Abstract
Background
Patients with chronic kidney disease have a markedly increased cardiovascular mortality compared with the general population. Long chain n-3 polyunsaturated fatty acids have been suggested to possess cardioprotective properties. This cross-sectional and comparative study evaluated correlations between hemodynamic measurements, resistance artery function and fish consumption to the content of long chain n-3 polyunsaturated fatty acids in adipose tissue, a long-term marker of seafood intake.
Methods
Seventeen patients with chronic kidney disease stage 5 + 5d and 27 healthy kidney donors were evaluated with hemodynamic measurements before surgery; from these subjects, 11 patients and 11 healthy subjects had vasodilator properties of subcutaneous resistance arteries examined. The measurements were correlated to adipose tissue n-3 polyunsaturated fatty acids. Information on fish intake was obtained from a dietary questionnaire and compared with adipose tissue n-3 polyunsaturated fatty acids.
Results
Fish intake and the content of n-3 polyunsaturated fatty acids in adipose tissue did not differ between patients and controls. n-3 polyunsaturated fatty acid levels in adipose tissue were positively correlated to systemic vascular resistance index; (r = 0.44; p = 0.07 and r = 0.62; p < 0.05, chronic kidney disease and healthy subjects respectively) and negatively correlated to cardiac output index (r = −0.69; p < 0.01 and r = −0.50; p < 0.05, chronic kidney disease and healthy subjects respectively). No correlation was observed between n-3 polyunsaturated fatty acid levels in adipose tissue and vasodilator properties in resistance arteries. n-3 PUFA content in adipose tissue increased with increasing self-reported fish intake.
Conclusions
The correlations found, suggest a role for n-3 polyunsaturated fatty acids in hemodynamic properties. However, this is apparently not due to changes in intrinsic properties of the resistance arteries as no correlation was found to n-3 polyunsaturated fatty acids.
Similar content being viewed by others
Background
Patients with chronic kidney disease (CKD) have a high risk of cardiovascular disease (CVD) compared with the general population and with declining renal function, the risk of CVD increases [1]. In the general population, cardiovascular mortality has been reduced substantially during the past years, but in patients with CKD the high mortality from CVD remains unchanged [2]. The mechanisms responsible for the accelerated atherosclerosis in CKD are somewhat distinct from patients with classic CVD with intima lesions. They include inflammatory processes leading to vascular and myocardial fibrosis and vascular media calcifications [3, 4]. Although renal transplantation halts the progression of CVD, it still remains the leading cause of death after renal transplantation [5]. Large artery stiffness, as estimated by either pulse wave velocity (PWV) or augmentation index, has proved to be an independent predictor of CVD in patients with CKD [6].
Long chain n-3 polyunsaturated fatty acids (PUFA) might have cardioprotective effects [7, 8], and several studies have suggested a beneficial effect of long chain n-3 PUFA on CVD, although data are not entirely consistent [9–11]. The most important n-3 PUFA, eicosapentaenoic acid 20:5 (EPA) and docosahexaenoic acid 22:6 (DHA), are primarily found in fatty fish and are also available in fish oil supplements.
Several trials in non-CKD populations have addressed whether n-3 PUFA supplementation has an effect on arterial stiffness [12–14], and a meta-analysis concluded that n-3 PUFA supplementation significantly reduces arterial stiffness when determined as PWV or systemic arterial compliance [15]. Studies on the immediate effect of a large intake of n-3 PUFA have also proved an attenuating effect on arterial stiffness [16]. Contradicting results have emerged from trials testing the effect of n-3 PUFA supplementation on the vascular endothelial function, evaluated as flow-mediated dilatation of the brachial artery. Thus, n-3 PUFA improved the endothelial function in patients with metabolic syndrome, whereas such an effect could not be documented in healthy adults [17, 18]. The resistance arteries of the body are of critical importance for blood pressure regulation, organ perfusion and peripheral vascular resistance [19] and are presumed to be of major importance in the development of CVD. Previous investigations have not shown difference in morphology or vasoconstrictor response when comparing CKD and control resistance arteries [20], whereas studies on endothelial function have shown contradicting results [21–24].
The aim of this explorative cross-sectional and comparative study of patients with CKD stage 5 + 5d and healthy kidney donors was to correlate n-3 PUFA concentration in adipose tissue with arterial stiffness, cardiac output, systemic vascular resistance and endothelium-mediated vasodilatation in resistance arteries. Furthermore self-reported fish intake was compared to n-3 PUFA concentration in adipose tissue.
