Abstract
Background
Mesenteric inflammatory veno-occlusive disease (MIVOD) is difficult to diagnose because of its rarity, nonspecific clinical findings, and frequent confusion with other diseases including inflammatory bowel disease. This report presents a very rare case of MIVOD that occurred during the course of ulcerative colitis (UC).
Case presentation
A 32-year-old man, who had been diagnosed with UC at the age of 29 and was in remission maintained by oral administration of 5-aminosalicylic acid (5-ASA), showed exacerbation of diarrhea and was admitted to the hospital. Since it was deemed an exacerbation of UC, intravenous steroid therapy and oral administration of tacrolimus were initiated, but his condition continued to worsen. Abdominal computed tomography (CT) was performed and showed intraperitoneal free air, leading to a diagnosis of gastrointestinal perforation and the performance of emergency surgery (subtotal colectomy and ileostomy). Histopathological examination of the resected colon of the patient showed mucosal inflammatory findings that were not typical of UC, including multiple organized thrombi with recanalization in the veins existing in the submucosal layer to the subserosal layer and an increased infiltration of inflammatory cells. These findings led to the pathological diagnosis of MIVOD.
Conclusion
We report a very rare case in which MIVOD occurred during the course of UC.
Similar content being viewed by others
Background
Mesenteric inflammatory veno-occlusive disease (MIVOD) is a rare disease characterized by lymphocytic inflammation of the intestinal wall and mesenteric veins and venules, without arterial involvement and without evidence of systemic vasculitis, which results in intestinal ischemia [1]. MIVOD is difficult to diagnose because of its rarity, nonspecific clinical findings, and frequent confusion with other diseases, along with the necessity for histopathological verification [2]. Drugs, cytomegalovirus, bone marrow transplants, and antiphospholipid antibody syndrome are all involved in the disease, but a detailed pathogenesis remains unknown [3,4,5,6]. MIVOD is mostly resistant to medical treatment, as anticoagulants and immunoregulatory drugs have proven ineffective for patients with MIVOD [7, 8]. In most cases, surgical resection of the involved bowel is required and prognosis is typically excellent [7, 8]. Here we report a very rare case in which MOVID occurred during the course of ulcerative colitis (UC).
Case presentation
A 32-year-old man initially visited our hospital with the chief complaint of bloody stool at the age of 29. A colonoscopy performed after hospitalization revealed loss of the vascular appearance, erythema, friability of the mucosa, and spontaneous bleeding in the sigmoid and the rectal colon (Fig. 1a). Histological examination of biopsies showed chronic inflammatory cell infiltration with distortions of crypt architecture and cryptitis (Fig. 1b). Based on these findings, the patient was diagnosed with moderate UC. Although his clinical symptoms were relatively controlled by the oral administration of 5-aminosalicylic acid (5-ASA).
a Colonoscopy revealed loss of the vascular appearance, erythema, friability of the mucosa, and spontaneous bleeding in the sigmoid and rectal colon. These endoscopic findings are consistent with active ulcerative colitis. b Pathological images showed chronic inflammatory cell infiltration, distortion of crypt architecture, and inflammation of the crypts. Hematoxylin-eosin staining
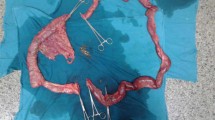
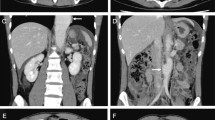
He was then hospitalized at the age of 32 for severe diarrhea and progressive epigastric pain. He had rectal bleeding and his disease activity was severe according to the Truelove and Witts’ severity index. On physical examination at the time of hospitalization, body temperature was 39.8°C and the abdomen was diffusely tender without muscular guarding or rigidity. Laboratory data, including complete blood count and coagulation profile, were within normal range aside from microcytic and hypochromic anemia with a hemoglobin level of 11.5 g/dL. Serum C-reactive protein (CRP) level was elevated at 23.9 mg/L. Serum tumor markers were within the normal range. Blood, stool, and urine cultures were negative. Cytomegalovirus (CMV) antigenemia test was negative, and antinuclear antibody and serum complement were both in the normal range. Contrast-enhanced computed tomography (CT) showed marked colonic wall thickening extending from the transverse colon to the rectum, but there was no abnormality in the mesenteric vessels and no abdominal free air (Fig. 2a). Colonoscopy revealed highly edematous mucosa, complete obliteration of the vascular pattern, and multiple deep ulcerations extending from the transverse colon to the rectum (Fig. 3a). Histopathology of biopsies obtained during colonoscopy showed findings consistent with possible UC. Basal plasmacytosis and eosinophil infiltration were unremarkable and there was no typical “owl eye” inclusion indicating CMV infection (Fig. 3b). Based on clinical findings, Buerger’s disease, Behçet’s disease, rheumatoid arthritis, and systemic lupus erythematosus were excluded. A diagnosis of severe UC was made and intravenous steroids and antibiotic