Abstract
Background
The effect of coupled plasma filtration adsorption (CPFA) for the treatment of sepsis or septic shock is controversial. A systematic review and meta-analysis was performed to evaluate the impact of CPFA on all-cause mortality in patients with sepsis or septic shock.
Methods
We searched the PubMed, Cochrane, and Embase databases for randomized controlled trials (RCTs) and cohort studies from inception to the 1st of May 2022. We included studies involving patients (˃ 14 years) with sepsis or septic shock. All authors reported our primary outcome of all-cause mortality (hospital mortality, 28-day mortality or 30-day mortality). Results were expressed as odds ratio (OR) with accompanying 95% confidence interval (CI).
Results
Six studies including 537 patients were included. The primary outcome of this meta-analysis showed that the all-cause mortality was about 54.2% (119/243 in the CPFA group and 172/294 in the control group). There was no statistically significant difference in the all-cause mortality between two groups (odds ratio [OR] = 0.75; 95% CI 0.53 to 1.06; P = 0.11; Chi2 = 14.04; I2 = 64%).
Conclusions
The treatment of CPFA failed to decrease all-cause mortality of sepsis or septic shock patients. Further large-scale randomized controlled trials (RCTs) evaluating the ability of this therapy to improve clinical outcomes are still required to confirm these results.
Key messages
-
The treatment of CPFA failed to decrease all-cause mortality of sepsis or septic shock patients.
-
Potential drawbacks of this technique are the unexpected elimination of antibiotics, worsened procoagulant state and oxidative stress, expensive cost.
-
Further rigorous investigation defining both the efficacy and safety of this otherwise promising hemopurification method on sepsis or septic shock is necessary.
Similar content being viewed by others
Background
Sepsis is still a leading cause of mortality in intensive care unit (ICU) patients, mortality of sepsis and septic shock remains incredibly high, ranging between 20 and 40%, depending on the severity of illness [1, 2]. The pathophysiology of sepsis and septic shock is only partly understood, circulating pro-inflammatory and anti-inflammatory mediators appear to participate in the complex cascade of events, which leads to cell and organ dysfunction and, in many cases, death [3, 4]. A systemic inflammatory response with massive cytokine and inflammatory mediator release and the activation of coagulation and complement systems can be induced by the endotoxin of Gram-negative bacteria, which is one of the key triggers of sepsis.
Sepsis or septic shock mainly involves immune cell dysfunction and mediator dysregulation in response to an infection [5]. Terms such as “cell hyporesponsiveness” or “immunoparalysis” have been used to illustrate the inability of cells to respond to lipopolysaccharide (LPS) stimuli ex vivo due to overproduction of anti-inflammatory cytokines [6,7,8,9]. Evidence has been accumulated that severe bacterial infections and septic shock are associated with increased levels of plasma cytokines such as tumor necrosis factor-α (TNF-α) and interleukins (IL)-1 [10]. These inflammatory mediators are important for the antimicrobial response to local body. However, excessive release of the body and overproduction lead to the diffuse tissue injury and multiple organ dysfunction syndrome (MODS) [11]. Therefore, extracorporeal blood purification therapies have been proposed for patients with sepsis in order to improve outcomes since these therapies can alter the host inflammatory response by non-selective removal of inflammatory mediators or bacterial products or both [12].
Theoretically, extracorporeal therapies can be used to remove septic mediators from the bloodstream of critically ill patients [13], coupled plasma filtration adsorption (CPFA) is one such technology. CPFA is an extracorporeal blood purification treatment, which combines a first stage of plasma separation and adsorption of cytokines, inflammatory mediators and/or toxins, followed by a second stage of haemofiltration for volume control and removal of small water-soluble mediators [14]. CPFA was originally developed as a treatment for sepsis in the mid-1990s to address the need to remove cytokines and inflammatory mediators that are not easily or effectively removed by conventional extracorporeal methods (plasma exchange, haemodiafiltration, haemodialysis) [10].
