Abstract
Aim
To elaborate the underlying mechanisms by which IL-1β promote progression of Dry eye disease(DED) through effect on pyroptosis and apoptosis of corneal epithelial cells(CECs).
Methods
400 mOsM solutions were used to establish the DED model (hCECs- DED). RT-qPCR was performed to measure IL-1β mRNA and miR-146a-5p in CECs. Western blotting was performed to measure STAT3, GSDMD, NLRP3, and Caspase-1 levels. Cell counting kit-8 assay was adopted to check cell viability. Apoptosis was detected by flow cytometry. ELISAs were performed to determine IL-18, IL-33 and LDH. The luciferase test detects targeting relationships.
Results
After treatment with 400 mOsM solution, cell viability decreased and apoptosis increased. Compared with hCECs, IL-1β was increased and miR-146a-5p was decreased in hCECs-DED. At the same time, GSDMD, NLRP3, Caspase-1, IL-18, IL-33 and LDH were significantly higher in hCECs-DED than in hCECs, while IL-1β silencing reversed this effect. In addition, IL-1β negatively regulated miR-146a-5p. MiR-146a-5p mimics eliminated the inhibition of hCECs-DED pyroptosis and apoptosis caused by IL-1β silencing. At the same time, miR-146a-5p reduced STAT3 levels in hCECs.
Conclusion
Highly expressed IL-1β promoted pyroptosis and apoptosis of hCECs- DED through downregulated miR-146a-5p and inhibited STAT3.
Similar content being viewed by others
Introduction
Dry eye disease (DED) is very common and widespread around the world, with a prevalence of up to 50% [1, 2]. Clinical manifestations of DED are persistent irritation, and can be subdivided into aqueous-deficient DED and hyperevaporative DED. If untreated, it can lead to inflammatory damage to the cornea or conjunctiva, which can have an adverse impact on patients’ quality of life and work productivity. Due to damage to corneal epithelial cells (CECs), it is one of the most important events in the progression of DED [3], and there was a study found that pyroptosis of CECs plays a pivotal role in DED [4]. Therefore, the aim of this paper is to investigate the mechanism of pyroptosis of CECs in dry eyes.
DED is associated with inflammation of the ocular surface [5]. Studies have found that levels of inflammatory cytokines are mostly elevated in patients with DED [6]. As one of the most important pro-inflammatory cytokines, the role of interleukin-1 beta (IL-1β) in DED has attracted much attention of researchers [7, 8]. There was a report indicated that inhibiting IL-1β relieves DED by suppresses cular surface injury [9]. While the mechanism of IL-1β promote the progress of DED is not clear.
miRNAs are small noncoding RNAs that regulate various physiological events or diseases [10]. First discovered in the 1990s, the function of miRNAs in DED has been explored [11]. Among them, miR-146a-5p (previously miR-146a) kindled our interest. It is in the second exon of the human LOC285628 gene. It suppresses the immune system and inflammation. It has also been shown to be associated with the pathogenesis of DED [12]. Nowadays, increasing evidences indicated that the expression of some miRNAs can be regulated by IL-1β [13]. Furthermore, miR-146a-5p is also an IL-1β-responsive miRNA [14]. Here, we studied the function of IL-1β and miR-146a-5p in DED, and the underlying mechanism by which they affect DED.
In order to provides a potential therapeutic target for DED, in this study, the mechanism that IL-1β regulate pyroptosis and apoptosis of CECs through miR-146a-5p/STAT3 axis were explored.
Methods
Cell treatment
Human corneal epithelial cell line (hCECs, #6510, Sciencell, USA) from ATCC was maintained in DMEM with 10% FBS, EGF, insulin, and antibiotics at 37 °C. Hyperosmotic treatment was used to establish hyperosmotic DED model in vitro. In brief, NaCl was used to prepare 400 mOsM solution, then hCECs were treated for 24 h as hCECs-DED). At the same time, 312 mOsM NaCl was used as the control group (hCECs group).
