Abstract
Background
Diverse microbiome communities drive biogeochemical processes and evolution of animals in their ecosystems. Many microbiome projects have demonstrated the power of using metagenomics to understand the structures and factors influencing the function of the microbiomes in their environments. In order to characterize the effects from microbiome composition for human health, diseases, and even ecosystems, one must first understand the relationship of microbes and their environment in different samples. Running machine learning model with metagenomic sequencing data is encouraged for this purpose, but it is not an easy task to make an appropriate machine learning model for all diverse metagenomic datasets.
Results
We introduce MegaR, an R Shiny package and web application, to build an unbiased machine learning model effortlessly with interactive visual analysis. The MegaR employs taxonomic profiles from either whole metagenome sequencing or 16S rRNA sequencing data to develop machine learning models and classify the samples into two or more categories. It provides various options for model fine tuning throughout the analysis pipeline such as data processing, multiple machine learning techniques, model validation, and unknown sample prediction that can be used to achieve the highest prediction accuracy possible for any given dataset while still maintaining a user-friendly experience.
Conclusions
Metagenomic sample classification and phenotype prediction is important particularly when it applies to a diagnostic method for identifying and predicting microbe-related human diseases. MegaR provides various interactive visualizations for user to build an accurate machine-learning model without difficulty. Unknown sample prediction with a properly trained model using MegaR will enhance researchers to identify the sample property in a fast turnaround time.
Similar content being viewed by others
Background
Metagenomics, studying microbial community and diversity from environmental samples directly without culture, is applied in many research projects for last two decades aiming to understand microbes’ impact on human, animal, plant, ocean, and environmental niches [1].
The human microbiota is the aggregated clusters of microorganisms that colonize on exposed surfaces such as skin, respiratory tract, and gastrointestinal tract. Human-microbes projects such as MetaHIT consortium and human microbiome project (HMP) sought to study microorganism diversity in and on healthy or sick cohorts via advanced metagenomic sequencing techniques [2, 3]. Not only human but also diverse ecosystems with microbes were studied using metagenomics including Tara Oceans project that is another big consortium to investigate the ocean microbiome to understanding its functional role at a global scale [4]. These large-scale metagenomics projects provide huge publicly available datasets. Deep analysis of such metagenomic datasets can reveal the secret of interactions between microbes and hosts in nature.
Analyzing metagenomic data is challenging because a sample can contain thousands of species, each with differing abundances, and multiple copies of the genomic sequences are sheared and fragmented as reads. The most often used technique to analyze the composition and diversity of microbes is 16S rRNA gene amplicon analysis that amplifies the 16S rRNA region to distinguish substantially identified gene regions [5]. Taxonomic assignment relies on the association of a specific 16S rRNA gene with a taxon; these associations are defined as operational taxonomic units (OTUs). Since OTUs are most commonly analyzed at the phyla or genera resolution, 16S rRNA sequencing technologies have a limited scope in analyzing microbial communities at the species and strain level. More recently, whole genome shotgun sequencing (WGS) has been adopted to increase sequence read depth and extend the range of capture to species level resolution and other microbes including viruses [6]. With extensive coverage provided, WGS allows for a more diverse picture of the microbes at the species and even strain level. Both sequencing techniques are currently being used to study the microbial landscape and have been evaluated for their inherent strengths and weaknesses [7]. The choice of 16S rRNA sequencing or WGS usually depends on the nature of the study: 16S is proper for large-scale analysis of a many samples such as longitudinal research and WGS provides a greater potential for higher resolution by identifying strains and even viruses that the 16S approach cannot. To address this technology gap, new advanced sequencing techniques are continuing to be developed and evaluated including shallow shotgun sequencing [8].
Taxonomy classification in metagenomics refers to identifying microbial genomes from closely related organisms in the metagenomic samples. QIIME (QIIME 2) is a widely used tools to analyze 16S rRNA gene sequences using OTU binning method from microbial communities [9]. In WGS, taxonomic profiles are examined by searching reads against reference genomes [10,11,12], analyzing k-mer frequency of reads [13, 14], or aligning reads with clade-specific marker genes including MetaPhlAn2 [15, 16]. A variety of tools including de novo assemblers, strain-level profilers, and functional analysis tools are also intensively used in metagenomics research [17,18,19,20,21]. Recently studied metagenomic research and related software tools are advanced compared to the standard metagenomics protocol such as identifying and quantifying microbial community composition. For example, the DIABIUMME project was designed to study the interactions and development of microbes, immune system, and diseases [22,23,24]. Another interesting metagenomic project is MetaSub designed for studying urban microbiomes their differences in the largest metro system in the world [25]. These types of research and challenges can be solved by investigating microbial patterns of the samples. Machine and deep learning techniques hold great promise in identifying such microbial patterns of the samples effectively and precisely [26, 27].
