Abstract
Introduction
Randomised controlled trials have shown that steroids reduce the risk of dying in patients with severe Coronavirus disease 2019 (COVID-19), whilst many real-world studies have failed to replicate this result. We aim to investigate real-world effectiveness of steroids in severe COVID-19.
Methods
Clinical, demographic, and viral genome data extracted from electronic patient record (EPR) was analysed from all SARS-CoV-2 RNA positive patients admitted with severe COVID-19, defined by hypoxia at presentation, between March 13th 2020 and May 27th 2021. Steroid treatment was measured by the number of prescription-days with dexamethasone, hydrocortisone, prednisolone or methylprednisolone. The association between steroid > 3 days treatment and disease outcome was explored using multivariable cox proportional hazards models with adjustment for confounders (including age, gender, ethnicity, co-morbidities and SARS-CoV-2 variant). The outcome was in-hospital mortality.
Results
1100 severe COVID-19 cases were identified having crude hospital mortality of 15.3%. 793/1100 (72.1%) individuals were treated with steroids and 513/1100 (46.6%) received steroid ≤ 3 days. From the multivariate model, steroid > 3 days was associated with decreased hazard of in-hospital mortality (HR: 0.47 (95% CI: 0.31–0.72)).
Conclusion
The protective effect of steroid treatment for severe COVID-19 reported in randomised clinical trials was replicated in this retrospective study of a large real-world cohort.
Similar content being viewed by others
Background
Currently, steroids are the main treatment for severe coronavirus disease 2019 (COVID-19) infection [1], which has infected over 540million people and caused over 6million deaths worldwide [2]. The RECOVERY trial [3, 4] was the first randomised controlled trial to show that in patients hospitalized with COVID-19, the use of dexamethasone resulted in lower 28-day mortality among those who were receiving either invasive mechanical ventilation or oxygen alone but not among those receiving no respiratory support. Some meta-analyses have shown a benefit of steroids at preventing mortality [5, 6] and reducing need for mechanical ventilation [6]. However, other meta-anlysis from both observational studies and randomised controlled trials have shown conflicting results [7, 8].
A guideline was issued by WHO on use of dexamethasone and other corticosteroids (hydrocortisone or prednisone) for treatment of severe and critically unwell COVID-19 patients in September 2020 [9]. After the RECOVERY trial and WHO guidelines, the use of steroids changed from being used in ICU for some very severe patients, to more consistent use in patients admitted to hospital requiring oxygen. Our objective was to determine whether the effect of steroids on outcomes for severe COVID-19 patients reported in randomised trials is replicated in a large real-world cohort spanning the duration of the pandemic.
Methods
Population of interest and setting
Guy’s and St Thomas’ NHS Foundation Trust (GSTT) is a multi-site inner-city healthcare institution providing general and emergency services predominantly to the South London boroughs of Lambeth and Southwark. NHS is the National Health Service in the UK. The acute-admitting site (St Thomas’ Hospital) has an emergency department with a large critical care service. A second hospital site (Guy’s Hospital) provides elective surgery, haemato-oncology, renal transplantation and other specialist services. There are also several community sites providing dialysis, rehabilitation and long-term care. Only COVID-19 cases admitted through the emergency department (ED) during March 13th 2020 and May 27th 2021 were included in this study. Patients dying or being discharged in the first 24h were considered most likely to have reached study endpoint independent of any steroid effect and were excluded from the primary analysis.
SARS-CoV-2 laboratory testing
GSTT has an on-site laboratory providing SARS-CoV-2 testing to all patients and hospital care workers (HCW). The policies and technologies employed for SARS-CoV-2 testing changed over time based on national and local screening guidance and improvements in diagnostics. Our laboratory began testing on 13th March 2020 with initial capacity for around 150 tests per day, before increasing to around 500 tests per day in late April during wave one, and up to 1000 tests per day during the second wave.
Assays used for the detection of SARS-CoV-2 RNA include PCR testing using Aus Diagnostics or by the Hologic Aptima SARS-CoV-2 Assay. Testing commenced during the first wave on 13th March 2020 limited to cases requiring admission or inpatients who had symptoms of fever or cough, as per national recommendation; guidance suggested cases who did not require admission should not be tested. Cases without laboratory confirmation of SARS-CoV-2 infection were not included.
