Abstract
Background
Primary open-angle glaucoma (POAG) is a progressive neurodegenerative disease which leads to irreversible blindness. An elevated intraocular pressure (IOP) is considered to be the main risk factor for the disease progression. It is known that retinal blood flow is altered in POAG eyes. Tafluprost, a prostaglandin analogue which lowers the IOP, has shown to also improve the retinal blood flow in animals.
Methods
The current study therefore evaluated the retinal vessel density in the peripapillary and macular region of POAG patients with normal IOP treated with topical Tafluprost (n = 20) compared to surgically treated patients with normal IOP (n = 22) using optical coherence tomography angiography (OCT-A). The retinal flow density was obtained after binarisation and evaluated in five sectors.
Results
There was a significantly higher peripapillary flow density in all sectors in Tafluprost treated eyes when compared to post-surgery eyes. The flow density in the inferior sector of the superficial plexus in the macular region was also significantly higher in the Tafluprost group.
Conclusions: These results indicate that Tafluprost not only lowers IOP, but may also enhance retinal blood flow in POAG patients with a normal IOP.
Similar content being viewed by others
Background
Primary open-angle glaucoma (POAG) is a leading cause for blindness worldwide [1, 2]. POAG shows no symptoms in early stages. Instead, patients present a slow, progressive deterioration of nerve fibre layer and increasing visual field losses. Glaucoma often affects both eyes. The pathophysiology of this chronic, multifactorial and neurodegenerative disease remains unclear [1].
An elevated IOP is the most crucial risk factor for POAG as well as disease progression [1]; hence, current treatment options focus on lowering it. However, treating glaucomatous eyes is challenging and requires lifelong and thorough follow-up examinations. Within the therapeutic alternatives, surgical interventions aim to enable improved drainage of the aqueous humour through the implementation of a mechanical drain. Medicamentous treatment involves either reducing aqueous humour production or increasing drainage.
However, the regulation of IOP in POAG does not ensure the stabilization of this irreversible disease and deterioration in the patient’s visual field remains possible. In addition to the mechanical pathophysiological theory of elevated IOP, retinal blood flow is reduced in glaucoma [3]. The damage caused by decreased retinal blood flow might contribute to the development and deterioration of POAG [4, 5]. Vascular dysregulation and the resulting insufficient blood supply may lead to a progressive loss of nerve fibres.
Tafluprost, a selective fluoroprostaglandin (FP) receptor agonist from the group of prostaglandins - used as a topical medication to lower the IOP - appears to offer a potential method for improving retinal perfusion. Animal testing in cats showed increased blood flow in larger retinal vessels following topical application of Tafluprost [6]. In the experimental glaucomatous eyes of monkeys treated with Tafluprost, optic nerve head (ONH) circulation increased significantly after application [7]. Furthermore, Tafluprost improved the ONH blood flow in human preperimetric glaucomatous eyes with ONH impairment [8]. In a case series of 11 patients with treatment-naïve POAG eyes, Tafluprost showed an increased parafoveal retinal blood flow velocity after up to 12 weeks of topical application [9]. In addition to its IOP lowering effects, Tafluprost may offer the possibility of an enhanced retinal perfusion.
Recently, the use of Optical Coherence Tomography Angiography (OCT-A) has been the favoured approach to visualising blood flow changes within the retina. OCT-A offers the possibility of a non-invasive illustration of capillary retinal vessels using the differentiation between moving components and static tissue [10]. Erythrocyte movement allows for acquisition of retinal perfusion in different layers both axially and transversally [11]. In addition to blood flow, retinal structures can also be visualized [11]. Changes within the retinal vascularisation can be found in OCT-A, for example, abnormal vessel formations, missing perfusion or atypical existence of newly formed vessels [10]. Acquisition is performed through undilated pupils. An intravenous implementation of drugs, as used in fluorescence-angiography, is not needed. Though a visualization of leakage is not possible with OCT-A, its easy and user-friendly handling offers many new possible diagnostic options.