Several studies have observed an improvement in haemodynamic parameters with CPFA in septic shock patients [15, 16]. However, the effect on mortality is still in controversy. Therefore, we conducted a meta-analysis which extracted results from published randomized controlled trials (RCTs) and cohort studies to evaluate the impact of CPFA on mortality in patients with sepsis or septic shock.
Methods
This systematic review and meta-analysis is reported according to the updated Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [17]. Ethical approval was not necessary for this study because it was a review of the published literature.
Search strategy
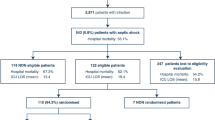
We searched the PubMed, Embase databases and Cochrane Library for studies from inception to the 1st of May 2022 using the following search terms: coupled plasma filtration adsorption, coupled plasma filtration and adsorption, coupled plasma filtration with adsorption, CPFA, plasma adsorption, blood purification, hemoadsorption, sepsis, septic shock. The search was slightly adjusted according to the requirements of the different databases. The authors’ personal files and reference lists of relevant review articles were also reviewed. The search strategy for each database is showed in Additional file 1. The flow chart of the search strategies is summarized in Fig. 1.
Types of outcome measures
The primary outcome was all-cause mortality, all-cause mortality included hospital mortality, 28-day mortality and 30-day mortality. Weighted means were calculated based on the number of patients in each study.
Study selection
The inclusion criteria were as follows: (1) RCTs as well as prospective and retrospective cohort studies; (2) patients (˃ 14 years) with a diagnosis of sepsis or septic shock; (3) all authors reported our primary outcome of all-cause mortality; (4) clearly comparing CPFA group versus control group with clinically relevant outcomes. We excluded studies without clear comparisons of the outcomes. In addition, we excluded review articles and studies about pediatric or animal.
Quality assessment
Two reviewers (Yuting Li and Hongxiang Li) independently performed quality assessment. The quality of studies was assessed using the Cochrane Collaboration’s tool for RCTs [18], and the Newcastle–Ottawa Scale (NOS) was used for cohort studies [19]. The specific elements to minimize bias of RCTs were: (1) randomization sequence (selection bias), (2) allocation concealment (selection bias), (3) blinding of study personnel and participants (performance bias), (4) blinding of outcome assessors (performance bias), (5) complete reporting of data without arbitrarily excluded patients and with low to minimal loss to follow-up (attrition bias), (6) selective reporting bias, and (7) other sources of bias. Satisfactory performance, unclear performance, and unsatisfactory performance of each domain from the tool is denoted by green, yellow, and red color respectively. The risk of bias summary for included RCTs is presented in Fig. 2, the risk of bias graph for included RCTs is presented in Fig. 3.
NOS allocates a maximum of 9 points according to the quality of the selection, comparability, and outcomes of the cohort study populations. Study quality was defined as poor (0–3), fair (4–6) or good (7–9). The quality of the included cohort studies is presented in Table 1.
Statistical analysis
Statistical analyses were performed using Review Manager Version 5.3 (RevMan, The Cochrane Collaboration, Oxford, United Kingdom). Odds ratio (OR) with 95% confidence intervals (CI) was calculated for dichotomous variables. A random-effects model was used to pool studies with significant heterogeneity, as determined by the Chi-squared test (P < 0.10) and inconsistency index (I2 ≥ 50%) [20]. A P-value < 0.05 was set as the threshold of statistical significance. To reduce bias, we performed a subgroup analysis of RCTs and cohort studies.
Result
Study characteristics
The search strategy identified 1316 studies, and the data were from four RCTs and two cohort studies comprising 537 patients (Table 2) [21,22,23,24,25,26]. The characteristics of the included studies are shown in Table 2. A total of six eligible studies were published between 2013 and 2021. Among these studies, one study was conducted in Malaysia, one study was conducted in Egypt, one study was conducted in Spain and three studies were conducted in Italy. Three of these studies were single-center studies and others were multicenter studies.