Definitions
Cases were identified by the first positive SARS-CoV-2 RNA test. The severe cases were measured by hypoxia upon admission to hospital. Cases were taken to be hypoxic if on admission they had oxygen saturations of < 94%, if they were recorded as requiring supplemental oxygen, or if the fraction of inspired oxygen was recorded as being greater than 0.21.
Determination of SARS-CoV-2 lineage
Whole genome sequencing of residual samples from SARS-CoV-2 cases was performed using GridION (Oxford Nanopore Technology), using version 3 of the ARTIC protocol [10] and bioinformatics pipeline [11]. Samples were selected for sequencing if the corrected CT value was 33 or below, or the Hologic Aptima assay was above 1000 relative light units (RLU). During the first wave sequencing occurred between March 1st − 31st, whilst sequencing restarted in November 2020 and is ongoing. Lineage determination was performed using updated versions of pangolin 2.0 [12]. Samples were regarded as successfully sequenced if over 50% of the genome was recovered and if lineage assignment by pangolin was given with at least 50% confidence.
Data sources, extraction and integration
Clinical, laboratory and demographic data for all cases with a laboratory reported SARS-CoV-2 PCR RNA test on nose and throat swabs or lower respiratory tract specimens were extracted from hospital electronic patient record (EPR) data sources using records closest to the test date (DXC Technology’s i.CM EPR, Philips IntelliVue Clinical Information Portfolio (ICIP) Critical Care, DXC Technology’s MedChart, e-Noting and Citrix Remote PACS - Sectra). Data was linked to the Index of Multiple Deprivation (IMD), with 1 denoting the least deprived areas, and 5 the most deprived ones. Age and sex were extracted from EPR. Self-reported ethnicity of cases was stratified to be White, BAME (Black, Asian and Minority Ethnic) and Unknown according to the 18 ONS categories of White (British, Irish, Gypsy and White-Other), Black (African, Caribbean, and Black-Other), Asian (Bangladeshi, Chinese, Indian, Pakistan, and Asian-Other), and Mixed/Other.
Comorbidities, medication history, and medicine data were extracted from the EPR and e-Noting using structured queries with corresponding dictionaries. Comorbidities were extracted from any of the databases covering the pathway of the cases from arrival in accident and emergency through inpatient general ward and critical care unit, where applicable, to hospital discharge or death. If a comorbidity was not recorded, we assume that it was not present. Cases were characterised as having/not having a past medical history of hypertension, cardiovascular disease (stroke, transient ischaemic attack, atrial fibrillation, congestive heart failure, ischaemic heart disease, peripheral artery disease or atherosclerotic disease), diabetes mellitus, chronic kidney disease, chronic respiratory disease (chronic obstructive pulmonary disease, asthma, bronchiectasis or pulmonary fibrosis) and neoplastic disease (solid tumours, haematological neoplasias or metastatic disease). Additionally, checks on free text data were performed by a cardiovascular clinician to ensure the information was accurate.
Steroids
Steroid treatment was measured by number of prescription-days with dexamethasone, hydrocortisone, prednisolone or methylprednisolone. Duration of treatment with steroids was calculated as cumulative days throughout first hospital admission after the first SARS-CoV-2 PCR positive test through to discharge or death during that admission. Analysis for lengths of steroid use were conducted in multivariate model with steroid use ≤ 3 days versus steroid use > 3 days. The cut-off for the steroid treatment days were chosen according to the interquartile range of steroid-days (3 to 10 days) in RECOVERY trial. Sensitivity analysis was conducted with continuous steroid days as the variable input in the Cox proportional hazards model.
Outcomes
The outcome was all-cause in-hospital mortality (WHO-COVID-19 Outcomes Scale 8), with patients still hospitalised at the end of the cohort considered censored.
Statistical analysis
The general statistics were summarised with mean and standard deviation (SD) for continuous variables if the distribution is normal and median and interquartile range (IQR) if the distribution is non-normal. Count and percentages were used for categorical variables. For the comparisons of the cohort statistics with different lengths of steroid use days (< 3 days vs. ≥ 3 days), Kruskal-Walllis test was used for continuous variables and Chi-squared test for categorical variables. The reference significant level was set to be p < 0.05.