Examination of glaucomatous eyes with OCT-A has been a recent topic of interest. Existing studies show a significantly reduced retinal vessel density and blood flow in POAG eyes compared to healthy ones [12,13,14]. These perfusion differences even allow the discrimination of healthy and POAG eyes through only the vessel density of the ONH acquired with OCT-A [14]. The loss of macula vessel density within 1 year in POAG eyes was significantly faster than in healthy eyes [15]. Vascular changes detected by OCT-A in POAG eyes might either cause optic nerve damage or result from it [16]. An improvement of retinal perfusion might decelerate the progression of POAG. Therefore, medication supporting perfusion could help delay the loss of retinal nerve fibres and visual field. There have been reports indicating that Tafluprost increased blood flow. However, investigating the effect of Tafluprost on retinal blood flow is challenging as intra-patient comparisons of the retinal blood flow before and after the start of IOP-lowering medication could be confounded by the IOP-lowering effect. Therefore, the study intends to evaluate potential differences in retinal vessel density through OCT-A between Tafluprost-treated eyes of POAG patients having reached IOP values between 10 and 21 mmHg either by topical treatment with Tafluprost (n = 20) or after successful surgical treatment (n = 22).
Methods
This was a prospective, cross-sectional study performed at the Department of Ophthalmology of the University Medical Centre Hamburg-Eppendorf, after approval of the ethics review board of the medical association Hamburg (registered study number: PV5467) and following the recommendations of the Declaration of Helsinki. Written informed consent was obtained from each patient.
Study population
Patients with POAG were prospectively included between May 2017 and July 2018. Suitable patients were over the age of 18 years and diagnosed with POAG. Following the proposed definition by the World Glaucoma Association, POAG was defined by a progressive thinned retinal nerve fibre layer thickness (RNFLT) and progressive narrowed retinal rim without mandatory visual field defects [17].
Patients were either topically treated with Tafluprost or had undertaken a pressure-lowering surgery. Applicable surgical treatments were deep sclerectomy, trabeculotomy and trabeculectomy. It is important to note that these patients received surgery due to malcompliance or multiple eye drops intolerance. Therefore, there was only early glaucomatous damage in these selected groups. Patients with retinal pathologies, additional topical medication, inconsistent medication, cardiovascular diseases and poor image quality were excluded. The following data was collected from all patients: age, gender, IOP and standard automated perimetry (SAP). Visual field measurements were evaluated by dividing the illustrated results into three segments (superior, inferior and central) and documenting visual field loss within each segment (yes/no). Mean deviation (MD) was also obtained.
OCT-A device and scanning protocol
Both study groups received OCT and OCT-A imaging using the Topcon DRI OCT Triton (Topcon Corporation, Tokyo, Japan; Software Version 10.13.003.06). The scans were performed through undilated pupils. For each eye three images were collected: An OCT-A 6 × 6 mm scan field centring the fovea, an OCT-A 6 × 6 mm scan field centring the optic nerve, and an OCT scan (3D Horizontal Wide Scan) of the retinal nerve fibre layer thickness. To assure adequate scan quality and comparability, OCT-A scans required an image quality of ≥60 and had to meet the consensus criteria for retinal OCT quality assessment (OSCAR-IB) [18].
OCT-A image processing
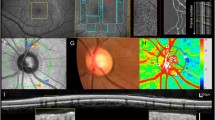
Blood flow analysis was obtained after binarisation. Pixel density was measured via an individual analysis with Matlab®. The scans of the superficial capillary plexus of the peripapillary region and the superficial and deep capillary plexus of the macular region were each divided into five sectors (according to the Early Treatment Diabetic Retinopathy Study (ETDR)): central, nasal, temporal, superior and inferior (central sector: 1 mm diameter; four sectors: 3.5 mm diameter. See Fig. 1a).