Methods
Study population
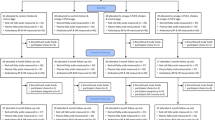
A total of 18 patients with chronic kidney disease stage 5 + 5d (CKD) and 27 healthy kidney donors were included in the study. One patient was excluded from statistical analysis because of missing values for n-3 PUFA in adipose tissue. Inclusion criteria were age above 18 years and scheduled for either living related-donor renal transplant (14 patients) or insertion of peritoneal dialysis catheter (3 patients) at the Department of Nephrology, Aarhus University Hospital, Denmark, during 1st of November 2010 until the 31st of October 2011. Inclusion ran consecutively and patients were approached when an operation date was scheduled. Exclusion criteria were severe congestive heart failure, persisting cardiac arrhythmias, reduced pulmonary function, leg amputation, severe psychiatric disease and acute infection. Three patients had diabetes mellitus, and 3 had previously documented CVD. Ahead of inclusion nine patients were not on dialysis, while 8 were treated with peritoneal dialysis. Median dialysis vintage was 294 days (range: 100–1154 days). All but one of the patients were treated with one or more antihypertensive drug: ACE-inhibitor (6 patients), angiotensin II receptor antagonist (7 patients), calcium channel antagonist (13 patients), beta-blocker (8 patients), furosemide (15 patients).
Patients prepared for living donor transplantation received immunosuppressive therapy 2 days in advance: Prednisolone 20 mg/d, Tacrolimus 0.2 mg/kg/d and Mycophenolat mofetil acid 1.5 g/d.
Twenty-seven healthy kidney donors who did not receive any medication served as controls. Control subjects were related to the patients (sibling, husband, wife, mother, father or friend).
The day before surgery, the participants’ hemodynamic data were measured along with height, weight and blood pressure. The participants filled out a questionnaire regarding fish intake, and fasting blood samples were drawn on the day of surgery. During surgery, a 2 × 3 cm biopsy from the abdominal wall containing skin, adipose tissue and subcutaneous resistance arteries was removed. Adipose tissue was submerged in liquid nitrogen and stored in a −80 °C freezer prior to analysis of fatty acid composition. Resistance arteries from the biopsy were isolated and mounted for isometric force measurements.
Hemodynamic study
Seventeen patients with CKD and 27 healthy subjects were part of the hemodynamic study. Blood pressure, pulse rate and cardiac output (CO) were measured with a portable non-invasive device consisting of a three-way respiratory valve with a mouthpiece and a rebreathing bag connected to an infrared photoacuostic gas analyzer, a pulse oxymeter and a device for automatic blood pressure measurement (Innocor®, Innovision, Odense, Denmark). CO was measured by rebreathing a gas mixture of sulfahexafluoride (SF6, 0.1%) and nitrous oxide (N20 0.5%) in an O2/N2 mixture. Rebreathing was done in 15 s with a gas volume of 1.8 l and a respiratory breathing rate of 14–18 min−1. Gas was sampled continuously from the mouthpiece and analyzed on-line by the IR gas analyzer. A constant ventilation rate and volume were ensured by synchrony between the graphical tachymeter on the computer screen and the study subject, who was instructed to empty the bag with each breath. The rebreathing software calculated pulmonary blood flow from the rate of uptake of N2O into the blood (slope of regression line through logarithmically transformation of expiratory N2O concentration plotted against time). The first two or three breaths were excluded from analysis if total lung volume changes measured by SF6 indicated incomplete gas mixing. After correction for system volume changes using SF6 concentration, the first two or three breath were excluded from the analysis due to initial incomplete gas mixing. For the majority of patients without pulmonary arterial-venous shunt (SaO2 ≥ 98%) the measured pulmonary blood flow value was considered equal to CO, whereas for patients with pulmonary shunt, the shunt was calculated and added to CO. The shunt fraction was calculated using the oxygen concentration. The calculations were performed under the assumption that gasses were mixed completely, that equilibration of gasses between alveoli and blood was rapid and that lung blood flow was constant.
Systolic blood pressure and diastolic blood pressure were measured using an automatic device connected to the Innocor®. Systemic vascular resistance index (SVRi) was calculated as: (Mean arterial blood pressure—central venous pressure)/Cardiac output and indexed to body surface. The measurement was performed twice, and the mean value was used for data analysis.