Cox proportional hazards models were used for time-to-event survival analysis in which the time was starting from hospital admission and events as the defined outcomes. Adjusted hazards ratios for the primary and secondary outcomes using Cox proportional hazards models were presented. The adjusted variables used in the model were selected via literature review [4] and clinical experts (Additional file Table A). Age, sex, Body Mass Index (BMI) > 30kg/m2, hypertension, cardiovascular disease, diabetes, respiratory disease, chronic kidney disease, sequenced SARS-CoV-2 variant and medications including steroids and tocilizumab/sarilumab were used as pre-defined covariates to adjust in multivariable models. As the distribution of steroid days is right skewed (steroid days ≥ 0), before modelling, the continuous steroid days were transformed with the log of steroid days plus one (log(steroid days + 1)). Missing values of the variant, BMI and ethnicity were imputed as a new category and cases with missing values in IMD were discarded. There were no missing values in other adjusted variables.
Data management was performed using SQL databases, with analysis carried out on the secure King’s Health Partners (KHP) Rosalind high-performance computer infrastructure [5] running Jupyter Notebook 6.0.3, R 3.6.3 and Python 3.7.6.
Results
Description of population, steroid use and outcomes
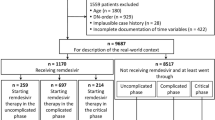
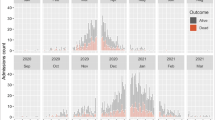
1120 patients were identified with hypoxia on admission of which 1100 were included in the analysis after removal of 20 cases that stayed for less than 24h after admission. 23 cases with missing data in the IMD variable were imputed with median. In-hospital mortality of the whole cohort was 15.0% (Table1). 793/1100 (72.1%) individuals were treated with steroids (> 0 days) and the median of steroid days was 6.0, IQR [3,9]. Before the WHO guideline, only 96/366 (26.2%) patients were treated with steroids compared to 697/734 (95%) after the WHO guideline (Table2; Fig.1). Overall, steroids were used for a median of 0 days [IQR: 0.0,1.0] before the WHO guideline, and 5.5 days [IQR: 3.0,9.0] after WHO guideline. Before the WHO guideline, 17.2% patients had more than 3 days steroids and 7.9% more than 10 days, whilst after the WHO guidelines 71.4% had more than 3 days and 14.3% had more than 10 days (Table2).
Hospital mortality was 20.8% amongst 307 patients who did not receive steroids and 12.7% amongst 793 patients who received steroids. For patients who received ≤ 3 days of steroids, 17.2% died in hospital compared to 13.1% who died in hospital for patients who received > 3days of steroids. A higher mortality rate for patients who received > 10 days of steroids (24.6%) compared to patients who received ≤ 10 days of steroids (13.7%) was observed (Table1).
Comparing patient characteristics between patients who had ≤ 3 days of steroids and who had > 3 days steroids (Table1), we found that patients who had steroids for > 3 days were less likely to be of BAME ethnicity (38.2% vs. 44.6%, p = 0.035), had more obesity (37.6% vs. 30.2%, p = 0.003), had more hypertension (39.4% vs. 32.4%, p = 0.019), a higher proportion with solid organ transplatation (3.6% vs. 1.0%, p = 0.008), higher use of tocilizumab (2.0% vs. 0%, p = 0.003), and had much more Alpha variant due to the emergence of Alpha in wave two (26.9% vs. 10.3%).
Cox proportional hazard model for the outcome of mortality
The Cox proportional hazard models showed significant protective effect of steroids used for more than 3 days compared to less steroids (HR: 0.47 (95% CI: 0.31–0.72)) for mortality. The protective effect of steroids was consistent when using steroids as a continuous variable (HR: 0.86 (95% CI: 0.76–0.96)) (Additional file Table B).
Other variables (Table3) including age, cardiovascular co-morbidity, and human immunodeficiency virus (HIV) infection had significant associations with death. The remaining variables including sex, ethnicity, IMD, hypertension, diabetes, respiratory disease, cancer, kidney disease and transplantation, Alpha variant, obesity (BMI > 30), and tocilizumab administration were not significantly associated with the outcome in the multivariable analysis.
Discussion
This study provides evidence for real-world effectiveness of steroids in reducing death amongst severe COVID-19 patients. The protective effect-size of treatment with steroids was similar to that reported in the RECOVERY clinical trial [3] for a comparable group of patients defined by receipt of oxygen therapy. This adds to the evidence base for a clinical benefit of steroid treatment in COVID-19.