Peripapillary OCT-A flow density [%] for patients with glaucoma undergoing Tafluprost therapy and post-surgery in the optic nerve area. An exemplary en-face OCT-A scan (a), OCT scan image (b) and the respective vessel density (c) are shown. The red dotted line in the OCT scan (b) highlights the layers included in the vessel density analysis. The values of patients undergoing Tafluprost therapy are marked with “_T” and white bars; the post-surgery patients with “_S” and dotted bars. The individual sectors are shown (central, superior, temporal, inferior, nasal). Presented p-values were calculated using unpaired t-tests
Statistical analysis
The comparison of retinal blood flow between the groups was performed through the parametric t-test with Sidák correction [19]. (Statistical analysis compared the different pixel densities of both groups in the following vessel plexus: the superficial capillary plexus of the macula (from the inner limiting membrane (ILM) + 2.6 μm to the inner plexiform layer/inner nuclear layer (IPL/INL) + 15.6 μm), the deep capillary plexus of the macula (from the IPL/INL + 15.6 μm to the IPL/INL + 70.2 μm) and the superficial capillary plexus of the peripapillary region (from top of the image to the IML + 130 μm). Statistical analysis of age was performed using the Mann-Whitney test. Gender distribution was analysed using the chi-square test with Yates’ correction. SAP MD, defect location and average RNFLT were compared using the independent samples t-test.
Results
Study participants
Seventy-one eyes of 42 patients were enrolled in this study. Thirty-three eyes from 20 patients were treated with topical Tafluprost. The post-surgery group included 38 eyes from 22 patients.
Demographics and ocular characteristics
There were no significant differences concerning age, gender, IOP, SAP and average RNFLT between the groups (Table 1). The mean age was 65.9 ± 11.9 years in the Tafluprost group and 70.3 ± 6.6 years in the surgically treated group. The male/female ratio was 23/10 among the Tafluprost-treated patients and 27/11 in the operated group. The IOP was 14.2 ± 0.5 mmHg in the Tafluprost group and 13.6 ± 0.4 mmHg in the post-surgery patients. SAP MD was − 4.4 ± 6.8 dB in the Tafluprost group and − 5.3 ± 3.8 dB in the post-surgery patients.
Flow density
Flow density measured with OCT-A was significantly higher in Tafluprost-treated eyes than in post-surgery eyes.
In the optic nerve area, each sector showed a significantly higher flow density in eyes treated with Tafluprost (central 39.3 ± 11.8% versus 32.8 ± 5.9%, p = 0.04; superior 41.1 ± 9.4% versus 32.1 ± 7.4%, p = 0.001; temporal 32.1 ± 14.9% versus 20.9 ± 5.9%, p < 0.0001; inferior 42.6 ± 12.4% versus 30.4 ± 8.9%, p < 0.0001; nasal 23.8 ± 10.1% versus 15.7 ± 4.9%, p = 0.03; Table 2 and Fig. 1).
The flow density of the macular area was divided into the superficial and the deep retinal plexus: Flow density of the superficial retinal plexus was significantly higher within the inferior sector (26.6 ± 10.5% versus 19.7 ± 3.2%, p = 0.03; Table 2 and Fig. 2), while all other sectors showed no significant differences. The deep retinal plexus in the macular area showed no significant differences in flow density between the groups (Table 2 and Fig. 3).
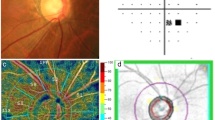
Macular superficial retinal plexus OCT-A flow density [%] for patients with glaucoma undergoing Tafluprost therapy and post-surgery in the optic nerve area. An exemplary en-face OCT-A scan (a), with OCT scan image (b) and the respective vessel density (c) are shown. The red dotted line in the OCT scan (b) highlights the layers included in the vessel density analysis. The values of patients undergoing the Tafluprost therapy are marked with “_T” and white bars; the post-surgery patients with “_S” and dotted bars. The individual sectors are shown (central, superior, temporal, inferior, nasal). Presented p-values were calculated using unpaired t-tests
Macular deep retinal plexus OCT-A flow density [%] for patients with glaucoma undergoing Tafluprost therapy and post-surgery in the optic nerve area. An exemplary en-face OCT-A scan (a), OCT scan image (b) and the respective vessel density (c) are shown. The red dotted line in the OCT scan (b) highlights the layers included in the vessel density analysis. The values of patients undergoing Tafluprost therapy are marked with “_T” and white bars; the post-surgery patients with “_S” and dotted bars. The individual sectors are shown (central, superior, temporal, inferior, nasal). Presented p-values were calculated using unpaired t-tests
Discussion
The present study found a significantly higher retinal flow density in all five sectors of the ONH area and in the inferior sector of the superficial retinal plexus in the macular region within Tafluprost-treated POAG eyes compared to non-treated post-surgery POAG eyes (both groups with IOP between 10 and 21 mmHg).