Transfection
Empty vector (Vector), IL-1β silencing vector (si-IL-1β), miR-146a-5p inhibitor, miR-146a-5p mimics and STAT3 overexpression vector (OE-STAT3) were obtained from Beyotime and transfected into cell by using lipo2000. In this study, according to the experimental group needs, miR-146a-5p was overexpressed in hCECs-DED 1 h before 24 h IL-1β treatment.
RT-qPCR
RNA was collected using RNeasy Mini Kit (QIAGEN). For mRNA quantification, the TruScript Reverse Transcriptase (#NGB-54440, Norgen Biotek, Canada) was used to reverse the total RNA to cDNA. Subsequently, RT-qPCR were performed by using real-time PCR 7500 (#4351104, Thermofisher, Singapore). For miRNA quantification, the miRNA first strand Cdna synthesis kit (PC4801, Aidlab Biotechnologies, China) was used to reverse the total RNA to cDNA. Real-time quantitative PCR was performed using the miRNA-Real Time PCR Assay kit (PC4901, Aidlab Biotechnologies, China). The primers (5’-3’) were: IL-1β F: ACTGAGGACGTTCACCGTCTA, R GTGGGTGAATCTTAACTGGTT; miR-146a-5p F: CTGAGAACTGAATTCCATGGGTT, R GTGCAGGGTCCGAGGT; GAPDH F: TCCACGGTAAGCGGCATATGCTCT, R GCGCATTACCACGAACTCCATTCA; U6 F: CTTGCATCCGCATCAGA, R AATGCATCATGAAGTTCCGA. The internal controls were GADPH and U6. Gene fold change was calculated by 2−ΔΔCt [15].
Western blotting
Proteins were isolated with a commercial kit (Millipore), and quantified with a BCA kit (FuShen, Shanghai). Proteins in equal volumes were separated using 10% SDS-PAGE (Ybscience) and electrophoretically blotted to PVDF, #YB101123-1, Ybscience) membrane. The blots were performed with 1st antibodies listed as follow, anti-GSDMD (1:2000, #XGK103775, Xige, Shanghai, China), anti-NLRP3 (1:2000, #XGK99167, Xige), anti-Caspase-1 (1:2000, #XGKflu99500, Xige) and anti-GAPDH (1:3000, #XGK104170, Xige). After treated with secondary antibody (1:1000, #XG-X10933, Xige), respective target proteins of the antibodies were developed by using electrochemiluminescence (ECL, #abs920, Absin, Shanghai, China). The amount of target proteins calculated by gray scanning [16].
Cell counting kit-8 assay
Cell counting kit-8 assay (CCK-8, Takara Bio, China) was used to measure cell growth [17]. Cells were cultured in 96-well plates, CCK-8 (10µL) was provided to each well and kept for 2 h incubation, and OD450 was read with a microplate reader.
ELISA
Human interleukin 18 (IL-18) ELISA Kit (#CK-E10092, Sino best, Wuhan, China), human interleukin 33 (IL-33) ELISA Kit (#EH0198, Fine test, Wuhan, China) and human lactate dehydrogenase (LDH) ELISA Kit (#CK-E10891, Sino best) were used to measure the level of IL-18, IL-33 and LDH in each group.
Double luciferase reporter genes assay
Briefly, wild type/ mutant 3’UTR of STAT3 (STAT3-WT, STAT3-MUT) were ligated into vectors, and then co-transfected each kind of them with miR-146a-5p mimics into hCECs using lipo2000. Luciferase was quantified by Nano-Glo® Dual-Luciferase® System (Promega) 48 h later [18].
Apoptosis analysis
Apoptosis was detected using flow cytometry. After treatment of cells according to different groupings, cells were stained with the Annexin V/FITC Kit (BD Biosciences, USA) according to the kit instructions.
Statistical analysis
Experiments in this study were independently performed 3 times, and data was shown as means ± SD. Data was analyzed by SPSS 22.0, and figure was mapped by using GraphPad Prism 7. Differences were analyzed by Student’s t test or one-way ANOVA. P < 0.05 was defined statistically significant.
