Abstract
Background
To assess the safety and efficacy of long-term administration of guanfacine extended-release (GXR) in adults with attention-deficit/hyperactivity disorder (ADHD).
Methods
In this open-label, long-term, phase 3 extension study in Japan, 150 patients transitioned from a double-blind trial, and 41 newly enrolled patients received once daily GXR (starting dose 2 mg/day, maintenance dose 4–6 mg/day) for 50 weeks. Primary outcome measures were the frequency and nature of treatment-emergent adverse events (TEAEs); secondary outcome measures included the change from week 0 in ADHD Rating Scale IV with Adult Prompts (ADHD-RS-IV; Japanese version) total and subscale scores, Conners’ Adult ADHD Rating Scales (CAARS), Clinical Global Impression-Improvement (CGI-I) and Patient Global Impression-Improvement (PGI-I) scales, and quality of life (QoL) and executive functioning measures.
Results
Of all patients, 94.2% (180/191) reported ≥1 TEAE and 19.9% (38/191) discontinued because of a TEAE. Most TEAEs were mild to moderate in severity; there were two serious TEAEs and no deaths. Commonly reported TEAEs (≥10% of patients) were somnolence, thirst, nasopharyngitis, decreased blood pressure, postural dizziness, bradycardia, malaise, constipation, and dizziness. Mean changes from week 0 in ADHD-RS-IV total and subscale scores and CAARS subscale scores were significantly improved in former placebo or GXR patients and new patients at last observation (p < .0001), and the percentage of patients with very much or much improved CGI-I and PGI-I scores increased.
Conclusions
There were no major safety concerns during long-term GXR administration in adults with ADHD. After long-term treatment, patients had significant improvements from baseline in ADHD symptoms, QoL, and executive functioning.
Trial registration
Japan Primary Registries Network (https://rctportal.niph.go.jp/en/): JapicCTI-163232, registered 04/21/2016.
Similar content being viewed by others
Background
Although attention-deficit/hyperactivity disorder (ADHD) is commonly considered a childhood disorder, it is estimated to affect up to 3% of adults worldwide [1, 2]. Adult ADHD can persist from childhood into adulthood or be newly diagnosed in adults [3] and differs from childhood ADHD in several respects. ADHD symptoms change as patients mature, with decreases in overt hyperactivity symptoms and increases in more subtle symptoms, such as inattention and disorganization [4,5,6]. Comorbid psychiatric and behavioral symptoms can be associated with ADHD in children and adults, which may obscure initial diagnosis of ADHD in adults [6, 7]. Nonpsychiatric comorbidities, particularly obesity, sleep disorders, and asthma, are also associated with ADHD in adults [8]. Overall, underdiagnosis and undertreatment of ADHD in adults can result in impaired quality of life (QoL) [9] and psychosocial functioning [10], addictive or risky behaviors (including substance use disorders) [6], high rates of accidental death [11], and suicide [12].
Guanfacine extended-release (GXR) is a nonstimulant, selective, α2A-adrenergic receptor agonist approved worldwide for ADHD in children and adolescents and was first approved for treatment of ADHD in adults in Japan in June 2019. As clinical trial data for the use of GXR in adults have only recently become available [13], GXR for adults was not included in a comprehensive systematic review and metaanalysis of medications for ADHD, published in 2018 [14], and is not included in current international guidelines [15]. In the first phase 3, double-blind, randomized trial conducted in adults, dose-optimized GXR treatment significantly reduced ADHD symptoms at week 10 compared with placebo, with improvements in QoL and functioning [13]. Compared with placebo, GXR was associated with an increased incidence of treatment-emergent adverse events (TEAEs) that were related to its effect on α2A-adrenergic receptors (somnolence, thirst, blood pressure decrease, postural dizziness, and constipation), but most were mild to moderate in severity and resolved during treatment [13]. Given the differences between children and adults in the clinical presentation of ADHD and associated comorbidities, assessment of the safety and efficacy of prolonged GXR treatment in adults is required.
The primary objective of this study was to assess the safety of long-term administration of once-daily GXR in adults with ADHD over 50 weeks of treatment. The secondary objective was to assess the efficacy of GXR.
