Abstract
Background
Due to the large surface area of green-synthesized TiO2@CuO@Chromite nanocatalysts (NCs) and accumulations of bioactive phytochemicals on its surface, it was used for an efficient and safe synthesis of nitriles and also an environmentally friendly process of water treatment. For the first time, a rapid, economic, one-pot, solventless and safe protocol is presented for ecosynthesis of TiO2@CuO@Chromite nanocatalysts (NCs) to efficient, ligand-free and solventless synthesis of aromatic nitriles through the cyanation of aldehydes at room temperature. Furthermore, the eco-NCs were used as a potent adsorbent for physical and biological treatment of sewage waters collected around the natural and residential area of northern parts of the Soran city in Iraq at room temperature.
Results
The structural elucidation of the NCs using the SEM (scanning electron microscopy), Cross-sectional EDS (electron dispersive spectroscopy), elemental mapping analysis, XRD (X-ray diffractions) and BET (Brunauer–Emmett–Teller) for detection of specific surface area of eco-NCs confirmed the formation of NCs with a large surface area. Application of green TiO2@CuO@Chromite NCs in solventless synthesis of aromatic nitriles shows high efficiency, time saving, economical aspect and ecofriendly and safe methodology. Also, the treatment process of sewage waters monitored using UV–Vis double beam spectrophotometer, optical microscopy and antibiogram tests demonstrated an efficient ability for the eco-NCs in physical and biological treatment of sewage samples.
Conclusions
The NCs employed in both ligand and solventless highly efficient and safe synthesis of aromatic nitriles through the cyanation of aldehydes at room temperature demonstrated the production of aryl nitriles in very good-to-excellent yields. This protocol indicated a green alternative to the existing methods since the reaction proceeds in solventless medium in the absence of any ligand and organic solvent with simple work-up procedure, low temperature, higher yield and shorter reaction time. Further, it was used in the physical and biological treatment of the real samples of sewage waters collected around the natural and residential area of northern parts of Iraq at room temperature, which shows a very good treatment ability in this process.
Similar content being viewed by others
Background
Nanotechnology is a rapid expanding zone of investigation which impression of employing matter at nano-scale level led to novel area of investigation and search inside the scientific community which created an acceptable number of novelties and findings. In nanotechnology science, a nanoparticle (NP) is well described as a tiny piece that acts by way of an entire component with regard to its transfer and characteristics. The discipline and manufacturing technology of nano-systems are accounted as the greatest urgent as well as rapid growth areas of nanotechnology [1,2,3]. Current progresses in the nanotechnology field, mainly the aptitude to make highly well-organized nanoparticles of any dimension and profile, have activated the improvement of its applications. Transition metal oxide nanostructure has attracted further arrangement of attention as a result of their exceptional physical and chemical characteristics emerging from big surface area/volume, quantum confinement consequence, that rely upon the outline and dimension of the substantial [4,5,6]. Despite all benefits of nanoparticles, they are typically more toxic than the bulk material of larger scales. Therefore, the green synthesis nanoparticle has been accomplished by means of ecologically adequate solvent schemes and ecofriendly reducing and capping agents. Conversely, the requisite for biosynthesis of nanoparticles rose since the physical and chemical methods are overpriced. Thus, in the exploration for low-cost method for nanoparticle synthesis, researchers utilized biosystems for production of NPs. Natural surroundings have created numerous procedures for the production of nano-scale and micro-scale climbed inorganic ingredients which have been added to the growth of comparatively novel as well as mainly uncultivated region of investigation built upon the green synthesis of nano materials [7,8,9,10,11,12].
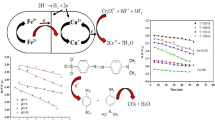
Nitriles are significant basements to prepare the industrial and pharmaceutical nitrogen-containing heterocycles. Previous methods to prepare the nitriles include the poisonous cyanide sources which suffer from harsh conditions, time-consuming reactions, tedious work-ups, noneconomic aspect following the poor yield and necessity of employing expensive ligands, ligand dependency, inevitability of employing expensive and moisture-sensitive reagents. Recently, usage of ecofriendly methods to introduce nitriles has been converted to a new gate in their synthesis. Some of these challenges were reduced by ligand-free green catalytic cyanation of aryl halides using K4Fe(CN)6 as an absolutely safe cyanide source [13,14,15,16,17,18]. Reports on the aldehydes cyanation in order to obtain the aryl nitriles indicated the aforementioned drawbacks of the methods [19,20,21], thus introducing economic and safe methods to obtain the aryl nitriles is of great attention. To develop safe methods of cyanation, as a part of this study, for the first time, we report the successful utilization of Crocus sativus L. plant in solventless and ligand-free catalysis of aldehyde cyanation under organic solventless condition at ambient temperature (Scheme 1).