Results
DED causes pyroptosis of hCECs
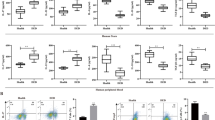
To establish the CEC DED model, hCECs were treated with 400 mOsM solutions and then labeled hCECs-DED. RT-qPCR results showed that compared with hCECs, expression IL-1β mRNA was increased in hCECs-DED (Fig. 1A), whereas miR-146a-5p were down-regulated in hCECs-DED (Fig. 1B). CCK-8 assay of cell viability revealed that treatment with 400 mOsM NaCl solution significantly inhibited cell viability (Fig. 1C). The same results were obtained by microscopic observation of cell numbers (Fig. 1D). Flow cytometry detection of apoptosis revealed a significant increase in apoptosis in the hCECs-DED group (Fig. 1E). Pyroptosis is characterized by Caspase-1-induced GSDMD-driven cell lysis and NLRP3 inflammasome activation. Therefore, we detected the pyroptosis-related proteins GSDMD, NRLP3 and Caspase-1 by Western blotting. The results showed that compared with the hCECs group, the expression of GSDMD, NRLP3 and Caspase-1 proteins in the hCECs-DED group was significantly increased (Fig. 1F). In addition, ELISA for IL-18, IL-33 and LDH concentrations showed a significant increase in IL-18 and IL-33 concentrations and LDH release in the hCECs-DED group (Fig. 1G). In summary, the above experiments showed that dry eyes increased the apoptosis of hCECs and promoted the expression of pyroptosis-related proteins.
DED causes pyroptosis of hCECs. A-B RT-qPCR to measure the expression of IL-1β and miR-146a-5p; C The viability was determined by CCK-8 assay; D Microscopic observation of cell morphology and numbe; E Apoptosis was detected by flow cytometry; F Western blot analysis to measure GSDMD, NLRP3 and Caspase-1. G IL-18, IL-33 and LDH were determined by ELISA Kit. Asterisks indicate statistical significance (*p < 0.05, **p < 0.01, ***p < 0.001)
si-IL-1β transfection inhibits pyroptosis and apoptosis of hCECs
To investigate whether si-IL-1β is associated with pyroptosis and apoptosis of hCECs, we transfected si-IL-1β in hCECs-DED. After transfected si-IL-1β into hCECs-DED, expression of IL-1β mRNA and protein were drastically declined in si-IL-1β group (Fig. 2A and B). Data also indicated that IL-1β silence elevated miR-146a-5p in hCECs-DED (Fig. 2C). Both CCK-8 and microscopic observation of cell numbers revealed significantly higher cell numbers in the si-IL-1β group than in the NC group (Fig. 2D and E). Cell apoptosis was detected by flow cytometry and the results showed that transfection with si-IL-1β significantly inhibited apoptosis of hCECs-DED (Fig. 2F). Similar results were obtained by Western blotting for pyroptosis-related proteins (Fig. 2G). Measurement of inflammatory factor (IL-18, IL-33) concentrations and LDH release revealed a decrease in inflammatory factor concentrations and relief of cellular damage (Fig. 2H). Therefore, the ability of IL-1β to affect pyroptosis and apoptosis of hCECs-DED.
si-IL-1β transfection inhibits pyroptosis of hCECs. A RT-qPCR to measure the expression of IL-1β; B Western blot to measure the expression of IL-1β; C RT-qPCR to measure the expression of miR-146a-5p; D The viability was determined by CCK-8 assay; E Microscopic observation of cell morphology and number; F Apoptosis was detected by flow cytometry; G Western blot analysis to measure GSDMD, NLRP3 and Caspase-1; H IL-18, IL-33 and LDH were determined by ELISA Kit. Asterisks indicate statistical significance (*p < 0.05, **p < 0.01, ***p < 0.001)
IL-1β effected on pyroptosis and apoptosis of hCECs-DED through miR-146a-5p
RT-qPCR data demonstrated that miR-146a-5p was markedly decreased in si-IL-1β and miR-146a-5p inhibitor group compared with si-IL-1β group (Fig. 3A). CCK-8 results suggested that compared with hCECs, cell viability memorably decreased in hCECs-DED, while IL-1β silence raised cell viability of hCECs-DED. However, cell viability of hCECs-DED in si-IL-1β + miR-146a-5p inhibitor treatment was suppressed compared to si-IL-1β treatment (Fig. 3B). Apoptosis results showed that miR-146a-5p inhibitor treatment significantly inhibited the effect of si-IL-1β (Fig. 3C). The same effect was found for Western blotting detection of pyroptosis-related proteins (Fig. 3D). ELISA results indicated that IL-18, IL-33 and LDH substantially increased in hCECs-DED group compared with hCECs, IL-1β silence decreased IL-18, IL-33 and LDH, but the levels of IL-18, IL-33 and LDH were higher in si-IL-1β + miR-146a-5p inhibitor treatment compared with si-IL-1β group (Fig. 3E). Thus, pyroptosis and apoptosis existed in hCECs-DED, IL-1β silence inhibited pyroptosis and apoptosis of hCECs-DED, while miR-146a-5p inhibitor abolished IL-1β silence caused suppression of hCECs-DED pyroptosis and apoptosis.
