Abstract
Background
The associations between fasting blood glucose and staging and overall survival of patients with pancreatic cancer are still controversial. This study aimed to investigate the association between fasting blood glucose levels and overall survival (OS) of patients with pancreatic cancer and to evaluate the impact of differentiation and staging of pancreatic cancer.
Methods
This was a retrospective study of patients with pathologically confirmed pancreatic cancer admitted to Shengjing Hospital of China Medical University between 01/2012 and 12/2016. The outcome was the OS. The factors associated with OS were examined using univariable and multivariable Cox and logistic regression analyses.
Results
A total of 253 patients were included. Preoperative blood glucose levels were not significantly associated with the OS of patients with pancreatic cancer (HR = 1.04, 95%CI: 0.78–1.40, P = 0.781). Only CA199 > 1000 was independently associated with OS (HR = 1.86, 95%CI: 1.15–3.02, P = 0.012). The median survival in the normal glucose group was 20.5 months (95% confidence interval (CI): 14.2–26.9). The median survival in the high glucose group was 14.2 months (95% CI: 9.7–18.6). There was no statistically significant difference between the two groups (P = 0.573). Multivariable logistic regression analyses were performed to determine if blood glucose levels influenced the 1- and 2-year OS. No significant association was observed for 1-year (OR = 1.27, 95%CI: 0.71–2.29, P = 0.418) or 2-year (HR = 1.37, 95%CI: 0.76–2.46, P = 0.296) OS.
Conclusions
Fasting blood glucose levels are not associated with the OS of patients with pancreatic adenocarcinoma.
Similar content being viewed by others
Background
Pancreatic adenocarcinoma (ductal and its variants) is a malignant exocrine tumor that is responsible for > 90% of all pancreatic cancers [1,2,3]. Most tumors (60–70%) occur in the head of the pancreas [1], and it most commonly affects patients > 55 years old [1,2,3]. The worldwide age-standardized annual incidence rate of pancreatic cancer is 5.5 per 100,000 men and 4 per 100,000 women [4, 5]. Pancreatic cancer is the tenth most common cancer in men and the ninth most common cancer in women [4, 5]. Major risk factors for pancreatic cancer include tobacco use, genetic predisposition and family history, obesity, chronic pancreatitis, and preexisting diabetes [1,2,3, 6]. The prognosis of pancreatic adenocarcinoma is very poor, with 5-year overall survival of < 10% [1,2,3].
Diabetes is both a risk factor and a complication of pancreatic cancer [1,2,3, 6]. Indeed, prediabetes and long-term diabetes are associated with an increased risk of pancreatic cancer [7, 8], and early-stage pancreatic cancer causes new-onset diabetes, but the pathogenesis is unclear. A systematic review also showed that elevated fasting blood glucose levels are associated with an increased risk of pancreatic cancer [9].
Diabetes is associated with a poor prognosis in patients with different solid tumors, such as head & neck, breast, liver, bladder, colorectal, and endometrial cancer [10,11,12]. High blood glucose levels are associated with the aggressiveness of colorectal cancer [13]. However, the association of fasting blood glucose with staging and overall survival (OS) of pancreatic cancer patients is still controversial [14, 15], and there are few studies in China or Asia that address this issue. The studies that explored the association between fasting blood glucose levels and the prognosis of pancreatic cancer have had inconsistent conclusions [14, 15]. These differences in results may be because of different populations with different genetic characteristics, cancer stage, or cancer differentiation. However, few studies have considered whether the differentiation and staging of pancreatic cancer influenced the results.
Therefore, the aim of the present study was to investigate the association between fasting blood glucose levels and OS of patients with pancreatic cancer and to evaluate the impact of differentiation and staging of pancreatic cancer on this association.
Methods
Study design and patients
This was a retrospective study of patients with pathologically confirmed pancreatic cancer admitted to Shengjing Hospital of China Medical University between January 2012 and December 2016. This study was approved by the Ethics Committee of Shengjing Hospital of China Medical University. Informed consent was waived because of the retrospective design.