There were several machine-learning-based software tools to analyze the relationship of microbial sequencing data and sample phenotype. MetAML utilizes microbiome features by means of different machine learning classifiers to study the association of the microbes and phenotypes [28]. MetaDprof fits smoothing spline regression model to identify differential abundances of samples [29]. MetaLonDa is an R package that is able to identify substantial time intervals of way different abundant microbial features in longitudinal studies [30]. MetaNN provides a neural network classifier to identify host phenotypes from metagenomic data [31].
Such proposed software tools provide some advantages for microbiome-phenotype association prediction, but there are some limits. MetAML only supports WGS data, not 16S rRNA data set analysis. 16S rRNA sequencing is still mostly used, and is a powerful sequencing technique in metagenomics, it is important to provide a tool utilizing both 16S and WGS data. MetaDprof and MetaLonDa can effectively perform for the data sets from longitudinal studies, but they were not designed for general classifications of samples for phenotype prediction. MetaNN only utilized only 16S rRNA sequences, not WGS sequences [31].
We therefore developed MegaR (https://github.com/BioHPC/MegaR) to study microbiome-phenotype associations effectively and precisely including disease prediction capability. Our proposed framework MegaR has the following three main contributions:
-
1.
MegaR supports both 16S rRNA and shotgun metagenomic sequencing data and can generate a model using different taxon level and different machine learning techniques.
-
2.
MegaR provides user-friendly features for data preprocessing, model development, and model cross validation by power of interactive web-supporting library, R-Shiny,
-
3.
MegaR classifies and predicts unknown samples based on the developed model precisely and speedily.
In this study, three different studies of DIABUMME project were used to assess the independent prediction accuracy of models for both 16S and WGS data and to compare strategies for practical use of the microbiome as a prediction tool. We also provide benchmark results of MegaR against MetAML using the data sets provided by MetAML to show the model accuracy, effectiveness, and user-friendly fine-tuning options to generate an optimized model with just a few clicks.
Implementation
MegaR data input
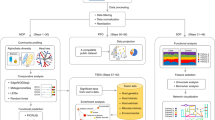
We developed MegaR as an R package that uses 16S or whole genome taxonomic profile data sets to train a machine learning model for classification of unknown profiles. An overview of the MegaR pipeline can be seen in Fig. 1. For testing our package, we used two widely used taxonomy profiling tools QIIME (QIIME 2) [9] for 16S rRNA data and MetaPhlAn2 [15] for whole metagenome data. QIIME suite is one of the major software tools for 16S rRNA microbiome analysis. QIIME takes raw sequencing data from users, preprocesses the data, identify OTUs, and assigns taxons. MetaPhlAn2 takes meta-genomic shotgun sequencing data to profile the composition of microbial communities by mapping the sequences to the built-in clade specific maker genes. Both QIIME and MetaPhlAn2 have been used in many microbiome research projects including well investigated HMP and DIABIUMME projects. Most metagenome taxonomic profilers including QIIME and MetaPhlAn2 generate taxonomy profile output as OTU table or BIOM (Biological Observation Matrix) format [32] by providing simple scripts to merge multiple taxonomy profiles together. MegaR takes a merged OTU table or BIOM format as input. The user will also need to provide a metadata file containing the class of each sample in the data set.
Machine learning methods
For the machine learning models in MegaR, we incorporated three machine learning classifiers: the first is generalized linear model (GLM), the second is support vector machine (SVM), and the third is random forest (RF). These approaches were implemented into MegaR by integrating the caret [33] and randomForest [34] packages.
The general linear model (GLM) is a statistical linear model also called as multivariate regression model [35]. GLM has several advantages over the other machine learning models. One is that it is easier to interpret since the coefficients are used within the mode. Many other accurate prediction models could be used to reduce error rates, but the ability of GLM to provide clarity while maintaining efficacy is the reason that this model has been adopted into MegaR.
