t-tests for categorical and continuous variables respectively. Given the within-subjects repeated-measures design used, an ANCOVA was used to compare SWE measures between the two groups for the left and right AT separately, while adjusting for covariates (Body Mass Index (BMI)). Bivariate Pearson correlation was used to determine any relationship between the number of tophi and SWE values. Statistical tests were significant for p < 0.05. SPSS v25 (IBM SPSS Statistics Version 25.0. Armonk, NY: IBM Corp.) used for all analyses.
Results
In total, 40 people with gout were recruited with 24 subsequently participating (60%). Of those not taking part, 8 declined and 5 subsequently excluded, two were subsequently not contactable and one did not attend their appointment. A population of 26 age/sex matched non-gout subjects were simultaneously recruited from the same clinical population.
Demographic and clinical data
Baseline demographic and clinical data are displayed in Table 3. Only 3 (13%) participants had experienced a gout attack within the last 3 months and 4 (17%) participants had clinically palpable gouty tophi over the foot/ankle region. Palpable subcutaneous tophi were located around the Achilles tendon for 3 subjects and on lesser digits for 1 subject. Chi square and Student’s t-tests showed that those with gout had significantly higher BMI (mdiff = 3.56 (95% CI 0.79 to 6.33; p = 0.013) and had a slightly higher proportion of comorbidities; but did not differ in respect of data concerning gender, ethnicity nor self-reported levels of activity.
Conventional ultrasound
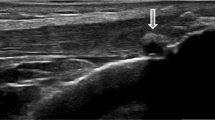
No subjects were noted to have either partial or complete tendon rupture. Table 4 highlights the main features of conventional ultrasound. There were no clinically meaningful differences in tendon grading and no significant difference in tendon thickness between groups. Small, but non-significant, differences in levels of neo-vascularity were noted between groups, with four (15%) non-gout participants exhibiting grade 1 or 2 neo-vascularity, whereas eight (33%) people with gout exhibited neo-vascularity between grades 1–3. Either gouty tophi and/or aggregates were visualised in nine (38%) participants with gout, these features being absent in the non-gout population – Fig. 3 illustrates a gout tophus on greyscale US.
Shear wave elastography (SWE)
SWE findings shown in Table 5 demonstrated significantly lower levels of tendon stiffness in those with gout compared with the non-gout group (right AT p = 0.003, left AT p = 0.025). Figure 4 illustrates a typical SWE scan from a participant without gout, whereas Fig. 5 exemplifies the SWE pattern seen in those with gout, where the tendon is more heterogeneous throughout, with a wider range of SWE readings noted.
Discussion
Using shear wave elastography, we have for the first time, demonstrated that people with gout exhibit significantly lower stiffness in the AT compared with age/sex matched non-gout participants. Previously, Aubrey and colleagues [45] reported that those with mid-portion AT tendinopathy demonstrated significantly lower mean SWE velocities than did those with a non-pathological AT. The loss of Achilles tendon stiffness we identified in those with gout was often greater than that demonstrated using a similar protocol in otherwise healthy runners with known Achilles tendinopathy [34]. The mean SWE values determined by Payne et al. [34] ranged from 7.91 m/s-9.56 m/s compared with mean SWE values in our participants with gout of 4.75 m/s-10 m/s. Recent work [46] suggests Achilles tendinopathy in healthy subjects involves both thickening of the tendon and softening (as reflected in lower SWE values), however our participants with gout did not report symptoms of AT.
Tendon pathology in the lower limbs has been shown to be more frequent in those with gout than in those with either osteoarthritis or healthy controls [13, 47]. A systematic review and meta-analysis revealed that abnormal ultrasound findings (i.e. tendon thickening, increased vascularity, hypoechogenicity) in the AT were predictive of tendinopathy [48]. However in our findings from those with gout, tendon thickening did not appear to be present, nor were considerably higher intra-tendon power Doppler readings noted, possibly suggesting a different pathological model from standard tendinopathy. A previous US study [13] demonstrated a much higher prevalence of intra-tendinous tophi (73%) than in non-gout participants. MSU deposition has been reported in 15–48% of the AT using dual energy CT scanning techniques [18, 49]. Our conventional ultrasound findings were toward the upper end of these results, but not as high as Carroll et al. [13], which may reflect different population sampling strategies. Previously, Chhana et al. [12] reported that MSU crystals directly interact with tenocytes to reduce cell viability and function, which may contribute to tendon damage. We did not identify any correlation between the number of tophi and SWE measures, which may further support a different pathological model. None of the studies reviewed above have reported SWE measurements. However currently, the lack of agreed and validated SWE values for tendinopathy may limit its use in the formal assessment of tendon damage [50].