Primary outcome
A total of five studies including 537 patients were included, and the all-cause mortality was about 54.2% (119/243 in the CPFA group and 172/294 in the control group). There was no statistically significant difference in the all-cause mortality between two groups (odds ratio [OR] = 0.75;95% CI 0.53 to 1.06; P = 0.11; Chi2 = 14.04; I2 = 64%) (Fig. 4). A funnel plot was used to assess the publication bias (Fig. 5).
Discussion
Sepsis is one of the main causes of death in critically ill patients worldwide, and in many cases it is associated with renal and/or other organ failure. However, we do not have a unique efficient therapy to reduce this extremely high mortality rate. Both pro-inflammatory and anti-inflammatory mediators participate in the pathogenesis of sepsis and explain the failure of specific therapies to improve survival. Continuous extracorporeal therapies have been proposed as a therapeutic option in sepsis [27]. One of the emerging treatments in patients with sepsis and septic shock is CPFA.CPFA is a technique that separates plasma from the blood using a plasma filter. The plasma is then passed through a synthetic resin cartridge and returned to the blood. A second blood filter is used to remove excess fluid and small molecular weight toxins [28]. The nonselective removal of inflammatory mediators is achieved by hydrophobic styrene resin, which has high affinity and capacity for many cytokines and mediators [29]. In vitro studies have demonstrated the efficacy of CPFA in adsorbing inflammatory mediators like IL-1β, IL-6, IL-8, IL-10, and TNF-α amongst others [27]. CPFA has also been shown to enhance early hemodynamic stability, reduce inotropic support requirement, and improve the immune response in septic patients [30]. However, these trials have so far failed to demonstrate any improvement in hard clinical outcomes.
Our systematic review and meta-analysis of six studies including 537 patients compared CPFA and control group in patients with sepsis or septic shock. We found that the overall all-cause mortality was about 54.2% and there was no statistically significant difference in the all-cause mortality between two groups. Guidelines, for example, state that ‘hemofiltration should not be used in patients with sepsis without renal indications unless ongoing studies provide positive results’ [31]. The role of plasma exchange remains equally controversial [32, 33]. The extracorporeal removal of septic mediators is not recommended in the 2016 edition of the Surviving Sepsis Campaign (SSC) due to the absence of large, randomized controlled trials demonstrating its efficacy [34]. Experimental study even showed that treatment with CPFA did not protect from progression of septic hypotension; failed to counteract the progressive alterations in microcirculatory perfusion, energy metabolism, and organ function; and even aggravated the sepsis-induced disturbances in coagulation and oxidative/nitrosative stress [29].
What are the implications of our meta-analysis’s results? Firstly, CPFA is a blood purification therapy aimed at modulating the host inflammatory response involved in sepsis pathogenesis. CPFA not only removes substances harmful to the body, but also removes beneficial substances. Piperacillin, tazobactam, and vancomycin, administered during CPFA, using the appropriate dosing regimens, achieved acceptable serum concentrations, despite adsorption on the resin cartridge [35]. However, a potential disadvantage of this technique is that it may accidentally eliminate other kinds of antibiotics. Any delay in receiving appropriate antibiotic therapy in severe sepsis or septic shock patients is associated with excess mortality [36,37,38]. Moreover, according to calculation, CPFA may removes 50% more antibiotics than does standard continuous renal replacement therapy, increasing the possibility of undertreatment. Increasing antibiotic clearance by adding the effect of at least 10 h’ renal replacement therapy to a well-functioning kidney could have caused treatment underdosing [26]. A significant dose–response effect of treated plasma on mortality was demonstrated in patients without severe renal failure. As a result, monitoring of antibiotics serum concentrations remains essential to avoid antibiotics underdosing. Secondly, even though previous studies have been promising, numerous questions, including the timing, duration, and frequency of these therapies in the clinical setting, remain unanswered. We hypothesize a connection to hemodynamic instability consequent on renal replacement therapy [39] that has been shown to increase mortality [40]. This instability may complicate the said therapy, especially when patients have not been fully stabilized, and may be related to early commencement of treatment(no more than 12 h from diagnosis) [26]. Thirdly, early treatment with CPFA failed to afford any protection against sepsis-mediated hemodynamic and physiological disturbances and tended to worsen procoagulant state and oxidative stress [29]. Fourthly, they did not take cost into account for each treatment. The cost of new sorbents may be one the main drawbacks in CPFA.