Pulse wave velocity (PWV) and augmentation index (AI) were measured in the supine position after 10 min of rest. Carotid–femoral PWV was measured with SphygmoCor® (AtCor Medical, TX, US), using the integral software.
Augmentation pressure was calculated as the difference between the second and first systolic peaks, and AI was calculated as the augmentation pressure expressed as percentage of pulse pressure. AI was measured for aorta. All of the measurements were made in duplicate by one trained study nurse, and the mean values were used in the subsequent analysis.
Microvascular study
In the microvascular study, 11 patients with CKD and 11 healthy subjects were examined; the included patients where those where dissection were successful and viable vessels found. Skin and subcutaneous tissue samples were taken during surgery and immediately placed in a 5 °C physiological salt solution (PSS). In the biopsies, 2 mm long resistance artery segments were dissected for measurement of endothelium-dependent vasodilatation. The artery segments were mounted on two stainless steel wires (40 μm diameter) in organ baths of a 4-channel wire myograph (model 610M, Danish Myo Technology, Aarhus, Denmark) or in a 2-channel wire myograph (model 410A, Danish Myo Technology) for isometric force measurements. The organ baths contained PSS at 37 °C, continuously bubbled with 5% CO2 in air to keep pH at 7.4. After mounting, the arteries equilibrated for 20 min before the elastic properties were characterized by stepwise increasing the artery circumference as previously described [25]. Experiments were performed at 90% of L100; where L100 is defined as the circumference of the relaxed artery when exposed to a transmural pressure of 100 mmHg. The artery viability was tested twice with 10 μM noradrenaline (NA) before beginning the protocol. Upon preconstriction with 3 μM NA, a concentration-response curve was performed with increasing concentrations of the endothelium-dependent vasodilator acetylcholine (ACh). After washout and 20 min of rest, the experiment was repeated in the presence of the cyclooxygenase(COX)-inhibitor indomethacin (3μM), and after another 20 min of rest, the protocol was repeated in the presence of indomethacin and the nitric oxide-synthase inhibitor NG-nitro-L-arginine methyl ester (L-NAME) (100 μM). Hence “total” endothelial function, non-COX-dependent and non-COX/nitric oxide-dependent endothelial function was tested, while nitric oxide-dependent endothelial function was calculated.
The composition of PSS: NaCl 119, KCl 4, KH2PO4 1.18, MgSO4 1.17, NaHCO3 25, CaCl2 1.6, EDTA 0.026, glucose 5.5 (mM). Indomethacin was dissolved in ethanol, all other chemicals in distilled water. Chemicals were acquired from Sigma-Aldrich (St. Louis, MO, US).
Evaluation of fish intake
Self-reported fish intake was obtained using a previously validated dietary questionnaire [26]. A score was given according to fish consumption at lunch and dinner as follows: 1 = never eating fish; 2 = eating fish once a month; 3 = eating fish twice a month; 4 = eating fish once a week; 5 = eating fish 2 to 3 times a week; 6 = eating fish every day. Fish scores were divided into three groups as low (2–5), moderate (6–8) and high (9–12) fish intake for further analysis.
Analysis of fatty acid composition of adipose tissue
Adipose tissue samples were obtained from the abdominal region and stored at −80 °C. Fatty acids were extracted in CHCl3/MeOH [27], dissolved in hexane and transesterified using 0.5 M of sodium methoxide and acetic acid [28]. Fatty acid analysis was performed by gas chromatography using a Chrompack CP-9002 gas chromatograph (Chrompack Int, Middelburg, the Netherlands) and expressed as percentage of total fatty acids.
Biochemistry
Estimated glomerular filtration rate (eGFR) was calculated from the 4-point MDRD formula [29]. Asymmetric dimethyl arginine (ADMA) was measured using ELISA (DLD Diagnostika GmbH, Germany). All other biochemistry was analysed at the Department of Clinical Biochemistry, Aarhus University Hospital.
Statistics
Data in tables are presented as mean ± standard deviation. Baseline characteristics were compared using Student’s t-test or rank-sum when appropriated; gender was compared using Fisher’s exact test. Normality was visual check of quintiles of the individual variables against quintiles of normal distribution and standardized normal probability plot and using Sharpiro-Wilks test. All 3 tests should suggest normality for it to be accepted. Only n-3 PUFA was not normally distributed and had to be logarithmically transformed and normality was confirmed by Sharpiro-Wilks test. Log n-3 PUFA concentrations in adipose tissue had equal median and variance for CKD patients and healthy subjects, and were pooled and divided into three fish intake groups, which were compared using Kruskal-Wallis test. Correlation tests were conducted using linear regression. Comparison of the regression coefficient for healthy subjects and CKD patients was done as described by the Educational Department of UCLA [30]. All statistical analyses were performed using STATA v. 13 SE (STATA Corp., TX, US).