therapy were started. Since the clinical symptoms and laboratory blood data were not improved by the systemic steroid therapy, the dose of steroids was decreased gradually on the 10th hospital day, while oral administration of tacrolimus was started and maintained at a high trough level. However, his clinical symptoms were completely unresponsive to tacrolimus therapy and surgical colectomy was recommended, but he strongly refused surgery, and consent was not obtained. We performed four sessions of granulocyte and monocyte adsorption apheresis as an additional therapy. On the 35th hospital day, his abdominal pain had worsened with rebound tenderness and ultimately led to a state of shock. CT revealed dilated loops of colon and abdominal free air (Fig. 2b). Following a diagnosis of perforation peritonitis complicated with toxic megacolon, subtotal colectomy and ileostomy were performed (Fig. 4a). Histopathology of the surgically resected colon showed only minor changes in the mucosal histological structure; however, many veins present from the submucosal layer to the subserosal layer demonstrated prominent myointimal hyperplasia with narrowed lumens in contrast to arteries that were essentially normal (Fig. 4b and c). Vasculitis of the small mesenteric veins and their intramural tributaries with thrombosis was also observed (Fig. 4d). These findings confirmed the diagnosis of MIVOD. Despite the occurrence of various postoperative complications, including acute respiratory distress syndrome (ARDS), CMV infection (Given the positive finding of CMV-C7HRP in his blood during management in the postoperative ICU, administration of ganciclovir was performed and we confirmed a negative result for CMV-C7HRP 14 days after administration of ganciclovir.), rectal hemorrhage, and renal failure, the patient gradually improved with multidisciplinary treatment and was discharged after 6 months of hospitalization. He is in remission with oral administration of 5ASA after discharge. The clinical course of this patient is shown in Fig. 5.
a Gross feature of surgical excision specimen. Deep longitudinal ulcers with fusion tendency were scattered in the large intestine. The yellow line indicates the site where the following tissue examination was performed. b Histopathology showed only minor changes in the mucosalstructure, but many veins in submucosal layer demonstrated prominent myointimal hyperplasia with narrowed lumens. Hematoxylin-eosin staining. c Arteries of submucosal layer were essentially normal (*). Venous thrombi of varying age were identified (arrow heads). d Small mesenteric vein and its intramural tributaries showed partly organized thrombi. Hematoxylin-eosin staining
Discussion
The first case of isolated mesenteric venous inflammation and associated thrombosis leading to mesenteric ischemia was described in 1976 [9]. Since then, various terminologies have been used to describe these pathological findings, including necrotizing and enterocolic lymphocytic phlebitis [10], idiopathic myointimal hyperplasia [11], intramural mesenteric venulitis, and most recently, MIVOD.
The clinical symptoms of patients with MIVOD are abdominal pain and mucus per rectum or bloody diarrhea of variable duration. Endoscopic findings are not typical. Several reports show that contrast-enhanced CT and angiography are useful [7, 12]. However, in our case, contrast-enhanced CT showed colonic wall thickening, but was unable to detect the abnormalities of the mesenteric veins. The diagnosis of MIVOD is based on histopathological findings combined with the exclusion of other systemic diseases such as Buerger’s disease, Behçet’s disease, rheumatoid arthritis, and systemic lupus erythematosus [13].
Histopathological findings of MIVOD show isolated vasculitis of the small mesenteric veins and their intramural tributaries with thrombosis as a secondary manifestation, but arterial involvement is not seen [13]. Some subacute cases show granulomatous phlebitis and myointimal hyperplasia, as observed in our case.
In addition to the similarity of the clinical symptoms and endoscopic images between MIVOD and UC, endoscopic biopsies from patients with MIVOD show nonspecific mucosal inflammation consistent with UC because these endoscopic biopsies may not include the submucosal vessels that are necessary for an accurate diagnosis [8]. Therefore, MIVOD is often misdiagnosed and treated as UC, and when the appropriate treatment for UC is unsuccessful, colectomy must be performed, resulting in the pathological diagnosis of MIVOD [14].
Although our case was clinically and pathologically consistent with UC at the disease onset, his clinical course suddenly worsened 3 years later, resulting in a subtotal colectomy and the pathologically proven diagnosis of MIVOD. Because UC and MIVOD have similar endoscopic findings and there are no endoscopic findings specific to MIVOD, it is very difficult to diagnose MIVOD with endoscopic findings alone. In addition, since the postoperative specimen revealed that the surface mucosal structure of the colon at the site where phlebitis and venous thrombus existed was relatively maintained, it seemed unlikely that secondary thrombus was formed by exacerbation of UC. Therefore, we contend that this case of MIVOD developed during the course of UC.