A support vector machine (SVM) is a widely used non-probabilistic supervised machine learning method that tries to find an optimal hyperplane with maximizing the margin around the separation by the hyperplane [36]. SVM supports both linear and non-linear classification based on labeled data. SVM structure shapes a hyperplane or hyperplanes in multi-dimensional space to separate the data points with maximum margin classifier.
Random forest (RF) is also a commonly used decision-tree based method for classification and regression due to high-accuracy in prediction [34]. Random forest establishes numerous decision trees that are trained by the bagging method and select the features randomly. The primary benefit of using the RF method is providing pretty strong prediction accuracy in general by not overfitting with many trees. Another important advantage of using the RF model is that important features can be pulled and extracted easily. Those important features can be crucial role in many research studies such as identifying target associated features and biomarkers.
Data processing and model development
The quantitative microbiome profiles in genus, species, and all levels can be selected by the user as features in the machine learning model. Because metagenomic datasets usually have different sizes and depths of sequences, MegaR provides four normalization options including Cumulative Sum Scaling (CSS), Quantile, Trimmed Mean of M-values (TMM), and none (NO) to normalize the aggregated metagenomic counts among samples [37]. The package also allows the user to set a minimum abundance threshold to filter out low abundance microbes that may not provide useful information.
After selecting the appropriate machine learning method for classification and modifying the parameters to best fit the data, the user can generate a model. MegaR provides an error rate for each prediction model generated that can be found under the Error Rate tab. The error rate of prediction on a test set is a better estimate of model accuracy, which can be estimated using a confusion matrix that is generated by the program under the Confusion Matrix tab. MegaR also provides an AUC graph of the model under the AUC tab. From a practical perspective, it is important to identify features that are useful in identifying the class of metagenomic samples. MegaR provides this data as a list of the top ten most important species or genera that are crucial in identifying the class of sample along with their variable importance under the Important Feature tab (Fig. 2). An additional feature of MegaR is “Class to remove” option that can improve prediction accuracy. When more than two classes are present in a data set, it is possible for the user not consider a specific class of the data set. Disregarding a class can also increase the prediction accuracy by narrowing down of the features.
Cross validation
Cross-validation is a manner to access, judge, and review the performance of machine learning models. First and foremost, cross validation is essential to validate the model accuracy and model bias. This implies that the developed model should not be overfitted and not having bias.
To make a better model, all data set is not usually used for the training purpose, but split into training and validating/testing sets. For example, in k-fold cross validation, the dataset is shuffled and divided into k sub samples. The k − 1 samples are used as a training dataset and the single partition is used for validation. This process is repeated k times to represent the model performance. MegaR provides cross validation options allowing for an accurate prediction measure. The variance in fitting the model tends to be higher if it is fitted to a small dataset, therefore k-fold cross validation can have a high variance. MegaR provides users to select N independent runs of the tenfold cross validation to minimize such a high variance.
Sample prediction
MegaR provides a Prediction tab for users to upload unknown samples and get a prediction on which category the unknown samples fall into among classes. Once a satisfactory model is created for the data set, the user can load a set of unknown samples into MegaR. Then MegaR generates a classification prediction for each sample in the set of classes, categories, or states. This function is useful for identifying the disease states of in individual which can provide a path towards precision medicine through the use of microbe composition as diagnostic biomarkers. MegaR also has a feature that allows the user to download the trained model for later use in Prediction. If a user clicks the Download Model button after training, the model (RDS type) file is generated and downloaded. The user can then load this model for prediction of unknown samples without re-training the model.
Results
Dataset
In order to demonstrate the efficacy of MegaR as a disease sample prediction tool, DIABIMMUNE (https://pubs.broadinstitute.org/diabimmune) microbiome project data sets were used to perform a sample pipeline execution in MegaR. The DIABIMMUNE project aims to find if the limitation in early exposure to bacteria and infections in western and developing country is related to increasing incidence of both autoimmune and allergic diseases. The DIABIMMUNE project provides three publicly available data sets.