Methods
This was an open-label, long-term, phase 3 study in adults with ADHD. The study (conducted at 71 Japanese centers from December 2016 through December 2018) was approved by the following local ethics committees: Mizuo Clinic Institutional Review Board (IRB); Ehime University Hospital IRB; IHL Shinagawa East One Medical Clinic IRB; Dr. Mano Medical Clinic IRB; Odori Park Mental Clinic IRB; Tokyo Midtown Clinic IRB; Tokyo-Eki Center-Building Clinic IRB; Riverside Internal and Circulatory Medical Clinic IRB; Goryokai Hospital IRB; Himorogi Psychiatric Institute IRB; Nanko Clinic of Psychiatry IRB; Iwata Buddy’s Clinic IRB; Suzuki Internal and Circulatory Medical Clinic IRB; Kojinkai Sapporo Skin Clinic IRB; Shoda Hospital IRB; Kondo Hospital IRB; Tomisaka Clinic IRB; Yokohama Sakae Kyosai Hospital IRB; Hokkaido University Hospital IRB; Yamate Dermatoligcal Clinic IRB; Chibune General Hospital IRB; IRB of Showa University Karasuyama Hospital; Yoyogi Mental Clinic IRB; Tokai University Hospital IRB; The Jikei University Hospital IRB for Medicinal Products; Non-Profit Organization Tokyo Allergy and Respiratory Disease Research Institute IRB; Review Board of Human Rights and Ethics for Clinical Studies; Nara Medical University Hospital IRB; University of Fukui Hospital IRB; Hayashi Diabetes Clinic IRB, and conducted in accordance with Good Clinical Practice and the Declaration of Helsinki. All patients provided written informed consent before participating in the study. The previous double-blind trial (DBT) [13] and this study were registered at the Japan Primary Registries Network (JapicCTI-163231).
Study population
Newly enrolled patients and the patients who completed the previous DBT and who consented to transition to this open-label study were eligible for inclusion. The main inclusion criteria for new patients were adult men or women (age ≥18 years) with a diagnosis of ADHD (Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition) [DSM-5]) [16], ADHD Rating Scale IV with Adult Prompts (ADHD-RS-IV; Japanese version) total score ≥24, and a Clinical Global Impression-Severity of Illness (CGI-S) scale score ≥4. Exclusion criteria were reported in detail previously [13]. In brief, the main exclusion criteria were a diagnosed or documented moderate/severe psychiatric disorder (based on DSM-5) requiring drug treatment, a history of substance use disorder or seizures, persons considered at risk of suicide, a history or evidence of cardiovascular disease, and use of medications affecting blood pressure or heart rate.
Study design
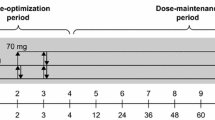
This open-label study was dose optimized and noncontrolled and comprised a 50-week treatment period, a 2-week tapered dose-reduction period, and a 1-week follow-up period (Additional file 1). All patients received a single dose of GXR once daily at approximately the same time (morning or afternoon), starting at a minimum dose of 2 mg/day and increasing to a maintenance dose of 4–6 mg/day for 50 weeks. Forced dose increments of 1-mg increases up to a total of 4 mg, followed by 1-mg increases or reductions at ≥5-day intervals to maintain the dose between 4 and 6 mg, were allowed at the investigator’s discretion for patients with no safety concerns and CGI-S scores ≥3. During the tapered dose-reduction period, doses were decreased by 1 mg at ≥3-day intervals over 2 weeks.
Outcome measures
Safety measures included the type and frequency of TEAEs (Medical Dictionary for Regulatory Activities, v19.0) and vital signs at each visit, and electrocardiogram (ECG) parameters and clinical laboratory tests (weeks 0, 10, 22, 34, 50, and study discontinuation).
Efficacy outcomes included physician-rated measures (ADHD-RS-IV total and subscale scores, Conners’ Adult ADHD Rating Scales [CAARS], and CGI-Improvement [CGI-I] and CGI-S scales) [17,18,19,20] and patient-rated measures (Patient Global Impression-Improvement [PGI-I] scale, the Adult ADHD Quality of Life Questionnaire [AAQoL], and the Behavior Rating Inventory of Executive Function-Adult Version [BRIEF-A]) [19, 21,22,23]. ADHD-RS-IV and CGI-S were assessed at each visit from weeks 0–50 or discontinuation. CGI-I and PGI-I were assessed at each visit from weeks 1–50 or discontinuation, CAARS was assessed at weeks 0, 22, and 50 or discontinuation, and AAQoL and BRIEF-A were assessed at weeks 0, 10, 22, 34, and 50 or discontinuation.