Tafluprost (AFP-168) is a synthesised Prostaglandin F2α analogue binding to the prostanoid FP receptor, which is present in human ocular tissues [20, 21]. This binding to FP receptors stimulates an increased uveoscleral outflow, which leads to an IOP-decrease [21]. Analysis in mice showed a dose-dependent effect with a saturating concentration [21]. With more than 10-fold higher affinity to FP receptors than latanoprost-acid, Tafluprost has also been shown to provide a longer lasting effect [6, 20, 21] and a greater night-time efficacy [22]. Tafluprost lowers IOP, at the latest, 4 hours after application and preserves its effect for at least 24 h [23]. In comparison to other prostanoid FP receptor agonists, Tafluprost demonstrated to induce less local side effects and may provide a more stable lowering of IOP [20, 24]. However, it may also lead to side effects similar to other prostaglandin analogues. These include trichiasis and pigmentation changes of the iris, periocular skin and eyelid [20]. In animal studies, Tafluprost has not only shown a vasodilatation on isolated rabbit arteries [25], but enhanced retinal blood velocity in cats [6], in experimental glaucoma in monkeys [7] and in humans [26].
In Glaucoma patients, ocular blood flow is altered [5, 27, 28]. It is not only mechanical pressure, but both reduced blood circulation and vascular dysregulations leading to reperfusion injury that may affect the incidence and progression of glaucoma [27]. Along with secondary influences, there are primary influences on disease development [5, 27]. For this reason, the retinal perfusion is of great interest in clinical glaucoma studies.
OCT-A offers a new diagnostic approach to image vascular changes in the retina. However, it must be mentioned that comparison between different devices and their vessel density measurements is challenging [29]. Nevertheless, within the collected data of POAG patients, there are consistent results concerning vascular changes when compared to healthy patients. Studies using OCT-A were able to show that glaucoma patients present with an impaired vessel density [12, 14, 30,31,32,33,34,35,36] as well as a significantly lower flow index [36, 37] and flow density [38] in the ONH area when compared to healthy subjects. In the macular area, an impaired vessel density [12, 15, 34, 39, 40] and flow density [38, 41] has also been shown in POAG patients when compared to healthy subjects. In mild to moderate glaucoma, Moghimi et al. [42] demonstrated an association between lower macular and lower ONH vessel density, as well as a faster rate of RNFL progression.
Our study aimed to investigate the potential effect of Tafluprost on flow density in POAG patients using OCT-A examination of both the optic nerve head and the macular region in a cross-sectional approach. Hee In et al. demonstrated that lowering the IOP could lead to an increased flow density correlated to the IOP reduction [43]. Therefore, an interventional approach to evaluate the effect of Tafluprost on flow density could be confounded by its IOP-lowering properties. Consequently, we compared the flow density between Tafluprost treated POAG patients and patients with POAG who had received IOP-lowering surgery due to malcompliance or multiple eye drops intolerance.
Our study was able to show a significantly higher flow density in Tafluprost treated eyes, especially within the peripapillary sectors and also in the inferior sector of the superficial retinal plexus of the macular region. Our results concerning the macular region were comparable with the findings of Iida et al. [9], who used adaptive optics scanner laser ophthalmoscopy to demonstrate an enhanced mean parafoveal blood flow velocity in a 12-week period after initiating topical Tafluprost treatment in POAG patients. Nevertheless, there was no significant difference in the mean blur rate in the tissue area of the ONH [9]. This is a discrepancy to our study, where significantly different results were observed - at the ONH in particular. Our findings with regard to the altered flow density of the superficial plexus in the macular region could be linked to visual field defects. A significant association between decreased vessel density and severity of visual field impairment has been shown [33]. Our study did not investigate the association between visual field impairment vessel density as any breakdown into a more specific analysis would not be reasonable considering the relatively small group size.