There are several limitations in our meta-analysis. First, the number of included studies is small. Further randomized clinical studies should be conducted in order to confirm the results. Second, many of the clinical outcomes such as ICU length of stay, hospital length of stay, hemodynamic parameters were not included in most of the studies examined in this meta-analysis. Therefore, we were unable to conduct a meta-analysis on secondary outcomes. Third, Organ dysfunction is also a very important clinical outcome. However, few included studies had showed this data. Fourth, although we had performed a subgroup analysis of RCTs and cohort studies, there was still substantial heterogeneity among the included studies. Very heterogeneous populations were included in both observational and randomized studies. In addition, inclusion/exclusion criteria and comorbidities were widely different among included studies which supposed a limitation to interpret results. Therefore, our findings should be interpreted with caution.
Conclusion
In our systematic review and meta-analysis, the treatment of CPFA failed to decrease all-cause mortality of sepsis or septic shock patients. This result indicates that further rigorous investigation defining both the efficacy and safety of this otherwise promising hemopurification method on sepsis or septic shock is necessary. Further large-scale RCTs evaluating the ability of this therapy to improve clinical outcomes are still required to confirm these results.
Availability of supporting data
All data generated or analyzed during this study are included in this published article.
Abbreviations
- CPFA:
-
Coupled plasma filtration adsorption
- ICU:
-
Intensive care unit
- LPS:
-
Lipopolysaccharide
- TNF-α:
-
Tumor necrosis factor-α
- IL:
-
Interleukins
- MODS:
-
Multiple organ dysfunction syndrome
- RCTs:
-
Randomized controlled trials
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- NOS:
-
Newcastle–Ottawa Scale
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- SSC:
-
Surviving Sepsis Campaign
References
Leligdowicz A, Dodek PM, Norena M, Wong H, Kumar A, Kumar A, Co-operative Antimicrobial Therapy of Septic Shock Database Research Group. Association between source of infection and hospital mortality in patients who have septic shock. Am J Respir Crit Care Med. 2014;189(10):1204–13.
Liu V, Escobar GJ, Greene JD, Soule J, Whippy A, Angus DC, et al. Hospital deaths in patients with sepsis from 2 independent cohorts. JAMA. 2014;312(1):90–2.
Camussi G, Montrucchio G, Dominioni L, Dionigi R. Septic shock: the unravelling of molecular mechanisms. Nephrol Dial Transplant. 1995;10(10):1808–13.
Pinsky MR. Sepsis: a pro- and anti-inflammatory disequilibrium syndrome. Contrib Nephrol. 2001;132:354–66.
King EG, Bauzá GJ, Mella JR, Remick DG. Pathophysiologic mechanisms in septic shock. Lab Invest. 2014;94(1):4–12.
Hotchkiss RS, Coopersmith CM, McDunn JE, Ferguson TA. The sepsis seesaw: tilting toward immunosuppression. Nat Med. 2009;15(5):496–7.
Volk HD, Reinke P, Krausch D, Zuckermann H, Asadullah K, Müller JM, et al. Monocyte deactivation: rationale for a new therapeutic strategy in sepsis. Intensive Care Med. 1996;22(Suppl 4):S474–81.
Brandtzaeg P, Osnes L, Ovstebø R, Joø GB, Westvik AB, Kierulf P. Net inflammatory capacity of human septic shock plasma evaluated by a monocyte-based target cell assay: identification of interleukin-10 as a major functional deactivator of human monocytes. J Exp Med. 1996;184(1):51–60.