Results
Study population
Baseline statistics are seen in Table 1. Fish score was similar in CKD patients and healthy subjects (CKD 7.5 ± 2.0, n = 17 vs. healthy subjects 7.1 ± 2.4, n = 27). Content of long chain n-3 PUFA in adipose tissue were comparable between CKD patients and healthy subjects (CKD 0.25 ± 0.18%, n = 17 vs. healthy controls 0.29 ± 0.21%, n = 23).
PWV was higher in CKD patients (CKD 9.53 ± 4.08 m/s, n = 15 vs. 7.24 ± 1.46 m/s, n = 25; p < 0.05), augmentation index was lower in CKD patients (CKD 70.5 ± 22.6%, n = 15 vs. healthy subjects 86.3 ± 17.5%, n = 25; p < 0.01) while cardiac output index and SVRi did not differ significantly between CKD patients and healthy subjects (Cardiac output index —CKD 3.25 ± 0.94 L/min/m2, n = 16 vs. healthy subjects 2.81 ± 0.66 L/min/m2, n = 26; SVRi —CKD 30.9 ± 9.85 mmHg/(L/min)/m2, n = 16 vs. 32.6 ± 6.9 mmHg/(L/min)/m2, n = 26). Baseline characteristics for the microvascular study group (11 vs. 11) did not differ from the whole group. No difference was seen between dialysis and non-dialysis patients (data not shown).
Fish score groups and n-3 PUFA in adipose tissue
All subjects (n = 44) were divided into three groups according to their fish intake. As shown in Fig. 1, n-3 PUFA content in adipose tissue increased with increasing fish intake and groups were significantly different from each other (p < 0.01).
Adipose n-3 PUFA and hemodynamic function
The content of long chain n-3 PUFA and SVRi was positively correlated in both CKD (r = 0.44; p = 0.07, n = 16) and control subjects (r = 0.62; p < 0.05, n = 26). No differences in hemodynamic function between CKD and controls were found (Fig. 2). Furthermore, n-3 PUFA were negatively associated with cardiac output index in both CKD and controls (r = −0.69; p < 0.01, n = 16 and r = −0.50; p < 0.05, n = 26; respectively) (Fig. 3).
The association between n-3 PUFA in adipose tissue and systemic vascular resistance index. Chronic kidney disease patients (r = 0.44; p = 0.07) (red symbols/red dash line, fitted line and 95% confidence interval); healthy subjects (r = 0.62; p < 0.05) (green symbols and solid line). Regression formulas were y = 8.0*x + 43.0 and y = 6.3*x + 40.0 for CKD patients and healthy subjects, respectively
The association between n-3 PUFA in adipose tissue and cardiac output index. Chronic kidney disease patients (r = −0.69; p < 0.01) (red symbols/red dash line, fitted line and 95% confidence interval); healthy subjects (r = −0.50; p < 0.05) (green symbols and green solid line). Regression formulas were y = −0.79*x + 2.06 and y = −0.68*x + 1.87 for ESRD patients and control subjects, respectively
Adipose n-3 PUFA and microvascular function
The “total” Ach-induced vasodilatation in the microvasculature was not associated with the content of n-3 PUFA in adipose tissue (Fig. 4). Furthermore, specific pathways such as non-COX-dependent, nitric oxide-dependent and non-COX/nitric oxide-dependent were not correlated to n-3 PUFA in adipose tissue (data not shown).
The association between n-3 PUFA in adipose tissue and acetylcholine-induced vasodilator response in resistance arteries. Chronic kidney disease patients red symbols/red dash line, fitted line and 95% coefficient interval); healthy subjects (green symbols and green solid line). Regression formulas were y = −0.07*x + 6.86 and y = −0.11*x + 6.81 for ESRD patients and control subjects, respectively
Discussion
n-3 PUFA content in adipose tissue and self-reported fish intake were both similar in CKD patients and controls. In both CKD and healthy subjects, n-3 PUFA levels and SVRi were positively associated, whereas n-3 PUFA and cardiac output index were negatively associated. The content of long chain n-3 PUFA in adipose tissue was not associated with endothelial function in small resistance vessels.
