Abstract
Backgrounds
Durability of androgen-deprivation therapy (ADT) for prostate cancer (PC) is limited. Additional selective estrogen receptor modulators (SERMs) may prolong the durability of ADT, because androgen and estrogen signaling drive PC progression.
Methods
Men with treatment-naïve bone metastatic PC were randomly assigned in 1:1:1 fashion to receive ADT, toremifene 60 mg plus ADT (TOPADT), or raloxifene 60 mg plus ADT (RAPADT). The primary endpoint was the biochemical recurrence (BCR) rate, and secondary endpoints were changes of scores of the visual analogue scale (VAS) and the functional assessment of cancer therapy (FACT).
Results
A total of 15 men, 5 each, were allocated to one of the three treatment arms. The basal serum prostate-specific antigen (PSA) level was 198 ng/mL (median, range; 30–8428). Bone metastases were graded as 1 (n = 11), 2 (n = 3), and 3 (n = 1) by the extent of disease. During the median follow-up period of 1370 days (range; 431–1983), BCR occurred in 3, 0 and 2 men in ADT, TOPADT and RAPADT group, respectively. The 5-year BCR-free rate was 30, 100 and 53 %, in ADT, TOPADT and RAPADT group, respectively (p = 0.04, ADT v.s. TOPADT, p = 0.48, ADT v.s. RAPADT and p = 0.12, TOPADT v.s. RAPADT). Scores of VAS improved in all groups and remained stable throughout the study. This analysis is limited as a preliminary result in a single center.
Conclusions
Toremifene with conventional ADT significantly improved the BCR rate in treatment-naïve bone metastatic PC. Further clinical trials are warranted to confirm the promising clinical efficacy of this combination therapy.
Trial registration
The protocol was registered at the University Hospital Medical Information Network (UMIN ID;0,000,064,000) in Sep 25, 2011.
Similar content being viewed by others
Background
Based on the pioneering work by Huggins [1], androgen deprivation therapy (ADT) has been the primary treatment for advanced prostate cancer (PC). Unfortunately, most advanced cases of PC eventually become castration-resistant (CRPC), despite the continued use of ADT [2]. Novel therapies such as docetaxel, enzaltamide, abiraterone, cabazitaxel and sipuleucel-T [2–5] have been developed to treat CRPC. However, the development of agents that inhibit progression to CRPC may represent alternative therapeutic options for PC.
The results of recent studies have revealed growth regulation of PC via steroid nuclear receptors, which included not only the androgen receptor (AR) [6, 7] but also members of the estrogen receptor (ER) family [8, 9]. ERα and ERβcx (ERβ2) in particular have been implicated in PC progression and PC-related mortality, whereas ERβ inhibits tumor growth [8, 9]. In this regard, selective estrogen receptor modulators (SERMs) are expected to change the clinical course of PC. For example, toremifene, an ERα antagonist in the prostate [10], decreased the incidence of PC in men with high-grade prostatic intraepithelial neoplasia (HGPIN) [11, 12]. Furthermoere, raloxifene inhibited androgen-independent PC growth in 5 (28 %) of 13 patients [13]. However, SERMs have not been fully investigated for use in those with treatment-naïve PC. We hypothesized that additional SERMs may prolong the durability of ADT, because androgen and estrogen signaling drive PC progression. In the present study, we conducted a prospective randomized clinical phase IIA trial to investigate the effects of SERMs (toremifene and raloxifene) when added to ADT in treatment-naïve bone metastatic PC.
Methods
Participants
The inclusion criteria were men aged ≥20 years if they had histological confirmed adenocarcinoma of the prostate and radiologically proven bone metastasis with performance status 0, and adequate hepatic, hematological and renal function. Patients who had previous ADT or chemotherapy for PC, deep vein thrombosis, pulmonary embolism or antiphospholipid antibody syndrome were excluded. Bisphosphonate, warfarin, phenobarbital, rifampicin, phenitoin, ampicillin or cholestyramine was not allowed during the study.