Our results show a higher flow density and therefore could insinuate an improved blood flow within the retina following from the topical application of Tafluprost. However, two issues must be emphasised: (1) The relevance of the flow density or retinal blood flow in terms of cause or effect for the development of POAG cannot be answered by this study. (2) Furthermore, a direct causal relationship between Tafluprost and flow density cannot be established due to the non-interventional approach of this study and the potential confounding factor of its IOP-lowering properties when used in an interventional study.
The strength of the present study is its status as the first investigating the connection between Tafluprost application in POAG patients and retinal blood flow using OCT-A in a cross-sectional approach. OCT-A is a widely spread, user-friendly, robust, reproducible and, recently, commonly used diagnostic approach in vascular retinal diseases [10, 44]. Both groups studied showed no significant differences concerning demographics and ocular characteristics, making comparison more meaningful. Further, our study followed international procedures and adhered to high quality standards to provide firm results. Patients were examined at least 4 weeks after the start of continual Tafluprost application and a minimum of 6 weeks after surgery to prevent distortion as a result of previously applied medication.
The weakness of the study is that no treatment-naïve flow density measurements were undertaken. It has been demonstrated that changes in IOP can alter flow density [45,46,47]; hence it would be challenging to differentiate the impact on the flow density induced by pressure differences rather than the chosen treatment. Furthermore, in patients with IOP-lowering surgery, a baseline measurement without treatment was not possible were on topical or systemic treatment before surgery as the refusal of therapy to glaucomatous eyes might be ethically difficult to argue nowadays. Therefore, our study included operated POAG eyes, as they carry similar vascular characteristics as well as a comparable IOP. Further, it has been demonstrated that flow density is significantly correlated with morphological and functional indices, and exhibits diagnostic capabilities comparable to currently employed clinical variables [48]. Consequently, we only included patients who underwent early surgery (e.g. due to multiple eye drops intolerance) to ensure that the disease was similarly advanced in both groups.
Conclusion
In conclusion, our results indicate that Tafluprost not only lowers IOP, but may also enhance retinal blood flow at a normal IOP. This effect might influence disease progression. A more accurate flow density could be estimated by applying statistical methods such as delta method incriminating all confusing factors (such as age, gender, intraocular pressure, spherical equivalent, physical activity, systemic diseases) [47]. Further studies are needed to investigate the long-term benefits of an improved flow density achieved via topical application of Tafluprost in POAG patients with normal IOP and whether different operations might have a varying impact on the flow density.
Availability of data and materials
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ETDR:
-
Early Treatment Diabetic Retinopathy Study
- FP:
-
Fluoroprostaglandin
- ILM:
-
Inner limiting membrane
- INL:
-
Inner nuclear layer
- IOP:
-
Intraocular pressure
- IPL:
-
Inner plexiform layer
- MD:
-
Mean deviation
- OCT-A:
-
Optical coherence tomography angiography
- ON:
-
Optic nerve head
- OSCAR-IB:
-
O: obvious problems; S: poor signal strength; C: centration of scan; A: algorithm failure; R: retinal pathology other than MS related; I: illumination; B: beam placement
- POAG:
-
Primary open-angle glaucoma
- RNFLT:
-
Retinal nerve fiber layer thickness
- SAP:
-
Standard automated perimetry
References
Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014;311(18):1901–11.
Quigley HA. Number of people with glaucoma worldwide. Br J Ophthalmol. 1996;80(5):389–93.
Chan KKW, Tang F, Tham CCY, Young AL, Cheung CY. Retinal vasculature in glaucoma: a review. BMJ Open Ophthalmol. 2017;1(1):e000032.