IL-1β effected on pyroptosis of hCECs-DED through miR-146a-5p. A RT-qPCR to measure the expression of miR-146a-5p; B The viability was determined by CCK-8 assay; C Apoptosis was detected by flow cytometry; D Western blot analysis to measure GSDMD, NLRP3 and Caspase-1. E IL-18, IL-33 and LDH were determined by ELISA Kit. Compared with hCECs, *p < 0.05, **p < 0.01; Compared with NC, #p < 0.05, ##p < 0.01; Compared with si-IL-1β, #p < 0.05, ##p < 0.01
Targeting relationship between miR-146a-5p and STAT3
The binding sites of miR-146a-5p in STAT3 were shown in Fig. 4A. To verify their relationship, miR-146a-5p mimics were introduced into hCECs (Fig. 4B). Luciferase assay results showed that miR-146a-5p mimics significantly inhibited STAT3-WT but not STAT3-MUT activity compared to hCECs-400 mOsM (Fig. 4C). Moreover, miR-146a-5p mimics dramatically suppressed STAT3 in hCECs (Fig. 4D). Therefore, miR-146a-5p inhibited STAT3 in hCECs.
Targeting relationship between miR-146a-5p and STAT3. A Binding sites of miR-146a-5p in STAT3; B RT-qPCR to measure the expression of miR-146a-5p; C Luciferase was quantified by Nano-Glo® Dual-Luciferase® System (Promega) 48 h later; D Western blots and quantification of STAT3. Asterisks indicate statistical significance (*p < 0.05, **p < 0.01, ***p < 0.001)
IL-1β/miR-146a-5p/STAT3 influenced pyroptosis and apoptosis of hCECs-DED
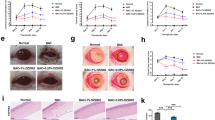
To verify that IL-1β can mediate miR-146a-5p/STAT3 to affect pyroptosis and apoptosis of hCECs-DED. Western blotting indicated that STAT3 was increased in hCECs-DED compared with hCECs, it was inhibited by miR-146a-5p overexpression, while this inhibition reversed by STAT3 overexpression or IL-1β treatment (Fig. 5A). Cell viability as measured by CCK-8 indicated that miR-146a-5p mimics promoted cell viability compared to hCECs-DED, and OE-STAT3 or si-IL-1β treatment inhibited this promotion (Fig. 5B). The results of flow cytometric detection of apoptosis were opposite to those of CCK-8 (Fig. 5C). Western blot detection of pyroptosis-related proteins revealed that miR-146a-5p mimics significantly inhibited the expression of pyroptosis-related proteins, while miR-146a-5p mimics + OE-STAT3 or miR-146a-5p mimics + si-IL-1β treatment was able to promote the expression of pyroptosis-related proteins compared with miR-146a-5p mimics (Fig. 5D). Meanwhile, trends in IL-18, IL-33 and LDH content were the same as those of pyroptosis-related proteins (Fig. 5E). Consequently, IL-1β promoted pyroptosis and apoptosis of hCECs-DED through downregulated miR-146a-5p and inhibited STAT3.