Extent of diseases (EOD) of bone metastasis was graded by bone scintigraphy using technetium-99 m-methylene diphosphonate as follows: 0, normal or abnormal due to benign bone disease; 1, number of bony metastases <6, each of which was <50 % of the size of a vertebral body (one lesion approximately the size of a vertebral body would be counted as two lesions); 2, number of bone metastases between 6 and 20, size of lesions as previously described; 3, number of metastases ≥20 but less than a “super scan”, and 4, “super scan” or its equivalent, i.e., more than 75 % of the ribs, vertebrae and pelvic bones [14]. The Japan Cancer of the Prostate Risk Assessment (J-CAPRA) score (range; 0–12) was calculated on the basis of GS, PSA levels and clinical stage [15].
The protocol was approved by the ethical committee (Internal Review Board) at the University of Tokyo Hospital in August 2008 (approval number; P2008054) entitled preliminary study of selective estrogen modulators (SERMs) combined with maximum androgen blockade for metastatic prostate cancer (see Additional file 1: Table S1). And the study was also registered at the University Hospital Medical Information Network (UMIN ID; 0000064000). All patients provided written informed consent. An analysis was performed and reported to the Internal Review Board in the University of Tokyo Hospital every year.
Study design
Figure 1 shows the consolidated standards of reporting trials (CONSORT) flow diagram of recruited patients and follow-up. Eligible patients were randomly allocated in a 1:1:1 fashion to receive ADT alone, toremifene plus ADT (TOPADT) or raloxifene plus ADT (RAPADT). ADT consisted of castration [bilateral orchiectomy or luteinizing hormone-releasing hormone (LHRH) agonists] combined with 80 mg of bicalutamide. The LHRH agonist was administered throughout, whereas bicalutamide was changed to flutamide on biochemical recurrence after denying anti-androgen withdrawal syndrome. Toremifene (Orion Corporation, Finland) and raloxifene (Eli Lilly Japan K.K.) were given at a dose of 60 mg orally every day combined with aspirin 100 mg daily for prophylactic anti-coagulation.
After PC became hormone-refractory, administration of flutamide was switched to systemic chemotherapy which was performed every 3–4 weeks. If PC became both hormone refractory and chemotherapy-refractory, patients received best supportive care.
Study end points
Patients were monitored every month during the first year, and every 3 months thereafter. The primary endpoint was the BCR, which was defined as consecutive increase in serum PSA levels to above the patient’s PSA nadir [16]. Secondary endpoints included pain on a visual analogue scale (VAS) and functional assessment of cancer therapy (FACT) in every 3 months [17].
Immunohistochemical analysis
The immunohistochemical analyses for AR, ERα and ERβ were performed using the streptavidin-biotin amplification method and an EnVision + visualization kit (Dako, Carpinteria, CA, USA) as previously described [9]. The primary antibody against AR (1:40 dilution), ERα (1:40 dilution) and ERβ (1:200 dilution) was applied and incubated at room temperature for 1 h. The sections were then rinsed in phosphate-bufferes saline and incubated at room temperature with EnVision + for 1 h. The antigen-antibody complex was visualized with 3, 3′-diaminobenzidine (DAB) solution [1 mM DAB, 50 mM Tris–HCl buffer (pH 7.69, and 0.006 % H2O2]. The monoclonal antibodies for AR (AR441) and ERα (NCL-ER-6 F11) were purchased from Dako (Dako, Carpinteria, CA, USA) and Novo-castra Laboratories (Newcastle upon Tyne, UK), respectively. A polyclonal antibody specific for ERβ was raised in rabbits against peptides synthesized to correspond to the C-terminal region of ERβ (CSPAEDSKSKEGSQNPQSQ) [9].
Immunohistochemical assessment
The labeling index (LI) was determined by counting the percentage of cells with positive immunoreactivity per 1000 cells [18]. Two trained pathologists (TF and YY) independently evaluated the tissue sections, and the average LI was used. We defined positive immunoreactivity as showing moderate or strong immunoreactivity.
Statistical analyses
Correlations between age, pretreatment serum PSA levels, J-CAPRA score [15], and LI were evaluated using the Wilcoxon rank sum test. Associations between the group and clinical parameters including Gleason score (GS) and clinical stage were assessed using chi-square tests. BCR-free survival curves were plotted using the Kaplan-Meier method and verified using the log-rank test and univariate Cox proportional hazards regression models. JMP 11.0.0 software (SAS Institute, Cary, NC, USA) was used for all statistical analyses, and p < 0.05 was considered to indicate statistical significance.