Fish intake and n-3 PUFA
n-3 PUFA content in adipose tissue (a long-term marker of n-3 intake [31]) and fish intake were similar in CKD patients and healthy controls. The majority of subjects were related to one of the CKD patients as a sibling, husband, wife, mother, father or friend, and hence a few of them shared household and therefore, the control group may tend to have the same fish intake. It should, however, be appreciated that the fish score gives a rather crude measure of consumption and particularly of intake of EPA and DHA, which is a limitation to the study. Previous studies have described that US hemodialysis (HD) patients have much lower fish intake than recommended [32] as well as lower levels of n-3 PUFA compared with controls [33]. In contrast, HD patients in South Korea [34] and peritoneal dialysis patients in Greece [35] had similar levels of n-3 PUFA compared with the background population. This elucidates the importance of regional differences in fish intake when comparing dialysis patients with the general population.
The duration of HD has also proved important for the general nutritional intake, since both total energy, protein and lipid consumption are negatively correlated with the duration of HD [36]. In the current study, both patients with CKD treated with dialysis and pre-dialysis patients were included. Participants were relatively young (median 45 years) with a median dialysis vintage of 294 days. The majority was undergoing renal transplant and hence in good condition with retained nutritional intake. This could help explain the similar fish intake between patients and controls seen in this study. Despite these considerations, the similarity in fish intake and n-3 PUFA levels between CKD patients and controls suggests that uptake, processing and storage of n-3 PUFA in CKD patients are comparable to healthy controls, as described earlier [37].
Hemodynamic function
A positive correlation between n-3 PUFA in adipose tissue and the SVRi was found in both CKD patients and control subjects. An earlier study in healthy adults reported that resting SVR 5 hours after consuming a meal rich in DHA or EPA was the same as in the controls given a meal without DHA or EPA [38]. A subsequent exercise test resulted in a lower SVR in the DHA-consuming group compared with both the control and the EPA-consuming group. A negative correlation was found between the content of n-3 PUFA in adipose tissue and cardiac output index in both controls and CKD patients. The aforementioned study [38] of n-3 PUFA acute effects did not find differences in cardiac output at rest or exercise after DHA/EPA/placebo ingestion. Resting cardiac output is reduced in patients with CVD [39]. However, since a correlation was found in both CKD and healthy controls, the current results could also suggest that long-term fish intake enhances tissue oxygen uptake resulting in a lowering of the cardiac output, as suggested recently [40]. Moreover, in animal models it is well known that n-3 PUFA lower the basic metabolic rate [41], which could also result in a lowering of cardiac output. The diminished cardiac output could subsequently result in a baroreceptor-mediated augmentation in SVR. A major part of the vascular resistance resides in the microvasculature. However, since we found no correlation between n-3 PUFA and the microvascular endothelial-mediated vasodilator function, this apparently is not due to changes in intrinsic properties of the resistance arteries. Rather the change in resistance may stem from continual in-vivo activation from nerves, paracrine or endocrine processes.
The current study showed associations between n-3 PUFA and cardiac index/SVRi. However, associations were not found with PWV or augmentation index. This contradicts an earlier study showing DHA to be inversely associated with PWV in non-diabetic CKD patients [42]. PWV is used as a marker of early-stage atherosclerosis [43], and the lack of coherence between results could possibly be explained by the young age of the CKD patients, the low dialysis vintage or our relatively small sample size.
It has been difficult to prove an effect of n-3 PUFA ingestion on mortality or CVD in CKD patients in clinical trials. However, an observational study [44] has suggested DHA in red blood cells to be an independent predictor of mortality in end stage renal disease patients on hemodialysis after 10 years of follow-up. Also, a clinical controlled trial found 2 years of n-3 PUFA treatment to be beneficial in secondary prevention of myocardial infarction in chronic HD patients, while no effect was seen on the primary endpoint, a composite of total cardiovascular events and death [45]. Longer prospective studies on large cohorts are needed to determine the effect of n-3 PUFA on hemodynamic function and mortality in CKD patients.
Microvascular function
Although several studies have addressed the effect of n-3 PUFA on endothelial function in large arteries [17, 18], this study is the first to correlate the content of n-3 PUFA in adipose tissue with microvascular function. The endothelial Ach-induced vasodilatation was not correlated to n-3 PUFA in adipose tissue. Furthermore, the individual Ach-induced vasodilator pathways were not associated with long chain n-3 PUFA levels (data not shown). These results are in line with animal studies performed on rat femoral resistance arteries [46] and suggest that the intrinsic properties of resistance arteries are not affected by n-3 PUFA.