Triolo G, Rabiolo A, Shemonski ND, Fard A, Di Matteo F, Sacconi R, et al. Optical coherence tomography angiography macular and Peripapillary vessel perfusion density in healthy subjects, glaucoma suspects, and glaucoma patients. Invest Ophthalmol Vis Sci. 2017;58(13):5713–22.
Flammer J, Mozaffarieh M. What is the present pathogenetic concept of glaucomatous optic neuropathy? Surv Ophthalmol. 2007;52(Suppl 2):S162–73.
Izumi N, Nagaoka T, Sato E, Mori F, Takahashi A, Sogawa K, et al. Short-term effects of topical tafluprost on retinal blood flow in cats. J Ocul Pharmacol Ther. 2008;24(5):521–6.
Mayama C, Ishii K, Saeki T, Ota T, Tomidokoro A, Araie M. Effects of topical phenylephrine and tafluprost on optic nerve head circulation in monkeys with unilateral experimental glaucoma. Invest Ophthalmol Vis Sci. 2010;51(8):4117–24.
Aizawa N, Kunikata H, Shiga Y, Tsuda S, Yokoyama Y, Omodaka K, et al. Preperimetric glaucoma prospective observational study (PPGPS): design, baseline characteristics, and therapeutic effect of tafluprost in preperimetric glaucoma eye. PLoS One. 2017;12(12):e0188692.
Iida Y, Akagi T, Nakanishi H, Ohashi Ikeda H, Morooka S, Suda K, et al. Retinal blood flow velocity change in Parafoveal capillary after topical Tafluprost treatment in eyes with primary open-angle glaucoma. Sci Rep. 2017;7(1):5019.
Gao SS, Jia Y, Zhang M, Su JP, Liu G, Hwang TS, et al. Optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016;57(9):OCT27–36.
Mansouri K. Optical coherence tomography angiography and glaucoma: searching for the missing link. Expert Rev Med Devices. 2016;13(10):879–80.
Chen HS, Liu CH, Wu WC, Tseng HJ, Lee YS. Optical coherence tomography angiography of the superficial microvasculature in the macular and Peripapillary areas in glaucomatous and healthy eyes. Invest Ophthalmol Vis Sci. 2017;58(9):3637–45.
Mansoori T, Gamalapati J, Sivaswamy J, Balakrishna N. Optical coherence tomography angiography measured capillary density in the normal and glaucoma eyes. Saudi J Ophthalmol. 2018;32(4):295–302.
Lommatzsch C, Rothaus K, Koch JM, Heinz C, Grisanti S. Vessel density in OCT angiography permits differentiation between normal and glaucomatous optic nerve heads. Int J Ophthalmol. 2018;11(5):835–43.
Shoji T, Zangwill LM, Akagi T, Saunders LJ, Yarmohammadi A, Manalastas PIC, et al. Progressive macula vessel density loss in primary open-angle glaucoma: a longitudinal study. Am J Ophthalmol. 2017;182:107–17.
Wan KH, Leung CKS. Optical coherence tomography angiography in glaucoma: a mini-review. F1000Res. 2017;6:1686.
Weinreb RN, Garway-Heath DF, Leung C, Medeiros FA, Liebmann J. Diagnosis of primary open angle glaucoma: world glaucoma association consensus series 10: Kugler Publications; 2017. ISB: 978-90-6299-262-1. https://www.kuglerpublications.com/index.php?p=322&page=publication.
Tewarie P, Balk L, Costello F, Green A, Martin R, Schippling S, et al. The OSCAR-IB consensus criteria for retinal OCT quality assessment. PLoS One. 2012;7(4):e34823.
Sidak Z. Rectangular confidence regions for the means of multivariate Normal distributions. J Am Stat Assoc. 1967;62(318):626–33.
Takagi Y, Nakajima T, Shimazaki A, Kageyama M, Matsugi T, Matsumura Y, et al. Pharmacological characteristics of AFP-168 (tafluprost), a new prostanoid FP receptor agonist, as an ocular hypotensive drug. Exp Eye Res. 2004;78(4):767–76.