Results
Patient characteristics
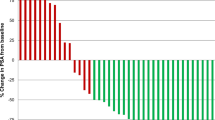
From August 14, 2008 to December 27, 2012, 15 patients were enrolled and randomly allocated to either of the three treatment groups as shown in Table 1. The median age was 74 years (range, 63–85). Pretreatment serum PSA levels were 30–8428 ng/mL (median, 198 ng/mL). The biopsy samples were evaluated by two pathologists as GS 7 (n = 3), 8 (n = 5), 9 (n = 5), or 10 (n = 2). The median J-CAPRA score was 9 (range, 6–11). There was no statistically significant difference in age, serum PSA level, stage, GS, EOD, J-CAPRA score or LI against the anti-AR, −ERα, and-ERβ antibodies among the three groups [ADT v.s. TOPADT and ADT v.s. RAPADT; Fig. 2].
Immunohistochemical staining for AR (a), ERα (b) and ERβ (c) in the tissue sections from the same area of a patient with PC. Strong (a) or moderate (b and c) staining was identified in the nuclei of cancer cells. The LI of AR (a), ERα (b) and ERβ (c) in cancer cells was 100, 35.4 and 26.4, respectively. Scale bar =100 μm
Primary endpoint
Table 2 shows the PSA response and outcome after ADT with or without SERMs. One patient discontinued toremifene becauseof a headache during the median follow-up period of 1370 days (range, 431–1983). Five (33 %) (2, 2, 1 in the three groups, respectively) patients achieved a PSA-nadir ≤0.01. At the end of the follow-up period, 5 (33 %) patients (3 in the ADT group and 2 in the RAPADT group) experienced BCR and were switched from bicaltamide to flutamide. One patient in the ADT group became hormone-refractory rapidly and died of PC on day 431 without chemotherapy. One patient in the TOPADT group died of gastric cancer without showing BCR on day 1371. The BCR-free survival rate was significantly higher in men treated with TOPADT than in those received ADT only (p = 0.04, ADT vs. TOPADT; p = 0.48, ADT vs. RAPADT;, and p = 0.12, TOPADT vs. RAPADT; Fig. 3).
Table 3 shows the results obtained from univariate Cox proportional hazards regression models for BCR associated with treatment and the clinicopathological characteristics of the patients, including age, serum PSA levels, J-CAPRA score and LI of AR, ERα and ERβ. TOPADT was only found to be significant in the univariate analysis (p = 0.023; hazard ratio, 1.1 e−9).
Hot flush was observed in all groups; ADT (n = 2), TOPADT (n = 3), and RAPADT (n = 3); although, all patients continued on therapy.
Secondary endpoint
The VAS scores were significantly decreased after treatment (p = 0.04, pre-treatment vs. 3 months thereafter), and showed no statistical differences among the three groups (Fig. 4a). Scores of physical well-being, social well-being, emotional well-being, functional well-being, as well as PC subscale scores of FACT questionnaire, were stable during the follow-up period and not statistically different among the three groups (Fig. 4b–f).
The change in VAS scores following treatment for PC (a). The VAS scores were significantly decreased after the treatment (p = 0.04, pre treatment vs. 3, 6, 9 and 12 months of treatment). Statistically significant differences were not detected among the three groups. The FACT of before treatment and after 3, 6, 9 and 12 months of treatment. Physical well-being (PWB; b), social well-being (SWB; c), emotional well-being (EWB; d), functional well-being (FWB; e) and prostate cancer (PC) scale scores (f) were stable during the follow-up period. Statistically significant differences were not detected among the three groups in PWB (p = 0.5, pre treatment v.s. 3, 6, 9 and 12 months of treatment), SWB (p = 0.5, pretreatment vs. 3, 6, 9 and 12 months of treatment), EWB (p = 0.75, pre treatment v.s. 3, 6, 9 and 12 months of treatment), FWB (p = 0. 5, pretreatment vs. 3, 6, 9 and 12 months of treatment), and PC sub scale (p = 0.25, pretreatment v.s. 3, 6, 9 and 12 months of treatment)
Discussion
The most common initial therapy for metastatic PC is ADT; however, the durability of ADT is limited and affected by various factors including pretreatment PSA level, GS, tumor stage and PSA nadir [19]. The durability of ADT is also influenced by the ER status of the tumor [18, 20].