The study design was cross-sectional and therefore cannot establish causal relationship between variables. Since all healthy kidney donors were related to an CKD kidney recipient, the two groups may tend to have the same fish intake. This could influence measurements of hemodynamic and microvascular properties and potentially mask differences which could have been found in non-related groups. Due to the rather low sample size, a risk of type II error exists. In this type of experiment confounding variables are also a hazard. Due to the size of the study, is has not been possible to adjust for confounders such as smoking, exercise or family history.
Conclusions
In conclusion, this study found similar fish intake and adipose content of long chain n-3 PUFA in CKD patients and control subjects. This is in contrast with earlier findings, but might be explained by young age of subject and the short duration of dialysis. Long chain n-3 PUFA were negatively correlated to cardiac output index, which may imply that n-3 PUFA enhance tissue oxygen uptake and lower metabolic rate. This could further explain the observed positive correlation between n-3 PUFA and systemic vascular resistance index although not through intrinsic properties of resistance arteries, as they did not correlate to long chain n-3 PUFA in adipose tissue.
Abbreviations
- ACh:
-
Acetyl choline
- ADMA:
-
Asymmetric dimethyl arginine
- AI:
-
Augmentation index
- CKD:
-
Chronic kidney disease
- CO:
-
Cardiac output
- COX:
-
Cyclooxygenase
- CVD:
-
Cardiovascular disease
- DHA:
-
Docosahexaenoic acid 22:6
- eGFR:
-
Estimated glomerular filtration rate
- EPA:
-
Eicosapentaenoic acid 20:5
- HD:
-
Hemodialysis
- L-NAME:
-
NG-nitro-L-arginine methyl ester
- n-3 PUFA:
-
n-3 Polyunsaturated fatty acids
- NA:
-
Noradrenaline
- PSS:
-
Physiological salt solution
- PWV:
-
Pulse wave velocity
- SVRi:
-
Systemic vascular resistance index
References
Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–305.
Roberts MA, Polkinghorne KR, McDonald SP, Ierino FL. Secular trends in cardiovascular mortality rates of patients receiving dialysis compared with the general population. Am J Kidney Dis. 2011;58:64–72.
Swaminathan S, Shah SV. Novel inflammatory mechanisms of accelerated atherosclerosis in kidney disease. Kidney Int. 2011;80:453–63.
Shanahan CM. Mechanisms of vascular calcification in CKD-evidence for premature ageing? Nat Rev Nephrol. 2013;9:661–70.
Meier-Kriesche HU, Schold JD, Srinivas TR, Reed A, Kaplan B. Kidney transplantation halts cardiovascular disease progression in patients with end-stage renal disease. Am J Transplant. 2004;4:1662–8.
Guerin AP, Pannier B, Metivier F, Marchais SJ, London GM. Assessment and significance of arterial stiffness in patients with chronic kidney disease. Curr Opin Nephrol Hypertens. 2008;17:635–41.
De CR. n-3 fatty acids in cardiovascular disease. N Engl J Med. 2011;364:2439–50.
Saravanan P, Davidson NC, Schmidt EB, Calder PC. Cardiovascular effects of marine omega-3 fatty acids. Lancet. 2010;376:540–50.
Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Lancet. 1999;354:447–55.
Mozaffarian D, Micha R, Wallace S. Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: a systematic review and meta-analysis of randomized controlled trials. PLoS Med. 2010;7:e1000252.
Rizos EC, Ntzani EE, Bika E, Kostapanos MS, Elisaf MS. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta-analysis. JAMA. 2012;308:1024–33.
Hjerkinn EM, Abdelnoor M, Breivik L, Bergengen L, Ellingsen I, Seljeflot I, et al. Effect of diet or very long chain omega-3 fatty acids on progression of atherosclerosis, evaluated by carotid plaques, intima-media thickness and by pulse wave propagation in elderly men with hypercholesterolaemia. Eur J Cardiovasc Prev Rehabil. 2006;13:325–33.
Root M, Collier SR, Zwetsloot KA, West KL, McGinn MC. A randomized trial of fish oil omega-3 fatty acids on arterial health, inflammation, and metabolic syndrome in a young healthy population. Nutr J. 2013;12:40.