IL-1β/miR-146a-5p/STAT3 influenced pyroptosis of hCECs-400 mOsM. A Western blot analysis to measure STAT3; B The viability was determined by CCK-8 assay; C Apoptosis was detected by flow cytometry; D Western blot analysis to measure GSDMD, NLRP3 and Caspase-1; E IL-18, IL-33 and LDH were determined by ELISA Kit. Compared with hCECs, *p < 0.05, **p < 0.01; Compared with NC, #p < 0.05, ##p < 0.01; Compared with miR-146a-5p mimics, #p < 0.05, ##p < 0.01
Discussion
DED is a major ophthalmic disease worldwide, characterized by loss of tear film homeostasis leading to eye discomfort and even visual impairment, which seriously affects the quality of life of patients [19]. A growing number of studies suggest that chronic immune processes play a key role in the pathogenesis of DED, characterized by elevated levels of pro-inflammatory cytokines and inflammation, which further leads to disruption of the corneal epithelial barrier [20]. Tear hyperpermeability leads to symptoms of dry eye affecting epithelial cell function. The osmolality of normal subjects ranged from 290 to 330 mOsm / L, while the osmolality of dry eye patients ranged from 315 to 365 mOsm/L [21]. Studies have shown that the use of 350–500 mOsM hypertonic medium to construct hCECs-DED [22].In our study, 400 mOsM hypertonic medium modeling effect is better. At the same time, this study found that high osmotic pressure can cause pyroptosis and apoptosis of hCECs (Fig. 1).
Pyroptosis is also known as cellular inflammatory necrosis and is a programmed cell death mediated by caspase-1. Caspase-1 is instrumental in the process of transforming two proinflammatory cytokines, IL-1β and IL-18, into mature forms within the inflammasome pathway. Caspase-1 also cleaves the pore-forming protein GSDMD into its active form N-GSDMD, which triggers a cascade of events that culminates in plasma membrane rupture and intracellular content release, resulting in a localized or systemic inflammatory response [23]. IL-1β is a key mediator of the inflammatory response [24]. It is recognized that inflammation such as high levels of IL-1β, is an underlying cause of DED. On the other hand, pyroptosis also leads to an increase in the expression of IL-1β [25, 26]. There were many researches demonstrated that IL-1β was up-regulated in DED patients [9, 27, 28]. In addition to pyroptosis, caspase-1 is also involved in apoptosis in inflammatory situations [29]. Our results showed that IL-1β was highly expressed in DED model hCECs, and IL-1β silencing inhibited the thermal degeneration of DED model hCECs (Fig. 2). Therefore, high level of IL-1β in DED patients promoted pyroptosis and apoptosis of hCECs, and then pyroptosis hCECs released pro-inflammatory cytokines that further worsen the condition of DED. This process formed an “inflammatory vicious cycle” in DED.
miRNAs are involved in a variety of physiological processes in cells. miR-146a-5p is a key regulator of inflammatory responses. In addition, miR-146a-5p is associated with a variety of diseases, such as cancer [30], renal ischemia/reperfusion injury [31], intracranial aneurysms [32], sjögren’s syndrome [33]. One study found that miR-146a-5p expression was reduced in dry eye patients [34]. In the present study miR-146a-5p was also found to be significantly reduced in hCECs-DED. The use of miR-146a-5p mimics treatment can increase cell viability and inhibit the occurrence of inflammation (Fig. 3).
STAT3 was identified as a target gene of miR-146a-5p by bioinformatics analysis, while luciferase assay and protein blotting confirmed this [35]. Signal transducer and STAT3 is a latent cytoplasmic protein associated with inflammation and cellular pyroptosis [36, 37]. One of the pathogenic mechanisms of Yorkren syndrome is STAT3-mediated epithelial cell dysfunction [38]. In addition, recent studies have found that IL-1β regulates wound healing in the corneal epithelium via p16Ink4a-STAT3 signaling [39]. STAT3 inhibitor ameliorates dry eye symptoms in mice [40]. In this study, we found that miR-146a-5p significantly decreased STAT3 expression (Fig. 4). Overexpression of STAT3 significantly inhibited the effects of miR-146a-5p on the expression of inflammatory factors and the proliferation of hCECs and pyroptosis and apoptosis (Fig. 5).