Generally, the postoperative course of MIVOD is good; however, in our case, certain issues remained, such as residual rectal inflammation and persistent small bleeding. Although it is advantageous to remove the residual rectum and close the ileostomy, the possibility of anastomotic leakage must be sufficiently evaluated. At the present time, we currently resort to conservative observation, with the need for careful, continued evaluation of the residual rectum and lower small intestine in the future.
Conclusion
MIVOD has no specific clinical findings and the final diagnosis is based on the histologic diagnosis of resected intestinal tract, resulting in a small number of reported cases of MIVOD. Furthermore, cases of MIVOD developing during the course of UC appear to be very rare. Further investigation of the mechanism of MIVOD onset and its relationship with UC is necessary through the accumulation of more cases in the future.
Abbreviations
- 5-ASA:
-
5-aminosalicylic acid
- ARDS:
-
Acute respiratory distress syndrome
- CMV:
-
Cytomegalovirus
- CRP:
-
C-reactive protein
- CT:
-
Computed tomography
- GMA:
-
Granulocyte Monocyte Apheresis
- LVFX:
-
Levofloxacin
- MIVOD:
-
Mesenteric inflammatory veno-occlusive disease
- PSL:
-
Prednisolone
- UC:
-
Ulcerative colitis
References
Huiberts AA, Donkervoort SC, Blok WL, Blaauwgeers HL. Enterocolic lymphocytic phlebitis: an oncologic surgical resection without a preoperative pathologic diagnosis. J Surg Case Rep. 2014;15:2014(5).
Lie JT. Mesenteric inflammatory veno-occlusive disease (MIVOD): an emerging and unsuspected cause of digestive tract ischemia. Vasa. 1997;26(2):91–6.
Saraga EP, Costa J. Idiopathic entero-colic lymphocytic phlebitis. A cause of ischemic intestinal necrosis. Am J Surg Pathol. 1989;13(4):303–8.
Ailani RK, Simms R, Caracioni AA, West BC. Extensive mesenteric inflammatory veno-occlusive disease of unknown etiology after primary cytomegalovirus infection: first case. Am J Gastroenterol. 1997;92(7):1216–8.
Pérez-Corral AM, Serrano D, Menarguez-Palanca J, Carrión R, Barreiro I, Gómez-Pineda A, et al. Mesenteric inflammatory veno-occlusive disease (MIVOD) after allogeneic peripheral blood stem cell transplantation (PBSCT). Bone Marrow Transplant. 2008;41(3):311–3.
Gül A, Inanç M, Ocal L, Koniçe M, Aral O, Lie JT. Primary antiphospholipid syndrome associated with mesenteric inflammatory veno-occlusive disease. Clin Rheumatol. 1996;15(2):207–10.
Miracle AC, Behr SC, Benhamida J, Gill RM, Yeh BC. Mesenteric inflammatory veno-occlusive disease: radiographic and histopathologic evaluation of 2 cases. Abdom Imaging. 2014;39(1):18–24.
Kao PC, Vecchio JA, Hyman NH, West AB, Blaszyk H. Idiopathic myointimal hyperplasia of mesenteric veins: a rare mimic of idiopathic inflammatory bowel disease. J Clin Gastroenterol. 2005;39(8):704–8.
Stevens SM, Gue S, Finckh ES. Necrotizing and giant cell granulomatous phlebitis of caecum and ascending colon. Pathology. 1976;8(3):259–64.
Haber MM, Burrell M, West AB. Enterocolic lymphocytic phlebitis. Clinical, radiologic, and pathologic features. J Clin Gastroenterol. 1993;17(4):327–32.
Genta RM, Haggitt RC. Idiopathic myointimal hyperplasia of mesenteric veins. Gastroenterology. 1991;101(2):533–9.
Lavu K, Minocha A. Mesenteric inflammatory veno-occlusive disorder: a rare entity mimicking inflammatory bowel disorder. Gastroenterology. 2003;125(1):236–9.
Eryigit E, Hoentjen F, Barbe E, van Meyel JJ. Intestinal ischaemia caused by mesenteric inflammatory veno-occlusive disease. Neth J Med. 2008;66(11):486–8.
Canavan JB, Coss A, Leader M, Patchett SE. Acute fulminant colitis caused by idiopathic mesenteric inflammatory veno-occlusive disease. Case Rep Gastroenterol. 2007;1(1):152–6.
Acknowledgements
Not applicable.
Funding
There is no funding support for this manuscript.
Availability of data and materials
Not applicable.
Author information
Authors and Affiliations
Contributions
YY1 and KS wrote the paper; YY2, YS and YH contributed to the paper design and coordination; YA, YO and TA contributed to the pathological examination. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Written consent was obtained from the patient. As a case report, approval from the institutional review board was not needed.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Yamada, Y., Sugimoto, K., Yoshizawa, Y. et al. Mesenteric inflammatory veno-occlusive disease occurring during the course of ulcerative colitis: a case report. BMC Gastroenterol 18, 9 (2018). https://doi.org/10.1186/s12876-018-0737-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12876-018-0737-7