Takaki A, Umemoto S, Ono K, Seki K, Ryoke T, Fujii A, et al. Add-on therapy of EPA reduces oxidative stress and inhibits the progression of aortic stiffness in patients with coronary artery disease and statin therapy: a randomized controlled study. J Atheroscler Thromb. 2011;18:857–66.
Pase MP, Grima NA, Sarris J. Do long-chain n-3 fatty acids reduce arterial stiffness? A meta-analysis of randomised controlled trials. Br J Nutr. 2011;106:974–80.
Chong MF, Lockyer S, Saunders CJ, Lovegrove JA. Long chain n-3 PUFA-rich meal reduced postprandial measures of arterial stiffness. Clin Nutr. 2010;29:678–81.
Tousoulis D, Plastiras A, Siasos G, Oikonomou E, Verveniotis A, Kokkou E, et al. Omega-3 PUFAs improved endothelial function and arterial stiffness with a parallel antiinflammatory effect in adults with metabolic syndrome. Atherosclerosis. 2014;232:10–6.
Sanders TA, Hall WL, Maniou Z, Lewis F, Seed PT, Chowienczyk PJ. Effect of low doses of long-chain n-3 PUFAs on endothelial function and arterial stiffness: a randomized controlled trial. Am J Clin Nutr. 2011;94:973–80.
Luksha L, Agewall S, Kublickiene K. Endothelium-derived hyperpolarizing factor in vascular physiology and cardiovascular disease. Atherosclerosis. 2009;202:330–44.
Aalkjaer C, Pedersen EB, Danielsen H, Fjeldborg O, Jespersen B, Kjaer T, et al. Morphological and functional characteristics of isolated resistance vessels in advanced uremia. Clin Sci (Lond). 1986;71:657–63.
Morris ST, McMurray JJ, Spiers A, Jardine AG. Impaired endothelial function in isolated human uremic resistance arteries. Kidney Int. 2001;60:1077–82.
Luksha N, Luksha L, Carrero JJ, Hammarqvist F, Stenvinkel P, et al. Impaired resistance artery function in patients with end-stage renal disease. Clin Sci (Lond). 2011;120:525–36.
Luksha L, Stenvinkel P, Hammarqvist F, Carrero JJ, Davidge ST, et al. Mechanisms of endothelial dysfunction in resistance arteries from patients with end-stage renal disease. PLoS One. 2012;7:e36056.
Borg MK, Ivarsen P, Brondum E, Povlsen JV, Aalkjaer C. Hemodynamics and function of resistance arteries in healthy persons and end stage renal disease patients. PLoS One. 2014;9:e94638.
Halpern W, Mulvany MJ. Tension responses to small length changes of vascular smooth muscle cells [proceedings]. J Physiol. 1977;265:21P–3P.
Christensen JH, Skou HA, Fog L, Hansen V, Vesterlund T, Dyerberg J, et al. Marine n-3 fatty acids, wine intake, and heart rate variability in patients referred for coronary angiography. Circulation. 2001;103:651–7.
Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–7.
Christoffersen SW, Glass RL. Preparation of milk fat methyl esters by alcoholysis in an essentially non-alcoholic solution. J Dairy Sci. 1969;52:1289–90.
Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–70.
Institute for digital research and education, UCLA: STATA FAQ: http://www.ats.ucla.edu/stat/stata/faq/compreg2.htm. Accessed 1 May 2014.
Marckmann P, Lassen A, Haraldsdottir J, Sandstrom B. Biomarkers of habitual fish intake in adipose tissue. Am J Clin Nutr. 1995;62:956–9.
Friedman AN, Moe SM, Perkins SM, Li Y, Watkins BA. Fish consumption and omega-3 fatty acid status and determinants in long-term hemodialysis. Am J Kidney Dis. 2006;47:1064–71.
Madsen T, Christensen JH, Svensson M, Witt PM, Toft E, Schmidt EB. Marine n-3 polyunsaturated fatty acids in patients with end-stage renal failure and in subjects without kidney disease: a comparative study. J Ren Nutr. 2011;21:169–75.
An WS, Kim SE, Kim KH, Lee S, Park Y, Kim HJ, et al. Comparison of fatty acid contents of erythrocyte membrane in hemodialysis and peritoneal dialysis patients. J Ren Nutr. 2009;19:267–74.