We adjusted for potential confounders (e.g. age, sex, ethnicity, comorbidities, BMI and IMD) as well as the characteristics of the virus (Alpha variant) and another treatment (tocilizumab) with the effect of steroids remaining statistically significant. Undoubtedly, we are unable to adjust for all confounders, including the vaccination status, other co-treatments and improvements introduced around the time of steroids e.g. thromboprophylaxis and proning which might compromise the practical use of the study findings even though the protective effects of steroids were significantly protective in the model. Vaccination could be a big confounder which was started from December 2020 and by the end of the study (17th May 2021), most of the adults had received one dose of vaccination. Regarding other co-treatments, during most of the study period, other drug therapeutics were not routinely deployed, and the effect size of newer treatments like tocilizumab were much less than steroids in clinical trials. No other SARS-CoV-2 variants that have been associated with altered severity of disease were circulating in our population during the study period.
It is notable the study was done in an institution that had good overall comparative NHS outcomes and an standardized mortality ratio (SMR) of 0.5 in ICU patients, with guidelines and practice recommending longer courses of steroids for severe patients. Over 80% of the > 10 steroid-days group were treated deliberately with long steroids and the remaining were on long term steroids as therapeutic immunomodulation for other conditions. Whether longer course of steroids has an additional benefit is not known.
Longer durations of steroids have not been systematically studied and might increase the risk/rate of adverse events, including delayed viral clearance [13]. Some studies are identifying other potential adverse events associated with steroids such as invasive mould infections including aspergillosis and mucormycosis [14], with work ongoing to assess the effect of steroids on risk of bloodstream infection [15].
In this study we investigated the association of steroid days with outcomes, however our analyses are agnostic to the dose of steroids used. There may be reasons why duration of steroid treatment mediates effects on outcomes independently of cumulative dose, for instance if a sustained period of immunosuppression is needed to prevent immune-mediated inflammation. In addition, as this study is retrospective and observational the link between steroids and the outcome is only an association and causality should not be inferred.
Many other studies on the real-world effectiveness of steroids have failed to reproduce the findings of clinical trials. Partly, this may be due to small sample size, heterogeneity of treatment and non-treatment groups, and incorrectly testing associations on individuals not expected to benefit, i.e. cases without evidence of hypoxia. Our study benefits from a wide time period for inclusion, allowing us to capture the changing treatment landscape before steroid use in COVID-19 was standardised in line with national and international guidelines. Additionally our adjustment accounts for many baseline variables which have previously been associated with severe outcomes. The validity of our analyses is supported by the findings that variables previously associated with severity, such as age and cardiovascular comorbidity retain significance in our modelling.
Other studies have found the Alpha variant of SARS-CoV-2 to be associated with severe disease, especially mortality [16,17,18] and hypoxia on admission [19]. However, another study in hospitalised patients did not find such an association [20]. To our knowledge, no studies on the severity of the alpha variant adjusted for newly introduced therapeutics. Interestingly, the association of alpha variant with severe disease as measured by mortality was not found in this study. This is in contrast to our initial findings in the same dataset that the Alpha variant was associated with severity as measured by hypoxia on admission [19]. It may be that severity of the alpha variant is ameliorated by efficacious treatment of hospitalised patients. This may be especially true as during the second wave steroid treatment had been introduced and standardised as the alpha variant emerged. This would also explain the disparity between findings of other published studies, with the only other study of variant status and death in hospitalised patients not finding an association.
Limitations of this study might include potential bias for patients who did not have a chance to receive steroids or received very short steroids because they were very severe and died soon after admission. This is an issue that is intractable with retrospective study, and we attempt to address this by excluding those who died in the first 24h after admission. Another limitation is that the choice of cut-offs for the steroid treatment days were chosen according to data from RECOVERY trial, our local recommendations, and WHO guidelines rather than pharmacological effect of steroids treatment in COVID-19.
Conclusion
The protective effect of steroids in severe COVID-19 seen in our cohort is similar to that seen in clinical trials, confirming the real world effectiveness.
Data Availability
The data that support the findings of this study are available from Guy’s and St Thomas’ NHS Foundation Trust (GSTT) but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of GSTT.
Abbreviations
- ICU:
-
Intensive Care Unit.
- COVID-19:
-
Coronavirus Disease 2019.