Ota T, Murata H, Sugimoto E, Aihara M, Araie M. Prostaglandin analogues and mouse intraocular pressure: effects of tafluprost, latanoprost, travoprost, and unoprostone, considering 24-hour variation. Invest Ophthalmol Vis Sci. 2005;46(6):2006–11.
Konstas AG, Boboridis KG, Kapis P, Marinopoulos K, Voudouragkaki IC, Panayiotou D, et al. 24-hour efficacy and ocular surface health with preservative-free Tafluprost alone and in conjunction with preservative-free Dorzolamide/Timolol fixed combination in open-angle glaucoma patients insufficiently controlled with preserved Latanoprost Monotherapy. Adv Ther. 2017;34(1):221–35.
Traverso CE, Ropo A, Papadia M, Uusitalo H. A phase II study on the duration and stability of the intraocular pressure-lowering effect and tolerability of Tafluprost compared with latanoprost. J Ocul Pharmacol Ther. 2010;26(1):97–104.
Sutton A, Gilvarry A, Ropo A. A comparative, placebo-controlled study of prostanoid fluoroprostaglandin-receptor agonists tafluprost and latanoprost in healthy males. J Ocul Pharmacol Ther. 2007;23(4):359–65.
Dong Y, Watabe H, Su G, Ishikawa H, Sato N, Yoshitomi T. Relaxing effect and mechanism of tafluprost on isolated rabbit ciliary arteries. Exp Eye Res. 2008;87(3):251–6.
Tsuda S, Yokoyama Y, Chiba N, Aizawa N, Shiga Y, Yasuda M, et al. Effect of topical tafluprost on optic nerve head blood flow in patients with myopic disc type. J Glaucoma. 2013;22(5):398–403.
Flammer J, Orgul S, Costa VP, Orzalesi N, Krieglstein GK, Serra LM, et al. The impact of ocular blood flow in glaucoma. Prog Retin Eye Res. 2002;21(4):359–93.
Grieshaber MC, Flammer J. Blood flow in glaucoma. Curr Opin Ophthalmol. 2005;16(2):79–83.
Wylegala A, Teper S, Dobrowolski D, Wylegala E. Optical coherence angiography: a review. Medicine (Baltimore). 2016;95(41):e4907.
Leveque PM, Zeboulon P, Brasnu E, Baudouin C, Labbe A. Optic disc vascularization in glaucoma: value of spectral-domain optical coherence tomography angiography. J Ophthalmol. 2016;2016:6956717.
Yarmohammadi A, Zangwill LM, Diniz-Filho A, Suh MH, Manalastas PI, Fatehee N, et al. Optical coherence tomography angiography vessel density in healthy, glaucoma suspect, and glaucoma eyes. Invest Ophthalmol Vis Sci. 2016;57(9):OCT451–9.
Mansoori T, Sivaswamy J, Gamalapati JS, Balakrishna N. Radial Peripapillary capillary density measurement using optical coherence tomography angiography in early glaucoma. J Glaucoma. 2017;26(5):438–43.
Yarmohammadi A, Zangwill LM, Diniz-Filho A, Suh MH, Yousefi S, Saunders LJ, et al. Relationship between optical coherence tomography angiography vessel density and severity of visual field loss in glaucoma. Ophthalmology. 2016;123(12):2498–508.
Rabiolo A, Gelormini F, Sacconi R, Cicinelli MV, Triolo G, Bettin P, et al. Comparison of methods to quantify macular and peripapillary vessel density in optical coherence tomography angiography. PLoS One. 2018;13(10):e0205773.
Manalastas PIC, Zangwill LM, Saunders LJ, Mansouri K, Belghith A, Suh MH, et al. Reproducibility of optical coherence tomography angiography macular and optic nerve head vascular density in glaucoma and healthy eyes. J Glaucoma. 2017;26(10):851–9.
Liu L, Jia Y, Takusagawa HL, Pechauer AD, Edmunds B, Lombardi L, et al. Optical coherence tomography angiography of the Peripapillary retina in glaucoma. JAMA Ophthalmol. 2015;133(9):1045–52.