The inclusion criteria were 1) > 18 years and 2) pathologically diagnosed with pancreatic cancer. The exclusion criteria were 1) severe primary diseases such as respiratory, cardiovascular, cerebrovascular, liver, kidney, or hematopoietic disease, psychosis, or long-term medication, or 2) incomplete clinical data.
Diagnostic criteria
The diagnosis and staging were made according to the NCCN guidelines using pancreatic computed tomography (CT) and/or magnetic resonance imaging (MRI) with contrast and a biopsy [6]. Pathological diagnosis and staging were made according to the WHO Classification of Tumours of the Digestive System (4th edition) [16], pancreatic adenocarcinoma included ductal adenocarcinoma and its variants: adenosquamous carcinoma, colloid carcinoma (mucinous non-cystic carcinoma), hepatoid carcinoma, medullary carcinoma, signet-ring carcinoma, undifferentiated carcinoma, and undifferentiated carcinoma with osteoclast-like giant cells.
Grouping and treatments
The patients were assigned to the normal blood glucose group and the hyperglycemia group according to a fasting blood glucose cutoff of 6.11 mmol/L. All patients were treated according to the current guidelines when they were diagnosed [6].
Data collection and outcomes
Demographic information (sex and age), current medical history (disease course, diameter of tumor, site of tumor, presence or absence of lymphatic metastasis, AJCC TNM 8 staging, and differentiation), medical history (hypertension, diabetes mellitus, smoking, and drinking), family history, pathological type, and surgical procedure were collected. Mortality was defined as death during the whole follow-up period. Perioperative complications and adverse reactions after surgical treatment were collected. OS was defined as the time from diagnosis to death from any cause.
Follow-up
Follow-up was conducted routinely by outpatient visits, hospitalization, and/or telephone. The follow-up information included death or survival and was extracted from the medical charts. Follow-up was censored on December 31st, 2019.
Statistical analysis
SPSS 22.0 (IBM, Armonk, NY, USA) was used for data analysis. The continuous data were first analyzed using the Kolmogorov-Smirnov test to determine whether they were normally distributed. The continuous variables conforming to the normal distribution are expressed as means ± standard deviation and were tested using the two-sample unpaired t-test; otherwise, they are presented as medians (minimum, maximum) and analyzed using the Mann-Whitney U test. Categorical data are presented as n (%) and were analyzed using the chi-square test or Fisher’s exact probability test. Univariable Cox regression was used to analyze the factors affecting OS. Variables with P < 0.05 or clinical significance were included in the multivariable Cox regression. Logistic regression was used to determine the variables independently associated with 1- and 2-year survival. Kaplan-Meier survival curves were plotted according to OS under different exposure factors and were compared using the log-rank test. Two-sided P-values < 0.05 were considered statistically significant.
Results
Characteristics of the patients
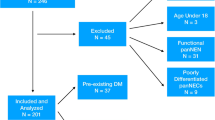
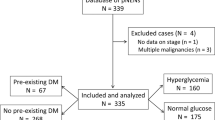
During the study period, 312 patients were admitted for pancreatic cancer, but 59 were excluded because of a lack of follow-up at the study hospital or missing blood glucose data. Therefore, 253 patients were included, of whom 208 were deceased, and 45 were alive or lost to follow-up. The demographic data are presented in Table 1.
Compared with the patients with normal glucose, the hyperglycemia group had a higher body mass index (BMI) (median, 23.5 vs. 22.3 kg/m2, P = 0.039), and higher proportions of females (49.4% vs. 28.7%, P = 0.002), diabetes (36.5% vs. 7.4%, P < 0.001), hypertension (32.1% vs. 20.2%, P = 0.042), allergies (16.4% vs. 7.4%, P = 0.042), and weight loss (66.7% vs. 53.8%, P = 0.043). Cancer differentiation degree and AJCC8 stage were not significantly different between the normal glucose and the hyperglycemia groups.