- GSTT:
-
Guy’s and St Thomas’ NHS Foundation Trust.
- EPR:
-
Electronic Patient Record.
- IMD:
-
Index of Multiple Deprivation.
- SD:
-
Standard Deviation.
- IQR:
-
Interquartile Range.
- BMI:
-
Body Mass Index.
- HIV:
-
Human Immunodeficiency Virus.
- HR:
-
Hazard Ratio.
References
Bhimraj A, Morgan RL, Shumaker AH, Lavergne V, Baden L, Cheng VC, Edwards KM, Gandhi R, Gallagher J, Muller WJ, O’Horo JC, Shoham S, Murad MH, Mustafa RA, Sultan S, Falck-Ytter Y. Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19. Infectious Diseases Society of America 2022; Version 9.0.1. Available at https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/.
WHO emergency response team. Weekly epidemiological update on COVID-19–17 August 2022, Edition 105, https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---17-august-2022.
The RECOVERY Collaborative Group. Dexamethasone in Hospitalized Patients with Covid-19 — Preliminary Report. N Engl J Med Published online July. 2020;17:NEJMoa2021436. https://doi.org/10.1056/NEJMoa2021436.
RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet Lond Engl. 2021;397(10285):1637–45. https://doi.org/10.1016/S0140-6736(21)00676-0.
The WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis. JAMA. 2020;324(13):1330–41. https://doi.org/10.1001/jama.2020.17023.
van Paassen J, Vos JS, Hoekstra EM, Neumann KMI, Boot PC, Arbous SM. Corticosteroid use in COVID-19 patients: a systematic review and meta-analysis on clinical outcomes. Crit Care. 2020;24(1):696. https://doi.org/10.1186/s13054-020-03400-9.
Sahilu T, Sheleme T, Melaku T. Severity and Mortality Associated with Steroid Use among Patients with COVID-19: A Systematic Review and Meta-Analysis. Interdiscip Perspect Infect Dis. 2021;2021:6650469. https://doi.org/10.1155/2021/6650469.
Yang Z, Liu J, Zhou Y, Zhao X, Zhao Q, Liu J. The effect of corticosteroid treatment on patients with coronavirus infection: a systematic review and meta-analysis. J Infect. 2020;81(1):e13–20. https://doi.org/10.1016/j.jinf.2020.03.062.
Corticosteroids for COVID-19. Accessed August 17. 2021. https://www.who.int/publications-detail-redirect/WHO-2019-nCoV-Corticosteroids-2020.1.
Quick J. nCoV-2019 sequencing protocol. Published online January 22, 2020. https://doi.org/10.17504/protocols.io.bbmuik6w.
Artic Network. Accessed August 17. 2021. https://artic.network/ncov-2019/ncov2019-bioinformatics-sop.html.
Pangolin CoV-lineages. 2021. Accessed August 17, 2021. https://github.com/cov-lineages/pangolin.
Yu WC, Hui DSC, Chan-Yeung M. Antiviral agents and corticosteroids in the treatment of severe acute respiratory syndrome (SARS). Thorax. 2004;59(8):643–5. https://doi.org/10.1136/thx.2003.017665.
Fernandes M, Brábek J. COVID-19, corticosteroids and public health: a reappraisal. Public Health. 2021;197:48–55. https://doi.org/10.1016/j.puhe.2021.05.028.
Snell LB, Irwin J, Buckingham C, et al. Increasing Rates of Intensive Care Unit Bloodstream Infections During the Second COVID-19 Pandemic Wave, Particularly Primary Endogenous Enterococcus Faecium, with High Crude Mortality and Associated with Introduction of Immunomodulatory Therapy. Social Science Research Network; 2021. https://doi.org/10.2139/ssrn.3895049.
Patone M, Thomas K, Hatch R, et al. Mortality and critical care unit admission associated with the SARS-CoV-2 lineage B.1.1.7 in England: an observational cohort study. Lancet Infect Dis Published online June 23, 2021. https://doi.org/10.1016/S1473-3099(21)00318-2.
Patone M, Thomas K, Hatch R, et al. Analysis of Severe Outcomes Associated with the SARS-CoV-2 Variant of Concern 202012/01 in England Using ICNARC Case Mix Programme and QResearch Databases.; 2021:2021.03.11.21253364.