Conclusion
In summary, this study found that IL-1β promotes pyroptosis and apoptosis in the DED model by downregulating miR-146a-5p and promoting STAT3. Our findings could provide a theoretical basis for the treatment of DED.
Data availability
All data is real and guarantee the validity of experimental results. If you need the results you can send an email (18987464191@163.com) to the corresponding authors.
References
Ræder S, Klyve P, Utheim TP. (2019). [Dry eye disease– diagnosis and treatment]. Tidsskrift for den Norske laegeforening: tidsskrift for praktisk medicin, ny raekke 139(11).
Markoulli M, Chandramohan N, Papas EB. (2021). Photobiomodulation (low-level light therapy) and dry eye disease. Clin Exp Optom 1–6.
Matsuda Y, Machida M, Nakagami Y, Nakajima T, Azuma M. NFE2L2 activator RS9 protects against corneal epithelial cell damage in dry eye models. PLoS ONE. 2020;15(4):e0229421.
Chen H, Gan X, Li Y, Gu J, Liu Y, Deng Y, Wang X, Hong Y, Hu Y, Su L, Chi W. NLRP12- and NLRC4-mediated corneal epithelial pyroptosis is driven by GSDMD cleavage accompanied by IL-33 processing in dry eye. Ocul Surf. 2020;18(4):783–94.
Fakih D, Zhao Z, Nicolle P, Reboussin E, Joubert F, Luzu J, Labbé A, Rostène W, Baudouin C, Mélik Parsadaniantz S, Réaux-Le Goazigo A. Chronic dry eye induced corneal hypersensitivity, neuroinflammatory responses, and synaptic plasticity in the mouse trigeminal brainstem. J Neuroinflamm. 2019;16(1):268.
Zhao H, Li Q, Ye M, Yu J. Tear Luminex Analysis in Dry Eye patients. Med Sci Monitor: Int Med J Experimental Clin Res. 2018;24:7595–602.
Akpek EK, Wu HY, Karakus S, Zhang Q, Masli S. Differential diagnosis of Sjögren Versus Non-Sjögren Dry Eye through tear Film biomarkers. Cornea. 2020;39(8):991–7.
Grosskreutz CL, Hockey HU, Serra D, Dryja TP. (2015). Dry Eye signs and symptoms persist during systemic neutralization of IL-1β by Canakinumab or IL-17A by Secukinumab. Cornea. 34(12):1551–6.
Chen Y, Zhang X, Yang L, Li M, Li B, Wang W, Sheng M. Decreased PPAR-γ expression in the conjunctiva and increased expression of TNF-α and IL-1β in the conjunctiva and tear fluid of dry eye mice. Mol Med Rep. 2014;9(5):2015–23.
Chen L, Heikkinen L, Wang C, Yang Y, Sun H, Wong G. Trends in the development of miRNA bioinformatics tools. Brief Bioinform. 2019;20(5):1836–52.
Rassi DM, De Paiva CS, Dias LC, Módulo CM, Adriano L, Fantucci MZ, Rocha EM. Review: MicroRNAS in ocular surface and dry eye diseases. Ocul Surf. 2017;15(4):660–9.
Xu WD, Lu MM, Pan HF, Ye D. Q.(2012). Association of MicroRNA-146a with autoimmune diseases. Inflammation. 35(4):1525–9.
Yang B, Ni J, Long H, Huang J, Yang C, Huang X. IL-1β-induced miR-34a up-regulation inhibits Cyr61 to modulate osteoarthritis chondrocyte proliferation through ADAMTS-4. J Cell Biochem. 2018;119(10):7959–70.
Sun Y, Zhou S, Shi Y, Zhou Y, Zhang Y, Liu K, Zhu Y, Han X. Inhibition of miR-153, an IL-1β-responsive miRNA, prevents beta cell failure and inflammation-associated diabetes. Metab Clin Exp. 2020;111:154335.