Previous clinical and laboratory-based studies have reported weaker foot/leg muscles in those with gout than in non-gout participants [51, 52]. This muscle weakness was also associated with greater levels of foot pain [53]. Those with gout also exhibited slower walking speeds than control participants [53]. Current international recommendations for gout management [54] highlight the importance of diet together with lifestyle changes such as increased levels of physical activity. In 2008 the UK National Institute for Health and Care Excellence (NICE) [55] produced management recommendations for osteoarthritis that focused on the lower limb and emphasized that muscle strengthening should be undertaken before increasing aerobic activity. While we did not measure muscle strength, our contention here is that those participants with considerable loss of tendon stiffness might find that some types of increased load-bearing exercise have the potential to hasten the end stages of the continuum model of tendon pathology [56].
A key strength of our work is that we recruited people from a community-based outpatient clinic, and as such our participants were broadly representative of patients with gout treated in primary care. None were experiencing symptoms of an acute gout flare, nor Achilles tendinosis, yet significant loss of tendon stiffness was demonstrated. The primary limitation of our study was that the researcher (SO) undertaking US scans and SWE readings was not blinded to participant diagnosis, which may have led to inadvertent bias. Pathologies consistent with gout when seen on ultrasound are highly characteristic, typically with excellent inter-rater reliability [13]. Consequently, it is difficult to completely blind sonographers to the underlying diagnosis, but the addition of a second sonographer may add to the reliability of SWE measures in future work. The US machine we used for SWE readings also has a limitation where a maximum SWE value of 10 m/s can be recorded, after which a value of HIGH is given. Consequently, there may be a ceiling effect to some of the measurements leading to a possible underestimation of the differences in AT stiffness. Moreover, there has been limited use of SWE to assess soft tissue stiffness in other inflammatory arthropathies, making direct comparisons difficult. For example, in Ankylosing Spondylitis significant structural impairment of the Achilles tendon was noted using sonoelastography [57]. However, rather than reporting SWE values, areas of the AT were simply colour coded into normal or pathological, making direct comparison impossible.
Conclusions
Subjects with gout demonstrated significantly reduced Achilles tendon stiffness compared to non-gout controls. From a clinical standpoint, our SWE findings for the AT in those with gout were similar to SWE measurements in subjects with Achilles tendinopathy who did not have gout. This finding suggests the AT in people with gout may be less effective at transmitting muscle force and potentially more susceptible to further pathology and injury.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available as some identifying information is present to enable patient re-referral if required, but are available from the corresponding author on reasonable request.
Abbreviations
- AT:
-
Achilles tendon
- MSU:
-
Monosodium urate
- OMERACT:
-
Outcome measures in rheumatology
- SWE:
-
Shear Wave Elastography
- US:
-
Ultrasongraphy
References
Kuo CF, Grainge MJ, Mallen C, Zhang W, Doherty M. Rising burden of gout in the UK but continuing suboptimal management: a nationwide population study. Ann Rheum Dis. 2015;74:661–7.
Kuo C-F, Grainge MJ, Zhang W, Doherty M. Global epidemiology of gout: prevalence, incidence and risk factors. Nat Rev Rheumatol. 2015;11:649–62.
Dehlin M, Jacobsson L, Roddy E. Global epidemiology of gout: prevalence, incidence, treatment patterns and risk factors. Nat Rev Rheumatol. 16(7):380–90.
Smith E, Hoy D, Cross M. The global burden of gout: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis. 2014;73:1470–6.