Factors associated with OS
In the univariable analyses, only CA199 and back pain were associated with OS. Cancer differentiation degree and AJCC8 stage were not identified as associated factors. Blood glucose was included in the Cox multivariable analysis along with CA199 and back pain. The results showed that preoperative blood glucose was not significantly associated with the OS of patients with pancreatic cancer (HR = 1.04, 95%CI: 0.78–1.40, P = 0.781). The glucose levels were tested as a continuous variable in the Cox model, and similar results were observed. Only CA199 > 1000 was independently associated with OS (HR = 1.86, 95%CI: 1.15–3.02, P = 0.012) (Table 2).
The relation between hyperglycemia and OS was also evaluated using a Kaplan-Meier analysis (Fig. 1). The median survival in the normal glucose group was 20.5 months (95% confidence interval (CI): 14.2–26.9). The median survival in the abnormal glucose group was 14.2 months (95% CI: 9.7–18.6). There was no statistically significant difference between the two groups (P = 0.573).
Kaplan-Meier survival curve. The median survival in the normal glucose group was 20.5 months (95% confidence interval (CI): 14.2–26.9). The median survival in the abnormal glucose group was 14.2 months (95% CI: 9.7–18.6). There was no statistically significant difference between the two groups (P = 0.573)
Univariable and multivariable logistic regression analyses were also performed for 1- and 2-year OS. No significant association was observed for glucose at 1-year OS (OR = 1.27, 95%CI: 0.71–2.29, P = 0.418); however, there was an association with CA199 > 1000 (OR = 3.68, 95%CI: 1.38–9.81, P = 0.009), moderate/high differentiation (OR = 0.51, 95%CI: 0.26–0.97, P = 0.039), and AJCC8 stage III-IV (OR = 2.51, 95%CI: 1.15–5.51, P = 0.021) (Table 3). There was also no significant association of glucose with 2-year OS (HR = 1.37, 95%CI: 0.76–2.46, P = 0.296); however, at this time point CA199 37–100 (OR = 2.46, 95%CI: 1.25–4.86, P = 0.009), CA199 > 1000 (OR = 3.33, 95%CI: 1.27–8.71, P = 0.014), and tail location (OR = 2.57, 95%CI: 1.19–5.53, P = 0.016) were significantly associated (Table 4).
Discussion
The association of fasting blood glucose with staging and overall survival of pancreatic cancer patients is still controversial [14, 15]. Therefore, this study aimed to investigate the association between fasting blood glucose levels and the OS of patients with pancreatic cancer and to evaluate the impact of differentiation and staging of pancreatic cancer. The results strongly suggest that fasting blood glucose levels are not associated with the OS of patients with pancreatic adenocarcinoma. Cancer differentiation degree and staging of pancreatic cancer did not influence the results for OS except at 1-year when they were both independently associated with OS.
In the present study, hyperglycemia and glucose levels were not associated with the OS of pancreatic cancer. This result suggests that pancreatic cancer is different to other solid tumors in general where hyperglycemia and diabetes are associated with prognosis [10,11,12]. The main mechanism involves insulin and insulin growth factor (IGF), which stimulate tumor growth [17,18,19]. The chronic inflammation state associated with hyperglycemia and diabetes also contributes to cancer progression [20]. However, the results of this study are supported by Dehayem et al. [21], who showed no association between diabetes and the prognosis of pancreatic cancer. Busaidy et al. [22] and Olowokure et al. [23] even showed that patients with pancreatic cancer and diabetes had a better OS than those without diabetes. On the other hand, some previous studies reported that hyperglycemia or diabetes was associated with poor survival of pancreatic cancer [24,25,26,27,28]. Those discrepancies and conflicting conclusions could be due to the sample size, ethnicity, and the fact that mortality can be due to diabetic complications rather than to cancer. In this study, we also considered that the degree of cancer differentiation or cancer stage might influence the results. So, we investigated whether these factors were associated with OS. The results suggest that for OS neither degree of differentiation nor cancer stage were associated factors. However, we did find that they were independently associated with 1-year OS. This might suggest that the results of other studies have been influenced by the differentiation degree or cancer stage of the patients, especially if the follow-up was only for 1 year. Larger case-control studies might be needed to fully evaluate the influence of these factors on OS.