Wierzbicki PM, Klacz J, Kotulak-Chrzaszcz A, Wronska A, Stanislawowski M, Rybarczyk A, Ludziejewska A, Kmiec Z, Matuszewski M. Prognostic significance of VHL, HIF1A, HIF2A, VEGFA and p53 expression in patients with clear–cell renal cell carcinoma treated with sunitinib as first–line treatment. Int J Oncol. 2019;55(2):371–90.
Fang JQ, Ou Q, Pan J, Fang J, Zhang DY, Qiu MQ, Li YQ, Wang XH, Yang XY, Chi Z, Gao W, Guo JP, Miethke T. Pan J. P.(2021). TcpC inhibits toll-like receptor signaling pathway by serving as an E3 ubiquitin ligase that promotes degradation of myeloid differentiation factor 88. PLoS pathogens 17(3):e1009481.
Luo Q, Yang J, Xu H, Shi J, Liang Z, Zhang R, Lu P, Pu G, Zhao N, Zhang J. Sorafenib-loaded nanostructured lipid carriers for topical ocular therapy of corneal neovascularization: development, in-vitro and in vivo study. Drug Delivery. 2022;29(1):837–55.
Choi H, Kwon J, Cho MS, Sun Y, Zheng X, Wang J, Bouker KB, Casey JL, Atkins MB, Toretsky J, Han C. Targeting DDX3X triggers Antitumor Immunity via a dsRNA-Mediated tumor-intrinsic type I Interferon Response. Cancer Res. 2021;81(13):3607–20.
Craig J, Nichols K, Akpek E, Caffery B, Dua H, Joo C, Liu Z, Nelson J, Nichols J, Tsubota K, Stapleton F. (2017). TFOS DEWS II definition and classification report. Ocul Surf.15(3):276–83.
Yamaguchi T. Inflammatory response in Dry Eye. Investig Ophthalmol Vis Sci. 2018;59(14):Des192–9.
Nelson J, Farris R. Sodium hyaluronate and polyvinyl alcohol artificial tear preparations. A comparison in patients with keratoconjunctivitis sicca. Archives Ophthalmol (Chicago Ill: 1960). 1988;106(4):484–7.
López-Cano J, González-Cela-Casamayor M, Andrés-Guerrero V, Herrero-Vanrell R, Benítez-Del-Castillo J, Molina-Martínez I. Combined hyperosmolarity and inflammatory conditions in stressed human corneal epithelial cells and macrophages to evaluate osmoprotective agents as potential DED treatments. Exp Eye Res. 2021;211:108723.
You H, Wang L, Meng H, Huang C, Fang G, Li J. Pyroptosis: shedding light on the mechanisms and links with cancers. Front Immunol. 2023;14:1290885.
Lopez-Castejon G, Brough D. Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor Rev. 2011;22(4):189–95.
Kovacs SB, Miao EA. Gasdermins: effectors of Pyroptosis. Trends Cell Biol. 2017;27(9):673–84.
Xu YJ, Zheng L, Hu YW, Wang Q. Pyroptosis and its relationship to atherosclerosis. Clin Chim Acta. 2018;476:28–37.
Roda M, Corazza I, Bacchi Reggiani ML, Pellegrini M, Taroni L, Giannaccare G, Versura P. (2020). Dry Eye Disease and Tear Cytokine Levels-A Meta-Analysis. Int J Mol Sci. 21(9).
Dai Y, Zhang J, Xiang J, Li Y, Wu D, Xu J. Calcitriol inhibits ROS-NLRP3-IL-1β signaling axis via activation of Nrf2-antioxidant signaling in hyperosmotic stress stimulated human corneal epithelial cells. Redox Biol. 2019;21:101093.
Li J, Yang K, Pan X, Peng H, Hou C, Xiao J, Wang Q. Long noncoding RNA MIAT regulates hyperosmotic stress-Induced corneal epithelial cell Injury via inhibiting the caspase-1-Dependent pyroptosis and apoptosis in Dry Eye Disease. J Inflamm Res. 2022;15:3269–83.
Iacona JR, Lutz CS. miR-146a-5p: expression, regulation, and functions in cancer.Wiley. Interdisciplinary Reviews RNA. 2019;10(4):e1533.