The first dataset consists of 812 metagenomics samples and 1584 16S samples from three different countries: Estonia, Finland and Russia. Some samples that did not have labels were dropped. We also analyzed 16S rRNA datasets, with 448 from Finland and 664 from Russia. The second cohort, named T1D cohort, consists of 126 metagenomic samples from 19 children in Estonia and Finland: 92 samples with T1D; 32 samples without T1D; 2 samples without T1D status were filtered out. This cohort consists of 28 samples from birth to age one, 62 samples from age one to two and 38 samples from age two to three. In the T1D cohort there were 777 16S rRNA samples, out of which 175 had T1D, and 85 samples did not have T1D. 314 samples were from children from birth to one age, 297 samples were from children from one to two, and 166 children were from two to three in terms of the age group. The third cohort is called antibiotic cohort and consists of 240 metagenomic samples, from 39 subjects. There were 139 samples from children who were treated antibiotics and 101 samples from children who were not treated with antibiotics. In the 16S rRNA data set, there are 528 samples from children who were not treated with antibiotics while 520 samples were from children who were treated with antibiotics.
In order to benchmark the performance of MegaR against other packages, we used the data set from the MetAML project [28]. From this dataset, we compared the performance of MegaR against MetAML for the T2D [38, 39] and Cirrhosis [40] data sets. The T2D dataset used was an aggregated dataset from two separate studies, totaling 490 participants, 345 being Chinese and 145 being European. Samples were obtained from fecal samples in these studies. The liver cirrhosis data set consists of 98 patients and 83 control individuals.
Taxonomy profiling
Preprocessed shotgun metagenomic data sets were downloaded from the DIABIMMUNE project. Left and right paired-end reads were concatenated together. The resulting data was run through MetaPhlAn2 using the parameter -t rel_ab_w_read_stats to obtain relative abundance and the number of reads derived from each clade. This estimate was extracted from each sample and merged into one file. The MetaPhlAn2 table that is available at the DIABIMMUNE project website had relative abundance as feature value. Our test shows that estimate of count as generated by MetaPhlAn2 option above is much better for classification. The associated metadata file was downloaded from the DIABIMMUNE website. All 16S rRNA taxonomy profiles were downloaded as an OTU table in BIOM format or tab separated format from the DIABIMMUNE project website.
Model and prediction accuracy
We used MegaR to analyze different datasets from the DIABIMMUNE research group. In the preliminary research, we tested each machine learning model available in MegaR; GLM, SVM and RF for each dataset (Table 1). Overall the model was tenfold more accurate when species was selected for feature rather than genus. So, all our analysis thereof uses species for the analysis. In the case of RF, the model is more accurate for WGS data than 16S rRNA data in the three-country cohort and the T1D cohort. In the case of SVM and GLM, all models from 16S rRNA metagenomics had a higher accuracy than WGS. Among RF, SVM and GLM, RF performed the best followed by SVM and GLM, with the exception of the 16S rRNA T1D cohort where SVM performs the best followed by RF and GLM.
We used MegaR to check if optimizing the threshold and percentage of sample with threshold as well as data split for training and testing improves the model (Fig. 3, Table 2). Our result showed a slight increase in accuracy than obtained from preliminary analysis in all the cases. We validated the improved model using cross validation. The validation accuracy for all the models was within 80- 90% range except for the antibiotic cohort using WGS, for which the cross validation accuracy was 72%.
We also checked if there is any age wise difference between our tool to classify the model. Although the overall performance of the model was within the accuracy of 77% to 90%, the 95% interval was very large (66–95% for 3 years) showing unreliable nature of the model. This could be due to the low number of samples available for building the model.
Benchmarking
We benchmarked MegaR against MetAML using the T2D [38, 39] and Cirrhosis [40] data sets provided by the MetAML project [28]. Using MegaR, we were able to obtain a slightly higher prediction accuracy for both datasets compared to the results reported by the MetAML project (Table 3). The model parameters used to achieve these results with MegaR were as follows. A threshold of 0.003 was used with a 90% 5 T 5P split. We believe that this slight increase is due to the ability of the MegaR package to fine tune the model parameters to easily optimize the model for each data set.
Conclusions
The MegaR package is an easy to use, versatile tool built with the intent of encouraging the use of machine learning analysis of metagenomic dataset for the purpose of phenotypic prediction and classification. The user-friendly interface allows users to fine tune the model for the specific data set in use in order to maximize the prediction accuracy, therefore increasing the potential functionality of machine learning for these tasks.