The only factor in this study that was independently associated with the OS to pancreatic cancer was CA199. This is supported by previous studies that showed that CA199 levels are associated with the survival and response to chemotherapy in patients with pancreatic cancer [29,30,31].
This study has limitations. This was a cross-sectional study, which precludes the determination of causality relationships. In addition, the sample size was small and was from a single center, which could introduce bias. Because this was a retrospective study, only the information found in the charts could be analyzed.
Conclusions
In conclusion, fasting blood glucose levels are not associated with the OS of patients with pancreatic adenocarcinoma. Only the CA199 levels are associated with OS. The results need to be confirmed using large-scale studies.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Abbreviations
- OS:
-
Overall survival
- CI:
-
Confidence interval
- CT:
-
Computed tomography
- MRI:
-
Magnetic resonance imaging
- BMI:
-
Body mass index
- IGF:
-
Insulin growth factor
References
McGuigan A, Kelly P, Turkington RC, Jones C, Coleman HG, McCain RS. Pancreatic cancer: a review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol. 2018;24:4846–61.
Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet. 2016;388:73–85.
Kleeff J, Korc M, Apte M, La Vecchia C, Johnson CD, Biankin AV, et al. Pancreatic cancer. Nat Rev Dis Primers. 2016;2:16022.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30.
NCCN. Clinical practice guidelines in oncology (NCCN guidelines). Pancreatic adenocarcinoma. Version 1.2020. Fort Washington: National Comprehensive Cancer Network; 2019.
Huang Y, Cai X, Qiu M, Chen P, Tang H, Hu Y, et al. Prediabetes and the risk of cancer: a meta-analysis. Diabetologia. 2014;57:2261–9.
Song S, Wang B, Zhang X, Hao L, Hu X, Li Z, et al. Long-term diabetes mellitus is associated with an increased risk of pancreatic Cancer: a meta-analysis. PLoS One. 2015;10:e0134321.
Liao WC, Tu YK, Wu MS, Lin JT, Wang HP, Chien KL. Blood glucose concentration and risk of pancreatic cancer: systematic review and dose-response meta-analysis. BMJ. 2015;350:g7371.
Wu CH, Wu TY, Li CC, Lui MT, Chang KW, Kao SY. Impact of diabetes mellitus on the prognosis of patients with oral squamous cell carcinoma: a retrospective cohort study. Ann Surg Oncol. 2010;17:2175–83.
Li W, Zhang X, Sang H, Zhou Y, Shang C, Wang Y, et al. Effects of hyperglycemia on the progression of tumor diseases. J Exp Clin Cancer Res. 2019;38:327.
Ramteke P, Deb A, Shepal V, Bhat MK. Hyperglycemia Associated Metabolic and Molecular Alterations in Cancer Risk, Progression, Treatment, and Mortality. Cancers (Basel). 2019;11(9):1402.
Cui G, Zhang T, Ren F, Feng WM, Yao Y, Cui J, et al. High blood glucose levels correlate with tumor malignancy in colorectal Cancer patients. Med Sci Monit. 2015;21:3825–33.
Li D. Diabetes and pancreatic cancer. Mol Carcinog. 2012;51:64–74.
De Souza A, Irfan K, Masud F, Saif MW. Diabetes type 2 and pancreatic Cancer: a history unfolding. JOP. 2016;17:144–8.
Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO classification of Tumours of the digestive system (4th edition). Lyon: IARC Press; 2010.
Grimberg A. Mechanisms by which IGF-I may promote cancer. Cancer Biol Ther. 2003;2:630–5.
Denduluri SK, Idowu O, Wang Z, Liao Z, Yan Z, Mohammed MK, et al. Insulin-like growth factor (IGF) signaling in tumorigenesis and the development of cancer drug resistance. Genes Dis. 2015;2:13–25.