Li X, Liao J, Su X, Li W, Bi Z, Wang J, Su Q, Huang H, Wei Y, Gao Y, Li J, Liu L, Wang C. Human urine-derived stem cells protect against renal ischemia/reperfusion injury in a rat model via exosomal miR-146a-5p which targets IRAK1. Theranostics. 2020;10(21):9561–78.
Zhang HL, Li L, Cheng CJ, Sun XC. Expression of miR-146a-5p in patients with intracranial aneurysms and its association with prognosis. Eur Rev Med Pharmacol Sci. 2018;22(3):726–30.
Sun HY, Lv AK, Yao H. (2017). Relationship of miRNA-146a to primary Sjögren’s syndrome and to systemic lupus erythematosus: a meta-analysis. Rheumatology International. 37(8):1311–6.
Wang X, Xin S, Wang Y, Ju D, Wu Q, Qiu Y, Niu X, Liu W, Li J, Ji P. (2021). MicroRNA-146a-5p enhances T helper 17 cell differentiation via decreasing a disintegrin and metalloprotease 17 level in primary sjögren’s syndrome. Bioengineered 12(1):310–24.
Jiang Z, Yin X, Wang M, Wang Y, Li F, Gao Y, Han G, Gao Z, Wang Z. β-Hydroxybutyrate alleviates pyroptosis in MPP/MPTP-induced Parkinson’s disease models via inhibiting STAT3/NLRP3/GSDMD pathway. Int Immunopharmacol. 2022;113:109451.
Jiang Z, Yin X, Wang M, Wang Y, Li F, Gao Y, Han G, Gao Z, Wang Z. (2022). β-Hydroxybutyrate alleviates pyroptosis in MPP+/MPTP-induced Parkinson’s disease models via inhibiting STAT3/NLRP3/GSDMD pathway. International Immunopharmacology 113(Pt B):109451.
Yao R, Chen Y, Hao H, Guo Z, Cheng X, Ma Y, Ji Q, Yang X, Wang Y, Li X, Wang Z. Pathogenic effects of inhibition of mTORC1/STAT3 axis facilitates Staphylococcus aureus-induced pyroptosis in human macrophages. Cell Communication Signaling: CCS. 2020;18(1):187.
Okuma A, Hoshino K, Ohba T, Fukushi S, Aiba S, Akira S, Ono M, Kaisho T, Muta T. Enhanced apoptosis by disruption of the STAT3-IκB-ζ signaling pathway in epithelial cells induces Sjögren’s syndrome-like. Autoimmune Disease Immun. 2013;38(3):450–60.
Wang X, Zhang S, Dong M, Li Y, Zhou Q, Yang L. The proinflammatory cytokines IL-1β and TNF-α modulate corneal epithelial wound healing through p16(Ink4a) suppressing STAT3 activity. J Cell Physiol. 2020;235(12):10081–93.
Qu M, Qi X, Wang Q, Wan L, Li J, Li W, Li Y, Zhou Q. Therapeutic effects of STAT3 inhibition on experimental murine Dry Eye. Investig Ophthalmol Vis Sci. 2019;60(12):3776–85.
Acknowledgements
Not applicable.
Funding
This study was supported by the Kangzefeng Expert Workstation of Yunnan Province (202105AF150044).
Author information
Authors and Affiliations
Contributions
Jian Liu designed and supervised the study. Xuejiao Li and Hua Peng performed the biochemical analyses, the western blot, and PCR analysis. Jian Liu, Jianshu Kang and Xiaomei Sun analyzed the data and wrote the paper. All authors have read and approved final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors have no conflicts of interest to declare.
Statement of ethics
Not applicable. There are no studies involving animals, humans or stem cells in this study.
Consent to publish
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, X., Peng, H., Kang, J. et al. IL-1β induced down-regulation of miR-146a-5p promoted pyroptosis and apoptosis of corneal epithelial cell in dry eye disease through targeting STAT3. BMC Ophthalmol 24, 144 (2024). https://doi.org/10.1186/s12886-024-03396-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12886-024-03396-8