Statistical analysis
The target sample size was 190 patients to allow for 100 patients completing 1 year of treatment. All patients who received at least one dose of GXR were included in the analyses. All TEAEs between the first intake of study drug and follow-up observation were analyzed. For analyses of ADHD-RS-IV total and subscale scores, CAARS scores, AAQoL scores, and BRIEF-A, mean (95% confidence intervals [CIs]) at each visit were reported. Mean differences in scores from week 0 (screening period) were assessed at each visit using two-sided t tests for ADHD-RS-IV total and subscale scores, CAARS scores, and AAQoL scores. Illness severity and improvement (CGI-S, CGI-I, or PGI-I) rates at each visit from week 0 were assessed using the Clopper–Pearson method. Missing data were not imputed for efficacy analyses; statistical analyses were performed using SAS Version 9.2 or higher (SAS Institute Inc., Cary, NC, USA).
Results
Patient disposition and baseline characteristics
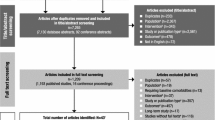
A total of 191 patients were enrolled, received at least one dose of study drug, and were included in the analyses (Fig. 1); 150 had transitioned from the previous DBT (former placebo or GXR patients) and 41 were newly enrolled (new patients). Of the enrolled patients, 124 (95 transitioned, 29 new) completed the study. The main reason for discontinuation was adverse events from all populations (Fig. 1).
During the study, mean (standard deviation) treatment duration was 254.9 (136.5) days for all patients (transitioned: 247.6 [140.1]; new: 281.7 [120.3]), and the most frequently taken doses of GXR were 6 mg (38% of patients), 4 mg (35% of patients), and 5 mg (17% of patients).
In all patients, approximately half had combined presentation or predominantly inattentive presentation, and approximately half had been treated with ADHD medication previously (Table 1). At the start of the DBT for those who transitioned and at the start of long-term treatment for new patients, mean ADHD-RS-IV total scores were approximately 32 among all patients, but there was a higher proportion of new patients (70.7%) with ADHD-RS-IV total scores ≥30 than former placebo (51.6%) or GXR (53.4%) patients.
Safety and tolerability
In general, no new or unexpected adverse events were reported during long-term treatment (Table 2). A total of 830 TEAEs were reported by 180 patients (94.2%), with most considered to be drug related (83.8% of all patients). Most TEAEs were mild to moderate in severity, and no deaths were reported (Table 2). Compared with former placebo patients and new patients, a smaller proportion of former GXR patients experienced treatment-related TEAEs or moderate severity TEAEs or discontinued because of a TEAE (Table 2).
Two patients experienced a serious TEAE. One continuing patient was diagnosed with acute myeloid leukemia 380 days after starting treatment (81 days after completing the tapering period), which was considered unrelated to study drug. One new patient, with a preexisting condition requiring prescription of verapamil, experienced supraventricular tachycardia of moderate severity 255 days after starting GXR; the patient recovered following treatment and discontinuation of GXR.
The most commonly reported TEAEs (incidence ≥10%) in all patients were somnolence, thirst, nasopharyngitis, decreased blood pressure, postural dizziness, bradycardia, malaise, constipation, and dizziness (Table 2). Except for nasopharyngitis, most events were considered related to GXR. Study drug discontinuation because of TEAEs was reported for 19.9% of all patients (Table 2). The main TEAEs resulting in GXR discontinuation were somnolence (nine patients), blood pressure reduction (eight patients), malaise (six patients), bradycardia (four patients), and postural dizziness (three patients) or dizziness (three patients). All events resulting in GXR discontinuation were of mild or moderate severity except for one event of severe bradycardia, which occurred 70 days after commencing treatment. The GXR dose at onset was 6 mg. The patient discontinued GXR and recovered without treatment.