Green technology covers a broad area including the monitoring and assessment, pollution prevention and control, and remediation and restoration of environmental challenges. Therefore, tracking those pollutants are essential to measure the release of natural or anthropogenic materials of harmful nature and also avoid the production of environmentally and industrially hazardous substances or alter human activities in ways that minimize damage to the environment. Thus, controlling the hazardous substances before entering the environment and also improving the condition of ecosystems are among the remediation process of green nanotechnology [22,23,24,25,26]. Water is the most precise natural resource and its application for human consumption is only around 1%. The reports of WHO reveal that over 1.1 billion people suffer from lack of accessibility to drinking water resources due to the growing living problems and variety of climatic and environmental concerns. Among the challenges leading to shortage of water supply is contamination of freshwaters by organic pollutants and these concerns can be reduced by treatment of wastewaters. However, the traditional and existing methods of treatment are not efficient enough to completely remove the emerging contaminants and have several drawbacks such as high energy requirement, incomplete pollutant removal and generation of toxic sludge [27,28,29,30]. A part of this study is related to the treatment of sewage waters around Soran city in northern part of Iraq including biological treatment and also decolonization and removing the suspended particles using bioactive green-synthesized TiO2@CuO@Chromite nano-adsorbent at natural temperature and then simple filtration to obtain the clean and treated water. In addition to converting the ugly appearance of sewage waters to a good one, this method efficiently can help in the recycling of these sources to the nature and human applications (Scheme 2).
Experimental
Chromitite: study area and geological setting
The chromitite deposit is located near Rayat village within the northeast corner of Iraq, about 120 km east of Erbil and near the Iraqi Zagros thrust zone (Fig. 1). The chromitites are podiform types; this type of chromite usually forms beneath mid-ocean ridge and during continental collision as they abducted on continental crust and observed in ophiolites and non-ophiolitic peridotite complexes. The Rayat chromitite is located in ophiolite belt inside peridotite rocks such as dunite and harzburgite and equivalent serpentinite [31,32,33].
Field observation and petrography
The field sample of chromitite can be seen at earth surfaces inside serpentinized peridotite (Fig. 2a). Due to carbonation which happened in the area, veins of calcite and dolomite can be seen in pods of chromitites (Fig. 2b). The hand samples are heavy with black color and massy textures (Fig. 2a, b).
Identification of chromitite ore body
For assessment of natural chromitite using X-ray diffraction (XRD), the samples were ground and then analyzed by analytical X-ray diffractometer (Fig. 3). Results of XRD show that the ore body comprises mostly chromite with minor amount of lizardite, magnetite, calcite, dolomite and clinochlore. According to mineralogical studies of ore body by polarized microscope and X-ray diffraction (XRD), the ore is composed of quartz, magnetite, hematite, and chromite.
In continuation of absolute identification of chromitite ore body, for more convenience about the geosubstrate, the collected sample was analyzed by SEM, EDS and elemental mapping analysis (Figs. 4, 5, 6). The results from mentioned analysis confirm the considerable presence of various mineral phases enriched in chromium, iron, silica and magnesium, aluminum calcium, dolomite and carbon. Thus based on the results, the collected ore body is certainly chromitite ore which can be efficiently used as a natural substrate due to the accumulation of Lewis acids in the structure of TiO2@CuO@Chromite NCs to increase the synergistic effect and as an anti-agglomeration agent.
Rapid synthesis of TiO2@CuO@Chromite NCs
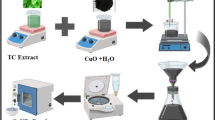
1 g TiO(OH)2, 2 g CuCl2·2H2O and 6 g chromitite ore body were mixed to 50 g fresh Crocus sativus L. plant at room temperature, then hardly milled using a pestle mortar for 30 min to provide the required temperature of the endothermic reaction. After change in color, the obtained mixture was washed with water and then separated precipitation analyzed to monitor the formation of NCs.
Sewage water samples collection
A total of 12 sewage water samples were collected around the northern parts of the Soran city during April 2019. The samples were collected mainly from the natural and residential area. The location of the samples is demonstrated in Fig. 7.