Adib-Conquy M, Adrie C, Moine P, Asehnoune K, Fitting C, Pinsky MR, et al. NF-kappaB expression in mononuclear cells of patients with sepsis resembles that observed in lipopolysaccharide tolerance. Am J Respir Crit Care Med. 2000;162(5):1877–83.
Tetta C, Cavaillon JM, Schulze M, Ronco C, Ghezzi PM, Camussi G, et al. Removal of cytokines and activated complement components in an experimental model of continuous plasma filtration coupled with sorbent adsorption. Nephrol Dial Transplant. 1998;13(6):1458–64.
Reinhart K, Sakka SG, Meier-Hellmann A. Haemodynamic management of a patient with septic shock. Eur J Anaesthesiol. 2000;17(1):6–17.
Rimmelé T, Kellum JA. Clinical review: blood purification for sepsis. Crit Care. 2011;15(1):205.
Panagiotou A, Gaiao S, Cruz DN. Extracorporeal therapies in sepsis. J Intensive Care Med. 2013;28(5):281–95.
La Manna G, Donati G. Coupled plasma filtration adsorption: a multipurpose extracorporeal detoxification therapy. Blood Purif. 2018;46(3):228–38.
Franchi M, Giacalone M, Traupe I, Rago R, Baldi G, Giunta F, et al. Coupled plasma filtration adsorption improves hemodynamics in septic shock. J Crit Care. 2016;33:100–5.
Formica M, Olivieri C, Livigni S, Cesano G, Vallero A, Maio M, et al. Hemodynamic response to coupled plasmafiltration-adsorption in human septic shock. Intensive Care Med. 2003;29(5):703–8.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. J Clin Epidemiol. 2021;134:178–89.
Higgins JPT, Sally G. Cochrane handbook for systematic reviews of interventions, version 5.1.0. 2016. http://training.cochrane.org/handbook. Accessed 23 Dec 2016.
Wells GA, Shea BJ, O’connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of non-randomized studies in meta-analysis. Appl Eng Agric. 2014;18:727–34.
Biggerstaff BJ, Jackson D. The exact distribution of Cochran’s heterogeneity statistic in one-way random effects meta-analysis. Stat Med. 2008;27(29):6093–110.
Hassan J, Cader RA, Kong NC, Mohd M, Rahman AR, Hod R. Coupled plasma filtration adsorption (CPFA) plus continuous veno-venous haemofiltration (CVVH) versus CVVH alone as an adjunctive therapy in the treatment of sepsis. EXCLI J. 2013;12:681–92.
Livigni S, Bertolini G, Rossi C, Ferrari F, Giardino M, Pozzato M, GiViTI: Gruppo Italiano per la Valutazione degli Interventi in Terapia Intensiva, et al. Efficacy of coupled plasma filtration adsorption (CPFA) in patients with septic shock: a multicenter randomised controlled clinical trial. BMJ Open. 2014;4(1):e003536.
Yaroustovsky M, Abramyan M, Krotenko N, Popov D, Plyushch M, Rogalskaya E. A pilot study of selective lipopolysaccharide adsorption and coupled plasma filtration and adsorption in adult patients with severe sepsis. Blood Purif. 2015;39(1–3):210–7.
Giménez-Esparza C, Portillo-Requena C, Colomina-Climent F, Allegue-Gallego JM, Galindo-Martínez M, Mollà-Jiménez C, et al. The premature closure of ROMPA clinical trial: mortality reduction in septic shock by plasma adsorption. BMJ Open. 2019;9(12):e030139.
Mariano F, Hollo’ Z, Depetris N, Malvasio V, Mella A, Bergamo D, et al. Coupled-plasma filtration and adsorption for severe burn patients with septic shock and acute kidney injury treated with renal replacement therapy. Burns. 2020;46(1):190–8.
Garbero E, Livigni S, Ferrari F, Finazzi S, Langer M, Malacarne P, GiViTI, et al. High dose coupled plasma filtration and adsorption in septic shock patients Results of the COMPACT-2: a multicentre, adaptive, randomised clinical trial. Intensive Care Med. 2021;47(11):1303–11.