For each analysis, MegaR provides various useful metrics in the forms of tables and graphs that allow the user to determine if (1) there is enough available data to build a model, (2) the error rate of the model by more of error graph and confusion matrix, (3) a list of the top 10 most important features identified by the model, which allows researchers to focus on these features for further research or drug development, (4) downloadable figures to be used in further publications.
Our results indicate that the RF model provides the highest accuracy in most metagenomic classification scenarios compared to SVM and GLM. GLM is useful for the examination of 16S rRNA due to the large number of samples compared to WGS data sets, although GLM is less efficient on datasets with high dimensionality. While the standard split criteria in machine learning is 80:10:10 for train:validation:test, we tested various split criteria and, depending on the data, obtained various accuracies. Many machine learning models do not perform well if features are very sparse. As anticipated, removing sparse features expressed in low numbers increased the machine learning model accuracy. Our cross validation of the improved model shows that the models are robust and can be used for prediction with the obtained confidence. In the near future, we plan to test other machine learning classifiers and deep learning methods to increase the prediction accuracy with fast turnaround time.
Availability and requirements
Project name: MegaR.
Project home page: https://github.com/BioHPC/MegaR.
Operating system(s): Windows, Mac, and Linux (Platform independent).
Programming language: R.
Other requirements: R 3.6 or higher.
License: GNU GPL-3.
Any restrictions to use by non-academics: None.
Availability of data and materials
We mainly used the data sets from DIABIMMUNE microbiome project (https://pubs.broadinstitute.org/diabimmune) [22,23,24]. The processed dataset for MegaR can be also found under the MegaR project website (https://github.com/BioHPC/MegaR). We also benchmarked MegaR using the T2D [38, 39] and Cirrhosis [40] data sets provided by the MetAML project [28].
Abbreviations
- 16S:
-
16S rRNA sequencing
- BIOM:
-
Biological Observation Matrix
- CAMDA:
-
Critical Assessment of Massive Data Analysis
- GLM:
-
Generalized linear model
- HMP:
-
Human microbiome project
- IMIDs:
-
Immune-mediated inflammatory diseases
- OTU:
-
Operational taxonomic unit
- RF:
-
Random forest
- SVM:
-
Support vector machine
- T1D:
-
Type I diabetes
- T2D:
-
Type II diabetes
- WGS:
-
Whole genome sequencing
References
Thomas T, Gilbert J, Meyer F. Metagenomics—a guide from sampling to data analysis. Microb Inform Exp. 2012;2(1):3.
Huttenhower C, Gevers D, Knight R, Abubucker S, Badger JH, Chinwalla AT, Creasy HH, Earl AM, FitzGerald MG, Fulton RS, et al. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486(7402):207–14.
Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59–65.
Sunagawa S, Coelho LP, Chaffron S, Kultima JR, Labadie K, Salazar G, Djahanschiri B, Zeller G, Mende DR, Alberti A, et al. Ocean plankton. Structure and function of the global ocean microbiome. Science. 2015;348(6237):1261359.
Sanschagrin S, Yergeau E. Next-generation sequencing of 16S ribosomal RNA gene amplicons. J Vis Exp. 2014;(90):51709.
Quince C, Walker AW, Simpson JT, Loman NJ, Segata N. Shotgun metagenomics, from sampling to analysis. Nat Biotechnol. 2017;35(9):833–44.
Jovel J, Patterson J, Wang W, Hotte N, O’Keefe S, Mitchel T, Perry T, Kao D, Mason AL, Madsen KL, et al. Characterization of the gut microbiome using 16S or shotgun metagenomics. Front Microbiol. 2016;7:459.
Hillmann B, Al-Ghalith GA, Shields-Cutler RR, Zhu Q, Gohl DM, Beckman KB, Knight R, Knights D. Evaluating the information content of shallow shotgun metagenomics. mSystems. 2018;3(6):e00069-18.