In fact, estrogens were initially used as one of the earliest forms of treatment agents; however, they were associated with thromboembolic and cardiovascular side effects [21]. SERMs are synthetic estrogen ligands that can exhibit either estrogenic or anti-estrogenic effects depending on tissue types [22]. Toremifene significantly reduced the incidence of PC in a transgenic adenocarcinoma mouse prostate model [23], as well as in men with HGPIN [11]. In addition, toremifene increased the bone mineral density of the hip and spine [24] and improved lipid profiles in men receiving ADT for PC [25]. Raloxifene, which acts as an ER agonist in the bone tissue [26], has been developed for the treatment of osteoporosis in women [27] and showed some tumor-inhibitory effects in CRPC in a pilot study [13]. To date, the anti-cancer effects of these SERMs have not yet been fully investigated in treatment-naïve PC patients. We hypothesized that concurrent use of SERMs would prolong the duration of efficacy of ADT in men with bone metastatic PC.
Currently, we have demonstrated that TOPADT significantly improved the biochemical recurrence rate in men with bone metastatic PC compared with men treated with ADT alone. The results of a recent study showed that the 5-year BCR-free rate was 30 % in men who received ADT plus docetaxel with median serum PSA levels of 26.7 (range, 5.0–106) [5]. In our study, similar rates were noted for men treated with ADT alone (30 %). Surprisingly, the 5-year BCR-free rate in the TOPADT group was 100 %.
Theoretically, the tumor inhibitory effects of toremifene would be mediated via the suppression of ERα-related signals [28, 10]. ERα expression in PC cells was confirmed by immunohistochemistry and quantitative reverse transcription polymerase chain reaction analyses [8, 9, 18]. The mRNA expression of ERα was much lower than AR in PC cells (1:100 ratio); however, ERα expression in cancer-associated stromal cells was significantly related to cancer-specific survival in men with bone metastatic PC [18]. ERα expression was negatively correlated with survival after radical prostatectomy in locally advanced PC [29]. Additionally, ERα promoted proliferation by regulating MYC expression and glucose sensitivity in phosphatase and tensin homolog (PTEN)-deficient mouse PC cells [8]. Conversely, depletion of ERα inhibited growth in PTEN-deficient mice via a reduction in MYC protein and alteration of glucose sensitivity [8]. The results of present study demonstrated that toremifene significantly improved the durability of ADT, suggesting blockade of ERα signaling as a potential target for advanced PC.
ERβ signaling has been associated with a tumor-inhibitory effect in PC through both the classical (ERβ and estrogen-response element complex) and non-classical pathways (ERβ, Krüppel-like zinc finger transcription factor 5, and adenosine 3′,5′-monophosphate response element-binding protein-binding protein complex) [30]. ERβ modulators are expected to inhibit PC growth. Raloxifene exhibits diverse activities via ER depending on whether ERα or ERβ is expressed in the target organ [26]. The results of the present study did not prove a distinct tumor-inhibitory effect mediated by RAPADT as compared to ADT alone. The difference in the reason tumor inhibitory effect between TOPADT and RAPADT may have been attributed to the potency of the drugs and the pattern of ER expression in PC cells. The tumor-inhibitory effect of fulvestrant, another ERβ modulator, was limited because the median time to progression was only 4.3 months in men with CRPC treated with fulvestrant [31]. Further investigations of additional ERβ modulators are warranted with respect to their potential role in the inhibition of human PC.
The known adverse events associated with the use of SERMs include hot flushes, sweating, nausea, dizziness, edema, vomiting and thrombosis [27]. In the present study, two men in the ADT group, two men in the TOPADT group, and three men in the RAPADT group complained of mild hot flushes; however, no medical intervention was deemed necessary. Only one man in the TOPADT group discontinued toremifene administration because of a headache. No events of liver dysfunction or thrombosis were observed.
The present study was not without limitations. The sample size was small, and the cohort was limited to a single institution in an all Asian population. A multicenter external validation study would be necessary to further elucidate the additional effect of toremifene on ADT that we found in patients with advanced PC.
Conclusions
Results from the present study we demonstrated the good clinical efficacy and tolerability of TOPADT in patients with treatment-naïve bone metastatic PC. Additional clinical trials with larger cohorts are warranted to confirm our promising phase IIA results.