Rome K, Frecklington M, McNair P, Gow P, Dalbeth N. Foot pain, impairment, and disability in patients with acute gout flares: a prospective observational study. Arthritis Care Res (Hoboken). 2012;64(3):384–8.
Carroll M, Boocock M, Dalbeth N, Stewart S, Frampton C, Rome K. Ankle joint function during walking in tophaceous gout: A biomechanical gait analysis study. Gait Posture. 2018;63:150–3.
Roddy E, Muller S, Rome K, Chandratre P, Hider SL, Richardson J, Blagojevic-Bucknall M, Mallen CD. Foot problems in people with gout in primary care: baseline findings from a prospective cohort study. J Foot Ankle Res. 2015;8:31.
Rome K, Survepalli D, Sanders A, Lobo M, McQueen FM, McNair P, Dalbeth N. Functional and biomechanical characteristics of foot disease in chronic gout: a case-control study. Clin Biomech. 2011;26(1):90–4.
Lindsay K, Gow P, Vanderpyl J, Logo P. Dalbeth N the experience and impact of living with gout: a study of men with chronic gout using a qualitative grounded theory approach. J Clin Rheumatol. 2011;17(1):1–6.
Chandratre P, Mallen CD, Roddy E, Liddle J, Richardson J. You want to get on with the rest of your life: a qualitative study of health-related quality of life in gout. Clin Rheumatol. 2016;35(5):1197–205.
Mallinson PI, Reagan AC, Coupal T, Munk PL, Ouellette H, Nicolaou S. The distribution of urate deposition within the extremities in gout: a review of 148 dual-energy CT cases. Skelet Radiol. 2014;43(3):277–81.
Chhana A, Callon KE, Dray M, Pool B, Naot D, Gamble GD, et al. Interactions between tenocytes and monosodium urate monohydrate crystals: implications for tendon involvement in gout. Ann Rheum Dis. 2014;73(9):1737–41.
Carroll M, Dalbeth N, Allen B, et al. Ultrasound characteristics of the Achilles tendon in Tophaceous gout: a comparison with age- and sex-matched controls. J Rheumatol. 2017;44(10):1487–92.
Magnan B, Bondi M, Pierantoni S, Samaila E. The pathogenesis of Achilles tendinopathy: A systematic review. Foot and Ankle Surgery. 2014;20:154–9.
Jarvinen TA, Kannus P, Maffulii N, Khan KM. Achilles tendon disorders: etiology and epidemiology. Foot & Ankle Clinics. 2005;10(2):255–66.
Foure A New Imaging Methods for Non-invasive Assessment of Mechanical, Structural, and Biochemical Properties of Human Achilles Tendon: A Mini Review Frontiers in Physiology 2016 7 Article 324.
Cortes DH, Suydam SM, Silbernagel KG, Buchanan TS, Elliott DM. Continuous Shear Wave Elastography: a New Method to Measure in-vivo Viscoelastic Properties of Tendons Biomedical. Ultrasound Med Biol. 2015;41(6):1518–29.
McAuliffe S, McCreesh K, Culloty F, et al. Can ultrasound imaging predict the development of Achilles and patellar tendinopathy? A systematic review and meta-analysis. Br J Sports Med. 2016;50:1516–23.
Arda K, Ciledag N, Aktas E, Aribas BK, Köse K. Quantitative assessment of normal soft-tissue elasticity using shear-wave ultrasound elastography. Am J Roentgenol. 2011;197(3):532–6.
Winn N, Lalam R, Cassar-Pullicino V. Sonoelastography in the musculoskeletal system: Current role and future directions. World J Radiol. 2016;8(11):868–79.
Martins-Rocha T, Azzolin I, Serban T, Massazza G, Iagnocco A. New applications of sonoelastography in rheumatology: where are we now? Rheumatology (Oxford). 2019;58(5):765–9.
Taljanovic MS, Gimber LH, Becker GW, Daniel Latt D, Klauser AS, Melville DM, et al. Shear-Wave Elastography: Basic Physics and Musculoskeletal Applications RadioGraphics. 2017;37:855–70.