There were no clinically relevant changes in blood pressure, pulse rate, or ECG parameters (Table 3) or clinical laboratory tests after 50 weeks of treatment with GXR. For all patients, the mean change from week 0 in systolic blood pressure and diastolic blood pressure between week 1 and week 50 ranged from −9.54 to −3.82 mmHg and from −8.37 to −2.93 mmHg, respectively; the mean change in pulse rate ranged from −9.04 to −2.12 beats/minute; and the mean change in body weight between week 4 and week 50 ranged from −0.33 to 0.28 kg. For all patients, small changes in ECG parameters were observed during long-term treatment, which gradually recovered to the levels observed at week 0 by the end of treatment (weeks 50–52). The mean change from week 0 at last observation in the treatment period was a decrease in heart rate of 6.75 beats/minute, an increase in RR interval of 115.12 msec, an increase in PR interval of 3.55 msec, an increase in QT interval of 12.96 msec, and a decrease in QTc corrected by Bazett’s formula (QTcB) interval of 9.91 msec and a decrease in QTc corrected by Fridericia’s formula (QTcF) interval of 2.36 msec. The changes in QRS interval were variable during long-term treatment.
Efficacy
ADHD-RS-IV
Significant improvements in ADHD symptoms were reported in all patient populations during long-term treatment with GXR (Table 4). ADHD-RS-IV total and subscale scores significantly decreased (improved) compared with week 0 up to last observation and week 50 (Table 4; all p < .0001). The mean (95% CI) ADHD-RS-IV total scores at last observation were 18.82 (16.47, 21.16) for former placebo patients, 14.44 (12.08, 16.79) for former GXR patients, and 16.27 (13.21, 19.32) for new patients. Rapid improvements in ADHD-RS-IV total scores were observed within the first 1–6 weeks of long-term treatment, which were sustained up to week 50 for all populations (Fig. 2).
Change from baseline in ADHD-RS-IV total scores. Data are the mean change from baseline (i.e., the start of the previous double-blind trial [DBT]) for patients who transitioned from the placebo arm and guanfacine extended-release (GXR) arm and the mean change from week 0 of the long-term treatment study for new patients. Error bars denote standard deviations. ADHD-RS-IV: Attention-Deficit/Hyperactivity Disorder Rating Scale IV with Adult Prompts
CAARS
The mean (95% CI) CAARS total scores at last observation were 20.61 (18.27, 22.95) for former placebo patients, 15.66 (13.20, 18.11) for former GXR patients, and 16.68 (13.84, 19.51) for new patients. In addition, there were significant decreases (improvements) from week 0 at last observation and week 50 in all CAARS subscale scores (p <.0001; Table 4).
CGI-I, PGI-I, and CGI-S
The percentage of patients with “very much improved” or “much improved” physician-rated (CGI-I) and patient-rated (PGI-I) scores, and with “normal” or “borderline mentally ill” physician-rated CGI-S scores, increased during long-term GXR treatment (Table 4). Eighteen patients were rated as severely ill at week 0 (eight former placebo patients, four former GXR patients, and six new patients). At week 50, three were markedly ill (two former placebo patients, one new patient) and three remained severely ill (all former placebo patients), with the remainder rated as borderline, mildly, or moderately ill.
AAQoL and BRIEF-A
Patient-reported QoL and executive functioning significantly improved in former DBT patients who transitioned and in new patients during long-term treatment (Table 4). AAQoL total scores increased (improved) significantly from week 0 to 49.11 for former placebo patients, 58.27 for former GXR patients, and 52.39 for new patients at last observation. At last observation and week 50, significant improvements from week 0 were reported for AAQoL life productivity for former placebo and GXR patients, life outlook for new patients, and relationships for former placebo patients (Table 4). In addition, significant improvements were reported for almost all BRIEF-A T-score subscales in all populations (Table 4).
Discussion
This is the first study to assess long-term safety and efficacy of dose-optimized GXR in adult ADHD. The safety findings during treatment for 50 weeks were consistent with the previous 10-week DBT [13] and the known safety profile of GXR, and no new or unexpected safety signals were identified. Adult patients experienced improvements in ADHD symptoms, QoL, and executive functioning that were sustained for up to 1 year. Given the complexity of treating ADHD, nonstimulant medication can be an important option for patients when other medications are not effective or well tolerated [6]. The findings from this study support the use of GXR as an alternative treatment for adult patients with ADHD in Japan.