Nikolakakis N, Kounali D, Tornaritis M, Anastassou A, Papadakis E, Kassotakis G, et al. Adipose tissue fatty acid composition, serum lipids, and serum alpha-tocopherol in continuous ambulatory peritoneal dialysis patients living on the island of Crete. Perit Dial Int. 1999;19:154–9.
Mekki K, Remaoun M, Belleville J, Bouchenak M. Hemodialysis duration impairs food intake and nutritional parameters in chronic kidney disease patients. Int Urol Nephrol. 2012;44:237–44.
Svensson M, Schmidt EB, Jorgensen KA, Christensen JH. The effect of n-3 fatty acids on lipids and lipoproteins in patients treated with chronic haemodialysis: a randomized placebo-controlled intervention study. Nephrol Dial Transplant. 2008;23:2918–24.
Rontoyanni VG, Hall WL, Pombo-Rodrigues S, Appleton A, Chung R, Sanders TA. A comparison of the changes in cardiac output and systemic vascular resistance during exercise following high-fat meals containing DHA or EPA. Br J Nutr. 2012;108:492–9.
Kattus AA, Rivin AU, Cohen A, Sofio GS. Cardiac output and central volume as determined by dye dilution curves; resting values in normal subjects and patients with cardiovascular disease. Circulation. 1955;11:447–55.
Zebrowska A, Mizia-Stec K, Mizia M, Gasior Z, Poprzecki S. Omega-3 fatty acids supplementation improves endothelial function and maximal oxygen uptake in endurance-trained athletes. Eur J Sport Sci 2014;1–10.
Jefimow M, Wojciechowski MS. Effect of dietary fatty acids on metabolic rate and nonshivering thermogenesis in golden hamsters. J Exp Zool A Ecol Genet Physiol. 2014;321:98–107.
Hamazaki K, Terashima Y, Itomura M, Sawazaki S, Inagaki H, Kuroda M, et al. The relationship between n-3 long-chain polyunsaturated fatty acids and pulse wave velocity in diabetic and non-diabetic patients under long-term hemodialysis. A horizontal study. Clin Nephrol. 2009;71:508–13.
Saijo Y, Utsugi M, Yoshioka E, Fukui T, Sata F, Nakagawa N, et al. Inflammation as a cardiovascular risk factor and pulse wave velocity as a marker of early-stage atherosclerosis in the Japanese population. Environ Health Prev Med. 2009;14:159–64.
Terashima Y, Hamazaki K, Itomura M, Tomita S, Kuroda M, Hirata H, et al. Inverse association between docosahexaenoic acid and mortality in patients on hemodialysis during over 10 years. Hemodial Int. 2014;18:625–31.
Svensson M, Schmidt EB, Jorgensen KA, Christensen JH. N-3 fatty acids as secondary prevention against cardiovascular events in patients who undergo chronic hemodialysis: a randomized, placebo-controlled intervention trial. Clin J Am Soc Nephrol. 2006;1:780–6.
MacLeod DC, Heagerty AM, Bund SJ, Lawal TS, Riemersma RA. Effect of dietary polyunsaturated fatty acids on contraction and relaxation of rat femoral resistance arteries. J Cardiovasc Pharmacol. 1994;23:92–8.
Acknowledgements
The authors wish to thank the included patients and the staff at Aarhus University Hospital. Special thanks to laboratory technician Jørgen Andresen and project nurse Karin Hansen.
Funding
The study was supported by the P Carl Petersen Foundation. The funding source was not involved in study design, data collection, analysis or preparation of the manuscript.
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Authors’ contribution
MB participated in design of the study, carried out the experiments, performed data analysis and drafted the manuscript. MS performed data analysis and drafted the manuscript. JP performed data analysis and drafted the manuscript. ES performed data analysis and drafted the manuscript. CA participated in design of the study, performed data analysis and drafted the manuscript. JC performed data analysis and drafted the manuscript. PI participated in design of the study, carried out the experiments, performed data analysis and drafted the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The study was performed in accordance with the Helsinki Declaration. Protocol and consent forms were approved by the local Research Ethics Committee of Central Denmark Region (M-20090131). All participants gave written informed consent before inclusion.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Borg, M., Svensson, M., Povlsen, J.V. et al. Long chain n-3 polyunsaturated fatty acids and vascular function in patients with chronic kidney disease and healthy subjects: a cross-sectional and comparative study. BMC Nephrol 17, 184 (2016). https://doi.org/10.1186/s12882-016-0393-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12882-016-0393-5