Treatment procedure of sewage waters
In case of sewage water treatment, after filtration of samples, 10 mL from each sample (W1, W2,…., W12) were mixed and stirred at 1000 rpm for 30 min at room temperature and then the obtained mixture was monitored to more convenience about its contaminations using the double beam UV–Vis spectrophotometer and optical microscopy (Figs. 8 and 9). For treatment of sewage water sample, after obtaining the optimum amount of nano-adsorbent and concentration of NaBH4 (Table 1), it was found that no efficient treatment occurred in the absence of NaBH4 or nano-adsorbent (Table 1, entries 10 and 11). Also the best result was demonstrated for 5 mg nano-adsorbent and 6 × 10−3M NaBH4 at room temperature (Table 1, entry 4). Therefore, 5 mg of TiO2@CuO@Chromite NCs was added to 100 mL of sewage mixture then 10 mL freshly prepared solution of NaBH4 (6 × 10−3M) was added to the mixture at room temperature and stirred using a magnetic stirrer. Moreover, for more convenience the advancement of the process was followed using UV–Vis spectra and microscopic monitoring. Finally, the catalyst was recovered by simple filtration and washed using ethanol and hot distilled water, respectively, then dried to use in the next cycle of the process.
Solventless synthesis of aryl nitriles
A mixture of TiO2@CuO@Chromite NCs (5.0 mol %), aldehyde (1.0 mmol) and K4Fe(CN)6 (1.0 mmol) were vigorously milled at room temperature using a pestle mortar for the required time. After monitoring by TLC, the obtained products were isolated by EtOAc, then dried, concentrated at reduced pressure and finally recrystallized. All obtained products were well known and their structure confirmed by comparison of their mp, IR, NMR data reported by previous works [18, 34,35,36,37,38,39,40,41,42,43,44,45,46,47].
Results and discussion
Ecosynthesis of TiO2@CuO@Chromite NCs
After absolute identification of chromitite ore body and synthesis of TiO2@CuO@Chromite NCs using the chromitite natural substrate and Crocus sativus L. plant through a simple, inexpensive, rapid and safe method, the obtained nanostructure was characterized using analytical identification techniques for more convenience about its application in environmentally friendly reactions. Scheme 3 shows the possible mechanism of nanoparticle formation using sugar moieties of the plant phytochemicals.
Among the bioactive components of the plant, crocetin and crocin are powerful antioxidants in which the crocin even shows stronger antioxidant capacity than alpha-tocopherol in nervous system concerning treatment of oxidative stress. In fact, the antioxidant behavior of crocin is related to the sugar moiety in crocin molecule which has a vital role in its chemical reactivity.
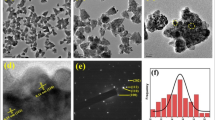
Figures 10, 11, and 12 show the absolute analysis of NCs. The SEM results of ecosynthesized NCs confirm the formation of NCs as it shows a nanosized structure with a semispherical shape and heterogeneous morphology including some agglomerations (Fig. 10). The elemental structure of the NCs was monitored using EDS and elemental mapping analysis, as can be seen in Figs. 11 and 12. Based on these analyses, besides the other elements involved in the composition of chromitite ore body, the presence of Ti, Cu, O, Cr elements as principal elements of the pure crystalline shape of the nanostructure is strongly demonstrated which evidently confirms the formation of nanostructure (Fig. 13).
The specific surface areas of TiO2@CuO@Chromite NCs were determined by BET (Brunauer–Emmett–Teller). Based on isotherm curve, the measured BET surface area of TiO2@CuO@Chromite NCs was 25.87 m2/g.
The results were obtained following the BET equation:
where Vm is monolayer adsorption amount and V is the adsorption amount at the equilibrium pressure P.
It is clear that the surface area of natural chromite substrate fairly increased after immobilization of CuO and TiO2 nanoparticles in the composition of nanocomposite. Since as all thermodynamic processes occur on the surface area, formation of an excellent surface area based on the obtained results by BET analysis probably provides numerous active sites on the surface unit of the green catalyst and nominate it as a potent nanocatalyst to the investigated processes in this study.
Treatment results of sewage waters using ecosynthesized TiO2@CuO@Chromite NCs
Application of optimum conditions for the process demonstrated a clear appearance of very opaque and blurred sewage sample in which no apparent contamination can be seen in the sample after its treatment during 1 h at room temperature even by optical microscopic monitoring. The catalyst was simply prepared using very low cost and abundant materials. Also, the process needs a very economic amount of NaBH4 and catalyst at ambient temperature. During the process to find the effect of temperature on treatment process while employing the optimum conditions, both room temperature and temperature of 50 °C were examined, but no significant difference in results was seen.
The UV–Vis monitoring of the sewage water sample at different times (0 h → 1 h) definitely shows the ability of the nanocomposite to purify the sample. According to the spectra with passing the time, the intensity, symmetry and shapes of the main peaks (625 nm, 455 nm, 295 nm and 220 nm, respectively) concerning the blurred and cloudy sewage water have been reduced and as can be seen in spectrum D, after 1 h there is almost no signal related to the colored physical and chemical contaminations (Fig. 14).