Consistent with the known safety profile of GXR in children [24,25,26,27], the most frequently reported TEAEs were sedative and included somnolence, decreased blood pressure, thirst, postural dizziness, bradycardia, malaise, constipation, and dizziness. Although nasopharyngitis was reported frequently, this TEAE was not considered related to GXR. Similar to the previous DBT [13], thirst was reported more frequently in adults than in studies of GXR in children [28, 29]. This finding was not considered to be clinically relevant or related to any differences in ethnicity between Japanese and non-Japanese populations because thirst (dry mouth) has been reported in studies conducted with GXR in adults in the United States [30, 31] and because direct comparison of the pharmacokinetics, safety, and tolerability of GXR showed no major differences in safety profiles between healthy Japanese and adults in the United States [32]. In line with the decreases in blood pressure and heart rate that have been observed during treatment with GXR in children [25,26,27], eight patients discontinued because of mild to moderate reductions in blood pressure and four discontinued because of bradycardia; only one case of bradycardia was severe and the patient recovered after treatment discontinuation. One patient experienced the serious TEAE, supraventricular tachycardia, which was moderately severe and for which relatedness to GXR was not excluded. GXR is not known to affect cardiac repolarization [31], and there were no clinically relevant changes in cardiovascular parameters, vital signs, or body weight for patients who continued treatment for 50 weeks. There were no substantial differences in the proportion of patients experiencing TEAEs among the treatment populations. However, former GXR patients reported fewer treatment-related TEAEs, fewer TEAEs leading to discontinuation, and fewer TEAEs of moderate severity compared with former placebo patients and new patients (Table 2), which is to be expected given that most sedative events are transitory, occur within the first few weeks of treatment, and resolve over time [13, 26, 27].
Treatments that provide sustained long-term improvements in ADHD symptoms are needed for adults because of the substantial impact of ADHD in adults on general health, psychosocial and neuropsychological functioning, and productivity [9, 10, 33]. During the previous DBT, significant improvements in ADHD symptoms (ADHD-RS-IV total and subscale scores) compared with placebo were observed for GXR-treated patients at 4 weeks [13]. In the current study, rapid improvement in ADHD symptoms was seen for GXR-treated patients within the first 6 weeks, which continued to improve for up to 50 weeks. These improvements were similar to the improvements in patient-reported QoL and all aspects of executive functioning.
The main strength of this study is that the flexible-dosing regimen allowed individualized treatment in all patients for 50 weeks of treatment. Furthermore, multiple physician- and patient-specific rating instruments were included to assess the effects of treatment. Although all patients underwent titration at the start of the long-term treatment, patients who transitioned from GXR in the previous DBT did not undergo a washout phase and received continuous GXR treatment through to the end of long-term treatment. There was a potential for observer bias because of the open-label nature of the study, and the findings may not be representative of real-world settings because patients with psychiatric or cardiovascular comorbidities, which are common in patients with ADHD, were excluded. In addition, there was a potential bias favoring safety and efficacy for continuing patients because those who discontinued owing to adverse events or lack of efficacy were not eligible for inclusion. However, these effects are balanced by the inclusion of newly enrolled patients.
Conclusions
In conclusion, there were no new or unexpected safety concerns during long-term administration of GXR in Japanese adults with ADHD. During long-term treatment for up to 50 weeks, patients who received dose-optimized GXR had improvements in multiple aspects of ADHD, including symptoms, QoL, and executive functioning.
Availability of data and materials
Researchers can request access to detailed information about Shionogi’s clinical trials, including trial protocols and individual patient data, on the portal site: clinicalstudydatarequest.com. Sharable information includes data about Shionogi’s clinical trials conducted in patients in Japan. The information will become sharable after the medicinal products for which the trials are performed have been approved in Japan. Note that all documents will be provided in Japanese language only as they have been prepared in Japanese.
Change history
22 December 2020
An amendment to this paper has been published and can be accessed via the original article.