Jia Y, Wei E, Wang X, Zhang X, Morrison JC, Parikh M, et al. Optical coherence tomography angiography of optic disc perfusion in glaucoma. Ophthalmology. 2014;121(7):1322–32.
Alnawaiseh M, Lahme L, Muller V, Rosentreter A, Eter N. Correlation of flow density, as measured using optical coherence tomography angiography, with structural and functional parameters in glaucoma patients. Graefes Arch Clin Exp Ophthalmol. 2018;256(3):589–97.
Penteado RC, Zangwill LM, Daga FB, Saunders LJ, Manalastas PIC, Shoji T, et al. Optical coherence tomography angiography macular vascular density measurements and the central 10-2 visual field in glaucoma. J Glaucoma. 2018;27(6):481–9.
Wan KH, Lam AKN, Leung CK. Optical coherence tomography angiography compared with optical coherence tomography macular measurements for detection of glaucoma. JAMA Ophthalmol. 2018;136(8):866–74.
Kromer R, Glusa P, Framme C, Pielen A, Junker B. Optical coherence tomography angiography analysis of macular flow density in glaucoma. Acta Ophthalmol. 2019;97(2):e199–206.
Moghimi S, Zangwill LM, Penteado RC, Hasenstab K, Ghahari E, Hou H, et al. Macular and optic nerve head vessel density and progressive retinal nerve fiber layer loss in glaucoma. Ophthalmology. 2018;125(11):1720–8.
In JH, Lee SY, Cho SH, Hong YJ. Peripapillary vessel density reversal after trabeculectomy in glaucoma. J Ophthalmol. 2018;2018:8909714.
Kashani AH, Chen CL, Gahm JK, Zheng F, Richter GM, Rosenfeld PJ, et al. Optical coherence tomography angiography: a comprehensive review of current methods and clinical applications. Prog Retin Eye Res. 2017;60:66–100.
Alnawaiseh M, Muller V, Lahme L, Merte RL, Eter N. Changes in flow density measured using optical coherence tomography angiography after iStent insertion in combination with phacoemulsification in patients with open-angle glaucoma. J Ophthalmol. 2018;2018:2890357.
Hollo G. Influence of large intraocular pressure reduction on Peripapillary OCT vessel density in ocular hypertensive and glaucoma eyes. J Glaucoma. 2017;26(1):e7–e10.
Brucher VC, Storp JJ, Eter N, Alnawaiseh M. Optical coherence tomography angiography-derived flow density: a review of the influencing factors. Graefes Arch Clin Exp Ophthalmol. 2020;258(4):701–10.
Geyman LS, Garg RA, Suwan Y, Trivedi V, Krawitz BD, Mo S, et al. Peripapillary perfused capillary density in primary open-angle glaucoma across disease stage: an optical coherence tomography angiography study. Br J Ophthalmol. 2017;101(9):1261–8.
Acknowledgments
Not applicable.
Funding
RK received funding (research support) as a donation from Santen. There was no influence on the conception and execution of the study. Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
HW made substantial contributions to the conception of the study, the acquisition, analysis and interpretation of data, and drafted the paper. MSS made substantial contributions to the conception of the study and substantively revised the paper. MS made substantial contributions to the conception of the study and substantively revised the paper. RK made substantial contributions to the conception of the study, the analysis and interpretation of data, and substantively revised the paper. The authors have approved the submitted version. All authors have agreed both to be personally accountable for the author’s own contributions and to ensure that questions related to the accuracy or integrity of any part of the work.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study design was approved by the appropriate ethics review board. Written informed consent was obtained from each patient.
Consent for publication
Consent for publication was obtained by each patient.
Competing interests
No further competing interests statement on behalf of all authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Weindler, H., Spitzer, M.S., Schultheiß, M. et al. OCT angiography analysis of retinal vessel density in primary open-angle glaucoma with and without Tafluprost therapy. BMC Ophthalmol 20, 444 (2020). https://doi.org/10.1186/s12886-020-01707-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12886-020-01707-3