Abbreviations
- ADT:
-
androgen-deprivation therapy
- AR:
-
androgen receptor
- BCR:
-
biochemical recurrence
- CONSORT:
-
consolidated standards of reporting trials
- EOD:
-
extent of disease
- ER:
-
estrogen receptor
- FACT:
-
functional assessment of cancer therapy
- J-CAPRA:
-
Japan Cancer of the Prostate Risk Assessment
- LI:
-
Labeling index
- PC:
-
prostate cancer
- PSA:
-
prostate-specific antigen
- PTEN:
-
phosphatase and tensin homolog
- RAPADT:
-
raloxifene plus ADT
- SERM:
-
selective estrogen receptor modulator
- TOPADT:
-
toremifene plus ADT
- VAS:
-
visual analogue scale
References
Huggins C, Hodges CV. Studies on prostatic cancer. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1941;1:293–7.
Cookson MS, Lowrance WT, Murad MH, Kibel AS. American Urological Association. Castration-resistant prostate cancer: AUA guideline amendment. J Urol. 2015;193:491–9.
Tannock IF, de Witt R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–12.
Petrylak DP, Tangen CM, Hussain MH, Lara PN Jr, Jones JA, Taplin ME, et al. compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–20.
Gravis G, Fizazi K, Joly F, Oudard S, Priou F, Esterni B, et al. Androgen-deprivation therapy alone or with or with docetaxel in non-castrate metastatic prostate cancer (GETUG-AFU15): a randomized, open-label, phase 3 trial. Lancet Oncol 2013;14:149–158.
Tammela T. Endocrine treatment of prostate cancer. J Steroid Biochem Mol Biol 2004;92:287–295.
Culig Z, Steiner H, Bartsch G, Hobisch A. Mechanism of endocrine therapy-responsive and –unresponsive prostate tumors. Endocr Relat Cancer. 2005;12:229–244.
Takizawa I, Lawrence MG, Balanathan P, Robello R, Pearson HB, Garg E, et al. Estrogen receptor alpha drives proliferation in PTEN-deficient prostate carcinoma by stimulating survival signaling, MYC expression and altering glucose sensitivity. Oncotarget 2014;6:604–616.
Fujimura T, Takahashi S, Urano T, Ogawa S, Ouchi Y, Kitamura T, et al. Differential expression of estrogen receptor beta (ERbeta) and its C-terminal truncated splice variant ERbetacx as prognostic predictors in human prostatic cancer. Biochem Biophys Res Commun 2001;289:692–699.
Taneja S, Smith MR, Dalton JT, Raghow S, Barnette G, Steiner M, et al. Toremifene-a promising therapy for the prevention of prostate cancer and complications of androgen deprivation therapy. Expert Opin Investig Drugs 2006;15:293–305.
Price D, Stein B, Sieber P, Tutrone R, Bailen J, Goluboff E, et al. Toremifene for the prevention of prostate cancer in men with high grade prostatic intraepithelial neoplasia: results of a double-blind, placebo controlled, phase IIB trial. J Urol 2006;176:965–971.
Riggs BL, Hartman LC. Selective estrogen-receptor modulators-mechanisms of action and application to clinical practice. N Engl J Med 2003; 348: 618–629.
Shazer RL, Jain A, Galkin AV, Cinman N, Nguyen KN, Natale RB, et al. Raloxifene, an oestrogen-receptor-β targeted therapy, inhibits androgen-independent prostate cancer growth: results from preclinical studies and a pilot phase II clinical trial. BJU Int 2006;97:691–697.
Soloway MS, Hardeman SW, Hickey D, Raymond J, Todd B, Soloway S, et al. Stratification of patients with metastatic prostate cancer based on disease on initial bone scan. Cancer 1988;61;195-202.
Cooperberg MR, Hinotsu S, Namiki M, Ito K, Broering J, Carroll PR, et al. Risk assessment among prostate cancer patients receiving primary androgen deprivation therapy. J Clin Oncol 2009;27:4306–4313.
Mottet N, Bellmunt J, Bolla M, Joniau S, Mason M, Matveev V, et al. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2011;59:572–583.
Hinotsu A, Niimi M, Akaza H, et al. Development of Japanese version of QOL questionnaire for bladder and prostate cancer patients using FACT-Bl and P: Pilot study. Gan To Kagaku Ryoho 1999;26:657–666 (In Japanese).