Abbreviations
- AAQoL:
-
Adult ADHD Quality of Life Questionnaire
- ADHD:
-
Attention-deficit/hyperactivity disorder
- ADHD-RS-IV:
-
ADHD Rating Scale IV with Adult Prompts
- BRIEF-A:
-
Behavior Rating Inventory of Executive Function-Adult Version
- CAARS:
-
Conners’ Adult ADHD Rating Scales
- CGI-S:
-
Clinical Global Impression-Severity of Illness scale
- CI:
-
Confidence interval
- DBT:
-
Double-blind trial
- DSM-5 :
-
Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition)
- ECG:
-
Electrocardiogram
- GXR:
-
Guanfacine extended-release
- PGI-I:
-
Patient Global Impression-Improvement scale
- TEAEs:
-
Treatment-emergent adverse events
- QoL:
-
Quality of life
References
Fayyad J, Sampson NA, Hwang I, Adamowski T, Aguilar-Gaxiola S, Al-Hamzawi A, Andrade LH, Borges G, de Girolamo G, Florescu S, et al. The descriptive epidemiology of DSM-IV adult ADHD in the World Health Organization world mental health surveys. Atten Defic Hyperact Disord. 2017;9(1):47–65.
Simon V, Czobor P, Bálint S, Mészáros A, Bitter I. Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. Br J Psychiatry. 2009;194(3):204–11.
Moffitt TE, Houts R, Asherson P, Belsky DW, Corcoran DL, Hammerle M, Harrington H, Hogan S, Meier MH, Polanczyk GV, et al. Is adult ADHD a childhood-onset neurodevelopmental disorder? Evidence from a four-decade longitudinal cohort study. Am J Psychiatry. 2015;172(10):967–77.
Biederman J, Mick E, Faraone SV. Age-dependent decline of symptoms of attention deficit hyperactivity disorder: impact of remission definition and symptom type. Am J Psychiatry. 2000;157(5):816–8.
Faraone SV, Biederman J, Mick E. The age-dependent decline of attention deficit hyperactivity disorder: a meta-analysis of follow-up studies. Psychol Med. 2006;36(2):159–65.
Kooij SJ, Bejerot S, Blackwell A, Caci H, Casas-Brugue M, Carpentier PJ, Edvinsson D, Fayyad J, Foeken K, Fitzgerald M, et al. European consensus statement on diagnosis and treatment of adult ADHD: the European network adult ADHD. BMC Psychiatry. 2010;10:67.
Piñeiro-Dieguez B, Balanzá-Martínez V, García-García P, Soler-López B, CAT Study Group. Psychiatric comorbidity at the time of diagnosis in adults with ADHD: the CAT study. J Atten Disord. 2016;20(12):1066–75.
Instanes JT, Klungsøyr K, Halmøy A, Fasmer OB, Haavik J. Adult ADHD and comorbid somatic disease: a systematic literature review. J Atten Disord. 2018;22(3):203–28.
Agarwal R, Goldenberg M, Perry R, IsHak WW. The quality of life of adults with attention deficit hyperactivity disorder: a systematic review. Innov Clin Neurosci. 2012;9(5–6):10–21.
Able SL, Johnston JA, Adler LA, Swindle RW. Functional and psychosocial impairment in adults with undiagnosed ADHD. Psychol Med. 2007;37(1):97–107.
Dalsgaard S, Østergaard SD, Leckman JF, Mortensen PB, Pedersen MG. Mortality in children, adolescents, and adults with attention deficit hyperactivity disorder: a nationwide cohort study. Lancet. 2015;385(9983):2190–6.
Furczyk K, Thome J. Adult ADHD and suicide. Atten Defic Hyperact Disord. 2014;6(3):153–8.
Iwanami A, Saito K, Fujiwara M, Okutsu D, Ichikawa H. Efficacy and safety of guanfacine extended release in treatment of attention deficit/hyperactivity disorder in adults: Results of a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2020;81(3):19m12979. https://doi.org/10.4088/JCP.19m12979.
Cortese S, Adamo N, Del Giovane C, Mohr-Jensen C, Hayes AJ, Carucci S, Atkinson LZ, Tessari L, Banaschewski T, Coghill D, et al. Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: a systematic review and network meta-analysis. Lancet Psychiatry. 2018;5(9):727–38.