Bowers LW, Rossi EL, O'Flanagan CH, deGraffenried LA, Hursting SD. The Role of the Insulin/IGF System in Cancer: Lessons Learned from Clinical Trials and the Energy Balance-Cancer Link. Front Endocrinol (Lausanne). 2015;6:77.
Li Y, Bian X, Wei S, He M, Yang Y. The relationship between pancreatic cancer and type 2 diabetes: cause and consequence. Cancer Manag Res. 2019;11:8257–68.
Dehayem YM, Phelip JM, Kengne AP, Choukem SP, Benhamou PY, Halimi S. Impact of diabetes mellitus on clinical presentation and prognosis of pancreatic cancer. Ann Endocrinol (Paris). 2011;72:24–9.
Busaidy N, Yazbeck C, Shah P, Evans D, Li D, Geraci JM, et al. Survival of resectable pancreatic cancer patients with diabetes. J Clin Oncol. 2006;24:4098.
Olowokure O, Beg MS, Ali S, Tandra A, Safa M, Havlin K, et al. Is diabetes mellitus (DM) associated with worse outcomes in pancreatic cancer (PC)? J Clin Oncol. 2010;28:4114.
Chu CK, Mazo AE, Goodman M, Egnatashvili V, Sarmiento JM, Staley CA, et al. Preoperative diabetes mellitus and long-term survival after resection of pancreatic adenocarcinoma. Ann Surg Oncol. 2010;17:502–13.
Chu CK, Mazo AE, Sarmiento JM, Staley CA, Adsay NV, Umpierrez GE, et al. Impact of diabetes mellitus on perioperative outcomes after resection for pancreatic adenocarcinoma. J Am Coll Surg. 2010;210:463–73.
Cannon RM, LeGrand R, Chagpar RB, Ahmad SA, McClaine R, Kim HJ, et al. Multi-institutional analysis of pancreatic adenocarcinoma demonstrating the effect of diabetes status on survival after resection. HPB (Oxford). 2012;14:228–35.
Kang SP, Saif MW. Clinical outcome of pancreatic cancer patients with diabetes mellitus: is diabetes a poor prognostic factor? Highlights from the "2010 ASCO annual meeting". Chicago, IL, USA. June 4-8, 2010. JOP. 2010;11:334–5.
Shama MA, Tanaka M, Curley SA, Abbruzzese JL, Li D. Association of diabetes with perineural invasion and overall survival in surgically resected patients with pancreatic cancer. J Clin Oncol. 2010;28:4117.
Mattiucci GC, Morganti AG, Cellini F, Buwenge M, Casadei R, Farioli A, et al. Prognostic impact of Presurgical CA19-9 level in pancreatic adenocarcinoma: a pooled analysis. Transl Oncol. 2019;12:1–7.
Dong Q, Yang XH, Zhang Y, Jing W, Zheng LQ, Liu YP, et al. Elevated serum CA19-9 level is a promising predictor for poor prognosis in patients with resectable pancreatic ductal adenocarcinoma: a pilot study. World J Surg Oncol. 2014;12:171.
Yang GY, Malik NK, Chandrasekhar R, Ma WW, Flaherty L, Iyer R, et al. Change in CA 19-9 levels after chemoradiotherapy predicts survival in patients with locally advanced unresectable pancreatic cancer. J Gastrointest Oncol. 2013;4:361–9.
Acknowledgements
Not applicable.
Funding
This study was funded by the National Natural Science Foundation of China (Grant No. 81773108). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
ZMM and YXH were major contributors in organizing and writing the manuscript. HXR and KY performed the data collection and the follow-up works. XWF analyzed and interpreted the patient data regarding the blood glucose levels. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Shengjing Hospital of China Medical University. Informed consent was waived because of the retrospective design.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, M., Hu, X., Kang, Y. et al. Association between fasting blood glucose levels at admission and overall survival of patients with pancreatic cancer. BMC Cancer 21, 131 (2021). https://doi.org/10.1186/s12885-021-07859-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-021-07859-9