Bolyen ERJ, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, Alexander H, Alm EJ, Arumugam M, Asnicar F, Bai Y, Bisanz JE, Bittinger K, Brejnrod A, Brislawn CJ, Brown CT, Callahan BJ, Caraballo-Rodríguez AM, Chase J, Cope EK, Da Silva R, Diener C, Dorrestein PC, Douglas GM, Durall DM, Duvallet C, Edwardson CF, Ernst M, Estaki M, Fouquier J, Gauglitz JM, Gibbons SM, Gibson DL, Gonzalez A, Gorlick K, Guo J, Hillmann B, Holmes S, Holste H, Huttenhower C, Huttley GA, Janssen S, Jarmusch AK, Jiang L, Kaehler BD, Kang KB, Keefe CR, Keim P, Kelley ST, Knights D, Koester I, Kosciolek T, Kreps J, Langille MGI, Lee J, Ley R, Liu YX, Loftfield E, Lozupone C, Maher M, Marotz C, Martin BD, McDonald D, McIver LJ, Melnik AV, Metcalf JL, Morgan SC, Morton JT, Naimey AT, Navas-Molina JA, Nothias LF, Orchanian SB, Pearson T, Peoples SL, Petras D, Preuss ML, Pruesse E, Rasmussen LB, Rivers A, Robeson MS, Rosenthal P, Segata N, Shaffer M, Shiffer A, Sinha R, Song SJ, Spear JR, Swafford AD, Thompson LR, Torres PJ, Trinh P, Tripathi A, Turnbaugh PJ, Ul-Hasan S, van der Hooft JJJ, Vargas F, Vázquez-Baeza Y, Vogtmann E, von Hippel M, Walters W, Wan Y, Wang M, Warren J, Weber KC, Williamson CHD, Willis AD, Xu ZZ, Zaneveld JR, Zhang Y, Zhu Q, Knight R, Caporaso JG. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol. 2019;37:852–7.
Liu B, Gibbons T, Ghodsi M, Treangen T, Pop M. Accurate and fast estimation of taxonomic profiles from metagenomic shotgun sequences. BMC Genomics. 2011;12(Suppl 2):S4.
Huson DH, Auch AF, Qi J, Schuster SC. MEGAN analysis of metagenomic data. Genome Res. 2007;17(3):377–86.
Ahn TH, Chai J, Pan C. Sigma: strain-level inference of genomes from metagenomic analysis for biosurveillance. Bioinformatics. 2015;31(2):170–7.
Brady A, Salzberg S. PhymmBL expanded: confidence scores, custom databases, parallelization and more. Nat Methods. 2011;8(5):367.
Patil KR, Haider P, Pope PB, Turnbaugh PJ, Morrison M, Scheffer T, McHardy AC. Taxonomic metagenome sequence assignment with structured output models. Nat Methods. 2011;8(3):191–2.
Truong DT, Franzosa EA, Tickle TL, Scholz M, Weingart G, Pasolli E, Tett A, Huttenhower C, Segata N. MetaPhlAn2 for enhanced metagenomic taxonomic profiling. Nat Methods. 2015;12(10):902–3.
Wu M, Scott AJ. Phylogenomic analysis of bacterial and archaeal sequences with AMPHORA2. Bioinformatics. 2012;28(7):1033–4.
Douglas GM, Maffei VJ, Zaneveld J, Yurgel SN, Brown JR, Taylor CM, Huttenhower C, Langille MGI. PICRUSt2: an improved and extensible approach for metagenome inference. bioRxiv 2019;672295.
Niu SY, Yang J, McDermaid A, Zhao J, Kang Y, Ma Q. Bioinformatics tools for quantitative and functional metagenome and metatranscriptome data analysis in microbes. Brief Bioinform. 2018;19(2):360.
Nurk S, Meleshko D, Korobeynikov A, Pevzner PA. metaSPAdes: a new versatile metagenomic assembler. Genome Res. 2017;27(5):824–34.
Truong DT, Tett A, Pasolli E, Huttenhower C, Segata N. Microbial strain-level population structure and genetic diversity from metagenomes. Genome Res. 2017;27(4):626–38.
Wood DE, Lu J, Langmead B. Improved metagenomic analysis with Kraken 2. Genome Biol. 2019;20(1):257.
Yassour M, Vatanen T, Siljander H, Hamalainen AM, Harkonen T, Ryhanen SJ, Franzosa EA, Vlamakis H, Huttenhower C, Gevers D, et al. Natural history of the infant gut microbiome and impact of antibiotic treatment on bacterial strain diversity and stability. Sci Transl Med. 2016;8(343):343–81.