Fujimura T, Takahashi S, Urano T, Takayama K, Sugihara T, Obinata D, et al. Expression of androgen and estrogen signaling components and stem cell markers to predict cancer progression and cancer-specific survival in patients with metastatic prostate cancer. Clin Cancer Res 2014;20:4625–4635.
Hussain M, Tangen CM, Higano C, Schelhammer PF, Faulkner J, Crawford ED, et al. Absolute prostate-specific antigen value after androgen deprivation is a strong independent predictor of survival in new metastatic prostate cancer: data from southwest oncology group trial 9346 (INT-0162). J Clin Oncol. 2006;24:3984–90.
Kawashima H, Nakatani T. Involvement of estrogen receptors in prostatic tissues. Int J Urol. 2012;19:512–22.
Turo R, Smolski M, Esler R, Kujawa ML, Bromage SJ, Oakley N, et al. Diethylstilboestrol for the treatment of prostate cancer: past, present and future. Scand J Urol. 2014;48:4–14.
Lonard DM, Smith CL. Molecular perspectives on selective estrogen receptors (SERMs): progress in understanding their tissue-specific agonist and antagonist actions. Steroids. 2002;67:15–24.
Raghow S, Hooshdaran MZ, Katiyar S, Steiner MS. Toremifene prevents prostate cancer in transgenic adenocarcinoma of mouse prostate model. Cancer Res. 2002;62:1370–6.
Smith MR, Malkowicz SB, Chu F, Forrest J, Price D, Sieber P, et al. Toremifene increase bone mineral density in men receiving androgen deprivation therapy for prostate cancer: Interim analysis of multicenter phase 3 clinical study. J Urol. 2008;179:152–5.
Smith MR, Malkowicz SB, Chu F, Forrest J, Sieber P, Barnette KG, et al. Toremifene improves lipid profiles in men receiving androgen deprivation therapy for prostate cancer: Interim analysis of multicenter phase 3 clinical study. J Clin Oncol. 2008;26:1824–9.
Dutertre M, Smith CL. Molecular mechanisms of selective estrogen receptors (SERM) action. J Pharmacol Exp Ther. 2000;295:431–7.
Draper MW, Flowers DE, Huster WJ, Neild JA, Harper KD, Arnaud C. A controlled trial of raloxifene (LY139481) HCI: impact on bone turnover and serum lipid profile in healthy postmenopausal women. J Bone Miner Res. 1996;11:835–42.
Kangas L. Review of the pharmacological properties of toremifene. J Steroid Biochem. 1990;36:191–5.
Megas G, Chrisofos M, Anastasiou I, Tsitlidou A, Choreftaki T, Deliveliotis C Estrogen receptor (α and β) but not androgen receptor expression is correlated with recurrence, progression and survival in post prostatectomy T3N0M0 locally advanced prostate cancer. Asian J Androl. 2015;17:98–105.
Nakajima Y, Akaogi K, Suzuki T, Osakabe A, Yamaguchi C, Sunahara N, et al. Estrogen regulates tumor growth through a nonclassical pathway that includes the transcription factors ERβ and KLF5. Sci Signal. 2011;4:1–12.
Chadaha MK, Ashraf U, Lawrence D, Tian L, Levine E, Silliman C, et al. Phase II study of fulvestrant (Faslodex) in castration resistant prostate cancer. Prostate. 2008;68:1461–6.
Acknowledgements
This work was supported by Grants from Yamaguchi Endocrine Research Foundation and by Grants from University of Tokyo Hospital. The sponsors of the study had no role in the study design, data collection, data analysis, data interpretation or writing the draft. Raw data was the property of the University of Tokyo. All authors had final responsibility for the decision to submit for publication.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare no conflict of interest.
Authors’ contributions
TF conceived of the study and wrote the draft. ST, SI and YH revised the draft. HK, YY, MS, HF and TN contributed to data collection and analysis. TU and KT contributed to data interpretation and analysis. All authors approved the final version.
Additional file
Additional file 1: Table S1.
CONSORT 2010 checklist of information to include when reporting a cluster randomised trial. (DOCX 30 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Fujimura, T., Takahashi, S., Kume, H. et al. Toremifene, a selective estrogen receptor modulator, significantly improved biochemical recurrence in bone metastatic prostate cancer: a randomized controlled phase II a trial. BMC Cancer 15, 836 (2015). https://doi.org/10.1186/s12885-015-1871-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-015-1871-z