Klauser AS, Miyamoto H, Bellmann-Weiler R, Feuchtner GM, Wick MC, Jaschke WR. Sonoelastography: Musculoskeletal. Radiology. 2014;272(3):622–33.
Schneider A, Feussner H, 2017 Biomedical engineering in gastrointestinal surgery (1st Ed) Elsevier Amsterdam p87-220.
Chen X, Cui L, He P, Shen W, Qian Y, Wang J. Shear wave elastographic characterization of normal and torn Achilles tendons a pilot study. J Ultrasound Med. 2013;32:449–55.
Pedersen M, Fredberg U, Langberg H. Sonoelastography as a diagnostic tool in the assessment of musculoskeletal alterations: a systematic review. Ultraschall Med. 2012;33(5):441–6.
Dirrichs T, Quack V, Gatz M, Tingart M, Kuhl CK, Schrading S. Shear Wave Elastography (SWE) for the Evaluation of Patients With Tendinopathies Acad Radiol. 2016 Oct;23(10):1204–13.
Dalbeth N, Doyle AJ. Imaging tools to measure treatment response in gout. Rheumatology (Oxford). 2018;57:i27–34.
Bursill D, Taylor WJ, Terkeltaub R, et al. Gout, Hyperuricaemia and Crystal-Associated Disease Network (G-CAN) consensus statement regarding labels and definitions of disease states of gout. Ann Rheum Dis. 2019;78:1592–600.
Neogi T, Jansen TLTA, Dalbeth N, et al. 2015 Gout classification criteria: an American College of Rheumatology/European league against rheumatism collaborative initiative. Ann Rheum Dis. 2015;74(10):1789–98.
Gill D, Jones GR, Zou G, Speechley M. Using a single question to assess physical activity in older adults: a reliability and validity study. BMC Med Res Methodol. 2012:12–20.
Bamber J, Cosgrove D, Dietrich CF, Fromageau J, Bojunga J, Calliadas F. EFSUMB Guidelines and Recommendations on the Clinical Use of Ultrasound Elastography. Part 1: Basic Principles and Technology. Ultraschall Med. 2013;34:169–84.
Payne C, Watt P, Cercignani M, Webborn N. Poor reproducibility of compression elastography in the Achilles tendon: same day and consecutive day measurements. Skeletal Radiol. 2017;46(7):889–95.
Payne C, Watt P, Cercignani M, Webborn N. Reproducibility of shear wave elastography measures of the Achilles tendon. Skelet Radiol. 2018;47:779–84.
De Zordo T, Chhem R, Smekal V, Feuchtner G, Reindl M, Fink C, et al. Real-time sonoelastography: findings in patients with symptomatic Achilles tendons and comparison to healthy volunteers. Ultraschall Med. 2010;31:394–400.
Domenichini R, Pialat J-B, Podda A, Aubry A. Ultrasound elastography in tendon pathology: state of the art. Skeletal Radiol. 2017;46:1643–55.
Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74.
Grassi W, Filippucci E, Farina A, Cervini C. Sonographic imaging of tendons. Arthritis Rheum. 2000;43:969–76.
Schmidt WA. Value of sonography in diagnosis of rheumatoid arthritis. Lancet. 2001;357:1056–7.
Archambault JM, Wiley JP, Bray RC, Verhoef M, Wiseman DA, Elliott PD. Can sonography predict the outcome in patients with achillodynia? J Clin Ultrasound. 1998;26(7):335–9.
Agudelo CA, Wise CM. Gout: diagnosis, pathogenesis, and clinical manifestations. Curr Opin Rheumatol. 2001;13:234–9.
Gutierrez M, Schmidt WA, Ralf G, Thiele RG, et al. International Consensus for ultrasound lesions in gout: results of Delphi process and web-reliability exercise. Rheumatology. 2015;54:1797–805.
Öhberg L, Alfredson H. Ultrasound guided sclerosis of neo-vessels in painful chronic Achilles tendinosis: pilot study of a new treatment. Br J Sports Med. 2002;36:173–7.
Tabachnick BG, Fidell LS. Using multivariate statistics (6th Ed). London: Pearson; 2013. p. 235–355.