Kostic AD, Gevers D, Siljander H, Vatanen T, Hyotylainen T, Hamalainen AM, Peet A, Tillmann V, Poho P, Mattila I, et al. The dynamics of the human infant gut microbiome in development and in progression toward type 1 diabetes. Cell Host Microbe. 2015;17(2):260–73.
Vatanen T, Kostic AD, d’Hennezel E, Siljander H, Franzosa EA, Yassour M, Kolde R, Vlamakis H, Arthur TD, Hamalainen AM, et al. Variation in microbiome LPS immunogenicity contributes to autoimmunity in humans. Cell. 2016;165(6):1551.
Meta SUBIC. The Metagenomics and metadesign of the subways and urban biomes (MetaSUB) international consortium inaugural meeting report. Microbiome. 2016;4(1):24.
Forbes JD, Chen CY, Knox NC, Marrie RA, El-Gabalawy H, de Kievit T, Alfa M, Bernstein CN, Van Domselaar G. A comparative study of the gut microbiota in immune-mediated inflammatory diseases-does a common dysbiosis exist? Microbiome. 2018;6(1):221.
Harris ZN, Dhungel E, Mosior M, Ahn TH. Massive metagenomic data analysis using abundance-based machine learning. Biol Direct. 2019;14(1):12.
Pasolli E, Truong DT, Malik F, Waldron L, Segata N. Machine learning meta-analysis of large metagenomic datasets: tools and biological insights. PLoS Comput Biol. 2016;12(7):e1004977.
Luo D, Ziebell S, An L. An informative approach on differential abundance analysis for time-course metagenomic sequencing data. Bioinformatics. 2017;33(9):1286–92.
Metwally AA, Yang J, Ascoli C, Dai Y, Finn PW, Perkins DL. MetaLonDA: a flexible R package for identifying time intervals of differentially abundant features in metagenomic longitudinal studies. Microbiome. 2018;6(1):32.
Lo C, Marculescu R. MetaNN: accurate classification of host phenotypes from metagenomic data using neural networks. BMC Bioinform. 2019;20(12):314.
McDonald D, Clemente JC, Kuczynski J, Rideout JR, Stombaugh J, Wendel D, Wilke A, Huse S, Hufnagle J, Meyer F, et al. The Biological Observation Matrix (BIOM) format or: how I learned to stop worrying and love the ome-ome. Gigascience. 2012;1(1):7.
Kuhn M. Building predictive models in R using the caret package. J Stat Softw. 2008;28(5):1–26.
Liaw A, Wiener M. Classification and regression by randomForest. R News. 2002;2(3):18–22.
Nelder JA, Wedderburn RWM. Generalized linear model. J R Stat Soc Ser A. 1972;135(3):370–84.
Cortes C, Vapnik V. Support-Vector networks. Mach Learn. 1995;20(3):273–97.
Paulson JN, Stine OC, Bravo HC, Pop M. Differential abundance analysis for microbial marker-gene surveys. Nat Methods. 2013;10(12):1200–2.
Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490(7418):55–60.
Karlsson FH, Tremaroli V, Nookaew I, Bergström G, Behre CJ, Fagerberg B, Nielsen J, Bäckhed F. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498(7452):99–103.
Qin N, Yang F, Li A, Prifti E, Chen Y, Shao L, Guo J, Le Chatelier E, Yao J, Wu L, et al. Alterations of the human gut microbiome in liver cirrhosis. Nature. 2014;513(7516):59–64.
Acknowledgements
Not applicable.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article. TA is supported by National Science Foundation (Accession ID: 1564894) and Saint Louis University President’s Research Fund. MR is supported by the Collaborative Genome Program of the Korea Institute of Marine Science and Technology Promotion (KIMST), funded by the Ministry of Oceans and Fisheries (MOF) [No. 20180430]. The funders had no role in the development of this software or writing of the manuscript.
Author information
Authors and Affiliations
Contributions
ED—Project design, implementation, documentation, and manuscript. YM—Implementation, testing, manuscript. HG—Testing and validation. AR—Testing and validation. MR—Conception of biologically relevant functionality, manuscript review. TA—Conceived the ideas, project design, prepared the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Dhungel, E., Mreyoud, Y., Gwak, HJ. et al. MegaR: an interactive R package for rapid sample classification and phenotype prediction using metagenome profiles and machine learning. BMC Bioinformatics 22, 25 (2021). https://doi.org/10.1186/s12859-020-03933-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12859-020-03933-4