Bellomo R, Tetta C, Brendolan A, Ronco C. Coupled plasma filtration adsorption. Blood Purif. 2002;20(3):289–92.
Ronco C, Brendolan A, Lonnemann G, Bellomo R, Piccinni P, Digito A, et al. A pilot study of coupled plasma filtration with adsorption in septic shock. Crit Care Med. 2002;30(6):1250–5.
Sykora R, Chvojka J, Krouzecky A, Radej J, Kuncova J, Varnerova V, et al. Coupled plasma filtration adsorption in experimental peritonitis-induced septic shock. Shock. 2009;31(5):473–80.
Cesano G, Livigni S, Vallero A, Olivieri C, Borca M, Quarello F, et al. Treatment of septic shock with the use of CPFA (associated plasma filtration and adsorption): impact on hemodynamics monitored with PiCCO. G Ital Nefrol. 2003;20(3):258–63.
Carlet J; International Sepsis Forum. Immunological therapy in sepsis: currently available. Intensive Care Med. 2001;27(Suppl 1):S93-103.
Reeves JH, Butt WW, Shann F, Layton JE, Stewart A, Waring PM, et al. Continuous plasmafiltration in sepsis syndrome. Crit Care Med. 1999;27(10):2096–104.
Busund R, Koukline V, Utrobin U, Nedashkovsky E. Plasmapheresis in severe sepsis and septic shock: a prospective, randomised, controlled trial. Intensive Care Med. 2002;28(10):1434–9.
Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med. 2017;45(3):486–552.
Page M, Cohen S, Ber CE, Allaouchiche B, Kellum JA, Rimmelé T. In vivo antibiotic removal during coupled plasma filtration adsorption: a retrospective study. ASAIO J. 2014;60(1):70–5.
Andersson M, Östholm-Balkhed Å, Fredrikson M, Holmbom M, Hällgren A, Berg S, et al. Delay of appropriate antibiotic treatment is associated with high mortality in patients with community-onset sepsis in a Swedish setting. Eur J Clin Microbiol Infect Dis. 2019;38(7):1223–34.
Seymour CW, Kahn JM, Martin-Gill C, Callaway CW, Yealy DM, Scales D, et al. Delays from first medical contact to antibiotic administration for Sepsis. Crit Care Med. 2017;45(5):759–65.
Weiss SL, Fitzgerald JC, Balamuth F, Alpern ER, Lavelle J, Chilutti M, et al. Delayed antimicrobial therapy increases mortality and organ dysfunction duration in pediatric sepsis. Crit Care Med. 2014;42(11):2409–17.
Douvris A, Zeid K, Hiremath S, Bagshaw SM, Wald R, Beaubien-Souligny W, et al. Mechanisms for hemodynamic instability related to renal replacement therapy: a narrative review. Intensive Care Med. 2019;45(10):1333–46.
Silversides JA, Pinto R, Kuint R, Wald R, Hladunewich MA, Lapinsky SE, et al. Fluid balance, intradialytic hypotension, and outcomes in critically ill patients undergoing renal replacement therapy: a cohort study. Crit Care. 2014;18(6):624.
Acknowledgements
Not applicable.
Funding
This work was supported by Finance Department of Jilin Province (JLSWSRCZX2020-022).
Author information
Authors and Affiliations
Contributions
YL searched the scientific literature and drafted the manuscript. HL contributed to conception, design and data interpretation. JG and YW helped to collect the data and performed statistical analyses. DZ contributed to conception, design, data interpretation, manuscript revision for critical intellectual content and supervision of the study. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Search strategy for each database.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, Y., Li, H., Guo, J. et al. Coupled plasma filtration adsorption for the treatment of sepsis or septic shock: a systematic review and meta-analysis. BMC Infect Dis 22, 714 (2022). https://doi.org/10.1186/s12879-022-07689-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-022-07689-5