National Institute for Care and Health Excellence. Attention deficit hyperactivity disorder: diagnosis and management. NICE guideline [NG87]. 2018, last updated September 2019. https://www.nice.org.uk/guidance/ng87/chapter/Recommendations. Accessed 3 Mar 2020.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 5th ed (DSM-5). Washington, DC: American Psychiatric Publishing; 2013.
Conners K, Erhardt D, Sparrow E. Conners’ adult ADHD rating scales (CAARS). New York: Multi-Health Systems; 1999.
DuPaul GJ, Power TJ, Anastopoulos AD, Reid R. ADHD rating scale-IV: checklists, norms, and clinical interpretation. New York: Guilford Press; 1998.
Guy W. Clinical global impressions. In: Guy W, editor. ECDEU Assessment Manual for Psychopharmacology. Rockville: National Institute for Mental Health; 1976. p. 218–22.
Ichikawa H, Saito K, Saito T, Kariya N, Kodaira M, Ohta H, Kishida I, Mikami K, Ota T, Kyo M, et al. Reliability and validity of the ADHD-RS-IV with adult prompts: the Japanese version. Clin Psychiatry. 2018;60(4):399–409.
Brod M, Johnston J, Able S, Swindle R. Validation of the adult attention-deficit/hyperactivity disorder quality-of-life scale (AAQoL): a disease-specific quality-of-life measure. Qual Life Res. 2006;15(1):117–29.
Matza LS, Johnston JA, Faries DE, Malley KG, Brod M. Responsiveness of the adult attention-deficit/hyperactivity disorder quality of life scale (AAQoL). Qual Life Res. 2007;16(9):1511–20.
Roth RM, Isquith PK, Gioia GA. Behavior rating inventory of executive function–adult version. Lutz: Psychological Assessment Resources; 2005.
Shire US. Intuniv® (guanfacine) [prescribing information]. Lexington; 2017.
Biederman J, Melmed RD, Patel A, McBurnett K, Donahue J, Lyne A. Long-term, open-label extension study of guanfacine extended release in children and adolescents with ADHD. CNS Spectr. 2008;13(12):1047–55.
Huss M, Dirks B, Gu J, Robertson B, Newcorn JH, Ramos-Quiroga JA. Long-term safety and efficacy of guanfacine extended release in children and adolescents with ADHD. Eur Child Adolesc Psychiatry. 2018;27(10):1283–94.
Sallee FR, Lyne A, Wigal T, McGough JJ. Long-term safety and efficacy of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2009;19(3):215–26.
Ichikawa H, Miyajima T, Yamashita Y, Fujiwara M, Okutsu D, Saito K. Efficacy and safety of guanfacine hydrochloride extended-release tablet for children and adolescents with ADHD: a phase 2/3 placebo-controlled, double-blind study in Japan [in Japanese]. Jpn J Clin Psychopharmacol. 2018;21(8):1093–117.
Ichikawa H, Miyajima T, Yamashita Y, Fujiwara M, Okutsu D, Saito K. Long-term safety and efficacy of guanfacine hydrochloride extended-release tablet for children and adolescents with ADHD: a phase 2/3 long-term, open-label extension study in Japan. [in Japanese]. Jpn J Clin Psychopharmacol. 2018;21(12):1645–61.
Butterfield ME, Saal J, Young B, Young JL. Supplementary guanfacine hydrochloride as a treatment of attention deficit hyperactivity disorder in adults: a double blind, placebo-controlled study. Psychiatry Res. 2016;236:136–41.
Martin P, Satin L, Kahn RS, Robinson A, Corcoran M, Purkayastha J, Youcha S, Ermer JC. A thorough QT study of guanfacine. Int J Clin Pharmacol Ther. 2015;53(4):301–16.
Matsuo Y, Okita M, Ermer J, Wajima T. Pharmacokinetics, safety, and tolerability of single and multiple doses of guanfacine extended-release formulation in healthy Japanese and Caucasian male adults. Clin Drug Investig. 2017;37(8):745–53.