Aubry S, Nueffer J-P, Tanter M, Becce F, Vidal C, Michel F. Viscoelasticity in Achilles Tendonopathy: Quantitative Assessment by Using Real-time Shear-Wave Elastography1. Radiology. 2015;274(3):821–9.
Payne C, Baker P, Louw M, Watt P, Webborn N, A 12 week eccentric exercise programme: clinical outcome measures and tendon stiffness measured by shear wave elastography in Achilles tendinopathy Journal of Orthopaedic Research (in review).
Ventura-Ríos L, Sánchez-Bringas G, Pineda C, Hernández-Díaz C, et al. Tendon involvement in patients with gout: an ultrasound study of prevalence. Clin Rheumatol. 2016;35(8):2039–44.
Dalbeth N, House ME, Aati O, Tan P, Franklin C, Horne A, McQueen FM. Urate crystal deposition in asymptomatic hyperuricaemia and symptomatic gout: a dual energy CT study. Ann Rheum Dis. 2015;74(5):908–11.
Yokose C, Dalbeth N, Wei J, Nicolaou S, Simeone FJ, Baumgartner S, Choi HK. Radiologic evidence of symmetric and polyarticular monosodium urate crystal deposition in gout - a cluster pattern analysis of dual-energy CT. Semin Arthritis Rheum. 2019;S0049-0172(19):30120–9.
Prado-Costa R, Rebelo J, Monteiro-Barroso J, Preto AS. Ultrasound elastography: compression elastography and shear-wave elastography in the assessment of tendon injury. Insights into Imaging. 2018;9:791–814.
Stewart S, Dalbeth N, Otter S, et al. Clinically-evident tophi are associated with reduced muscle force in the foot and ankle in people with gout: a cross-sectional study. J Foot Ankle Res. 2017;10:25.
Stewart S, Mawston G, Davidtz L, et al. Foot and ankle muscle strength in people with gout: a two-arm cross-sectional study. Clin Biomech. 2016;32:207–11.
Stewart S, Morpeth T, Dalbeth N, et al. Foot-related pain and disability and spatiotemporal parameters of gait during self-selected and fast walking speeds in people with gout: a two-arm cross sectional study. Gait Posture. 2016;44:18–22.
Richette P, Doherty M, Pascal E, Barskova V, Becce F, Nastaneda-Sanabria J, et al. 2016 updated EULAR evidence-based recommendations for the management of gout. Ann Rheum Dis. 2017;76:29–42.
National Institute for Health & Clinical Excellence. NICE clinical guideline [CG59] osteoarthritis: the care and management of osteoarthritis in adults. London: National Institute for Health & Clinical Excellence 2008. Available from: https://www.nice.org.uk/guidance/cg59 (last accessed 26 July 2020).
Cook JL, Rio E, Purdam CR, Docking SI. Revisiting the continuum model of tendon pathology: what is its merit in clinical practice and research? Br J Sports Med. 2016;50:1187–91.
Turan A, Tufan A, Rıdvan Mercan R, et al. Real-time sonoelastography of Achilles tendon in patients with ankylosing spondylitis. Skeletal Radiol. 2013;42:1113–8.
Acknowledgements
We wish to thank our participants for their time, clinical co-workers for helping with recruitment together with our reception colleagues for clinic bookings.
Funding
None.
Author information
Authors and Affiliations
Contributions
SO and PW designed the study. SO, CP and PW developed the methodology. SO performed ultrasound examinations, and was a major contributor in writing the manuscript. SO and A-M J analyzed and interpreted the patient data. NW oversaw the study. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval was granted by the University of Brighton (17–009) and Office for Research Ethics Committees Northern Ireland (ORECNI) (232339). All participants provided informed, written consent.
Consent for publication
No applicable – no identifying data are presented.
Competing interests
None.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Otter, S., Payne, C., Jones, AM. et al. Differences in Achilles tendon stiffness in people with gout: a pilot study. BMC Musculoskelet Disord 21, 658 (2020). https://doi.org/10.1186/s12891-020-03598-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12891-020-03598-3