Davies NG, Jarvis CI, Edmunds WJ, Jewell NP, Diaz-Ordaz K, Keogh RH. Increased mortality in community-tested cases of SARS-CoV-2 lineage B.1.1.7. Nature. 2021;593(7858):270–4. https://doi.org/10.1038/s41586-021-03426-1.
Snell L, Wang W, Alcolea-Medina A, et al. Descriptive comparison of admission characteristics between pandemic waves and multivariable analysis of the association of the Alpha variant (B.1.1.7 lineage) of SARS-CoV-2 with disease severity in inner London. BMJ Open. 2022;12:e055474. https://doi.org/10.1136/bmjopen-2021-055474.
Frampton D, Rampling T, Cross A, et al. Genomic characteristics and clinical effect of the emergent SARS-CoV-2 B.1.1.7 lineage in London, UK: a whole-genome sequencing and hospital-based cohort study. Lancet Infect Dis Published online April 12, 2021. https://doi.org/10.1016/S1473-3099(21)00170-5.
Acknowledgements
The authors acknowledge use of the research computing facility at King’s College London, Rosalind (https://rosalind.kcl.ac.uk), which is delivered in partnership with the National Institute for Health Research (NIHR) Biomedical Research Centres at South London & Maudsley and Guy’s & St. Thomas’ NHS Foundation Trusts and NIHR Applied Research Collaboration (ARC) South London at King’s College Hospital (KCH) NHS Foundation Trust and King’s College London, and part-funded by capital equipment grants from the Maudsley Charity (award 980) and Guy’s & St. Thomas’ Charity (TR130505). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, King’s College London, or the Department of Health and Social Care.
Funding
This work was supported by the King’s Together Multi and Interdisciplinary Research Scheme (Wellcome Trust Revenue Retention Award). LBS, and YW are supported by the National Institute for Health Research (NIHR) Biomedical Research Centre programme of Infection and Immunity (RJ112/N027) based at Guy’s and St Thomas’ National Health Service NHS) Foundation Trust and King’s College London and was funded by the National Institute for Health Research (NIHR) [Programme Grants for Applied Research (NIHR202339)]. This work was also supported by The Health Foundation and the Guy’s and St Thomas’ Charity. COG-UK is supported by funding from the Medical Research Council (MRC) part of UK Research & Innovation (UKRI), the National Institute of Health Research (NIHR) and Genome Research Limited, operating as the Wellcome Sanger Institute. VC is supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ National Health Service Foundation Trust and King’s College London, and the Public Health and Multi-morbidity Theme of the National Institute for Health Research’s Applied Research Collaboration (ARC) South London. VC is also supported by the EPSRC CONSULT grant (EP/P010105/1). VC is partly funded by the EPSRC project Consult: Collaborative Mobile Decision Support for Managing Multiple Morbidities, EP/P000339/1. LBS receives funding from the Medical Research Council (MR/W025140/1). DF is also partly funded by DRIVE-Health, KCL funded Centre for Doctoral Training (CDT) in Data-Driven Health.
The funding body did not participate in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
WW and LBS contributed to conceptualisation, data curation, methodology, formal analysis, and writing – original draft and editing. DF performed data curation and visualisation. ALG and NMP provided data interpretation and review&editing. VC performed supervision, funding acquision, project administration, and review&editing. JDE and YW performed conceptualisation, supervision, funding acquision, methodology, project administration, data interpretation, and review&editing. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval for data informatics was granted by The London Bromley Research Ethics Committee (reference (20/HRA/1871)) to the King’s Health Partners Data Analytics and Modelling COVID-19 Group to collect clinically relevant data points from patient’s electronic health records.
Whole genome sequencing of residual viral isolates was conducted under the COVID-19 Genomics UK (COG-UK) consortium study protocol, which was approved by the Public Health England Research Ethics and Governance Group (reference: R&D NR0195).
All methods were carried out in accordance with relevant guidelines and regulations. Patient consent was waived by The London Bromley Research Ethics Committee (reference (20/HRA/1871)).
Consent for publication
Not applicable.
Competing interests
None
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jonathan D Edgeworth and Yanzhong Wang contributed equally and are joint senior authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, W., Snell, L.B., Ferrari, D. et al. Real-world effectiveness of steroids in severe COVID-19: a retrospective cohort study. BMC Infect Dis 22, 776 (2022). https://doi.org/10.1186/s12879-022-07750-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-022-07750-3