Biederman J, Petty CR, Woodworth KY, Lomedico A, Hyder LL, Faraone SV. Adult outcome of attention-deficit/hyperactivity disorder: a controlled 16-year follow-up study. J Clin Psychiatry. 2012;73(7):941–50.
Acknowledgments
The authors would like to thank Brigitte Robertson, MD, for her scientific contributions to the trial. Dr. Robertson is an employee of Shire HGT, Inc. (Lexington, MA, USA), a member of the Takeda group of companies, and owns stock in the company. The authors would also like to thank all study participants.
Medical writing assistance was provided by Serina Stretton, PhD, CMPP, and Hiroko Ebina, BPharm, PhD, MBA, of ProScribe – Envision Pharma Group, and was funded by Shionogi & Co., Ltd. ProScribe’s services complied with international guidelines for Good Publication Practice (GPP3).
Funding
This study was funded by Shire International GmbH (manufacturer/licensee of Intuniv® [guanfacine extended-release]), a member of the Takeda group of companies, and Shionogi & Co., Ltd. GXR is approved for the treatment of ADHD in adults in Japan and in children (<18 years of age) throughout the world. Shionogi & Co., Ltd. was involved in the study design, data collection, data analysis, and preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
HI was an expert medical advisor for the study, AI and KS were coordinating investigators in the study and involved in data collection, DO was the study leader, and MF was involved in the statistical analyses. All authors participated in the interpretation of the study results and in the drafting, critical revision, and approval of the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the following local ethics committee: Mizuo Clinic Institutional Review Board (IRB); Ehime University Hospital IRB; IHL Shinagawa East One Medical Clinic IRB; Dr. Mano Medical Clinic IRB; Odori Park Mental Clinic IRB; Tokyo Midtown Clinic IRB; Tokyo-Eki Center-Building Clinic IRB; Riverside Internal and Circulatory Medical Clinic IRB; Goryokai Hospital IRB; Himorogi Psychiatric Institute IRB; Nanko Clinic of Psychiatry IRB; Iwata Buddy’s Clinic IRB; Suzuki Internal and Circulatory Medical Clinic IRB; Kojinkai Sapporo Skin Clinic IRB; Shoda Hospital IRB; Kondo Hospital IRB; Tomisaka Clinic IRB; Yokohama Sakae Kyosai Hospital IRB; Hokkaido University Hospital IRB; Yamate Dermatological Clinic IRB; Chibune General Hospital IRB; IRB of Showa University Karasuyama Hospital; Yoyogi Mental Clinic IRB; Tokai University Hospital IRB; The Jikei University Hospital IRB for Medicinal Products; Non-Profit Organization Tokyo Allergy and Respiratory Disease Research Institute IRB; Review Board of Human Rights and Ethics for Clinical Studies; Nara Medical University Hospital IRB; University of Fukui Hospital IRB; Hayashi Diabetes Clinic IRB. All patients provided written informed consent before participating in the study.
Consent for publication
Not applicable.
Competing interests
AI has received honoraria and other payments from Eisai, Eli Lilly Japan, Janssen Japan, Kyowa, Meiji Seika Pharma, Mitsubishi Tanabe Pharma, MSD K.K., Otsuka, Pfizer Japan, Sumitomo Dainippon Pharma, and Yoshitomiyakuhin Corporation. KS has received honoraria and other payments from Eli Lilly Japan, Hisamitsu, Janssen Japan, Meiji Seika Pharma, Mitsubishi Tanabe Pharma, Otsuka, Shionogi, Shire Japan (now part of the Takeda group of companies), Sumitomo Dainippon Pharma, Taisho, and Yoshitomiyakuhin Corporation. MF and DO are employees of and own shares in Shionogi. HI has received honoraria and other payments from AbbVie GK, Eli Lilly Japan, Hisamitsu Pharmaceutical, Janssen Japan, Meiji Seika Pharma, Otsuka, Shionogi, and Taisho.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised: table 4 has been updated.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Iwanami, A., Saito, K., Fujiwara, M. et al. Safety and efficacy of guanfacine extended-release in adults with attention-deficit/hyperactivity disorder: an open-label, long-term, phase 3 extension study. BMC Psychiatry 20, 485 (2020). https://doi.org/10.1186/s12888-020-02867-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12888-020-02867-8