Furthermore, for more convenience the treatment process of sewage sample was monitored again using optical microscopy at various times (at zero time (A), after 20 min (B), after 40 min (C), after 1 h (D)). According to Fig. 15, with passing time the amount and concentration of impurities decreased and physical appearance of sample is improved. The surface of green-synthesized NCs including a multi-mineral natural substrate (chromite ore body) provides a very suitable surface including many active sites on the surface area of the NCs (25.87 m2/g) to adsorb the suspended contaminations in the sewage sample. Besides these characteristics of the NCs, deposition of plant phytochemicals on the surface of the NCs as capping agents increased the synergistic effects and efficient capturing of the suspended contaminations in the treatment of the impurities.
The presence of bacteria and pathogenic (disease-causing) organisms (especially coliform bacteria) is a concern when considering the safety of drinking water. Pathogenic organisms can cause intestinal infections, dysentery, hepatitis, typhoid fever, cholera, and other illnesses. After filtration of sewage sample, it was stirred thoroughly at 1000 rpm for 20 min, then a 10 ppm of sample was prepared using sterile distilled water. 1 ml of the prepared sewage sample was inoculated into 20 ml of melted, cooled nutrient agar. The nutrient agar was mixed thoroughly and poured into a sterile petri plate. This was allowed to solidify and then incubated at 37 °C for 24 h. The number of discrete colonies were counted and expressed as colony forming unit per ml (cfu/mL). 1 mL of sterile water in 20 mL agar was used as control. The same protocol was repeated for the treated sewage sample and results were assessed to evaluate the biological treatment of sewage sample using eco-NCs.
Figure 16 demonstratively shows the efficient biological treatment effect for the bacterial colonies of the sewage sample. According to the result obtained by the treatment on nutrient agar, after 24 h a large number of bacterial colonies disappeared. The green approach employed during this study benefits from the deposition of bioactive phytochemicals on the large surface of ecosynthesized NCs. The accumulation of phytochemicals coated on the NCs caused the antibacterial activity of nanocomposite and its potential in biological treatment of sewage sample.
Solventless synthesis of aryl nitriles
This study reports for the first time of solventless process in the ligand-free cyanation of aldehydes through a safe and efficient pathway. The ecocatalytic solventless cyanation of benzaldehyde (1.0 mmol) by K4Fe(CN)6 (1.0 mmol) as a green CN− source at room temperature assessed as model substrate with TiO2@CuO@Chromite NCs (5.0 mol %) to screen optimum conditions. Employment of the optimum conditions gave 96% yield as the best result for the product during 25 min (Table 1, entry 1). Increasing the amount of the catalyst showed no considerable change in the output and time of the reaction. There is no reaction progress without application of the catalyst even after a lot of time. The obtained optimum conditions were applied for various types of aldehydes including electron-donating and electron-withdrawing substituents (Table 2). The results evidently showed that application of aldehyde containing electron-donating groups produced excellent-to-very good yields (Table 2, entries 2–5), whereas aldehydes including electron-withdrawing substituents demonstrated lower efficiency. Of course in this case electron-withdrawing substituents with inductive effects show lower yield than the same substituents with resonance effect (Table 2, entries 6–11). Table 3 presents a comparison between our method using eco-nanocatalyst with other reported methodologies concerning the synthesis of benzonitriles by using various catalysts. Our method using TiO2@CuO@Chromite NCs exhibited the best result for this reaction with a 96% yield at room temperature in a lower reaction time and solventless system while other systems presented lower yields even at high temperature and harsh reaction conditions.
Following our literature survey there is no report on the solventless catalytic cyanation of aldehydes to synthesis of nitriles and this study is the first report of this methodology in this subject. Higher yield, faster reaction rate, reaction at room temperature, ligand-free, simple, solventless and very economical preparation of the reaction catalyst are other important benefits of the method in contrast with the yield and conditions reported by the previous methods. Thus, our absolutely safe system introduces the best yield, time and economy for the synthesis of aryl nitriles.
Conclusion
We successfully examined the first time solventless and safe protocol for ecosynthesis of TiO2@CuO@Chromite nanocatalyst (NCs) which was then characterized using SEM, EDS, elemental mapping, XRD and BET analysis. The NCs were employed on both ligand and solventless highly efficient and safe synthesis of aromatic nitriles through the cyanation of aldehydes at room temperature that demonstrated the production of aryl nitriles in very good-to-excellent yields. This protocol indicated a green alternative to the existing methods since the reaction proceeds in a solventless medium in the absence of any ligand and organic solvent with simple work-up procedure, low temperature, higher yield and shorter reaction time. Besides the mentioned application of ecosynthesized NCs, it was used for the physical and biological treatment of real samples of sewage waters collected around the natural and residential area of northern parts of Iraq at room temperature. The process was assessed using UV–Vis spectrophotometer, optical microscopy and antibiogram tests that demonstrated the efficient ability of the eco-NCs in physical and biological treatment of sewage samples in a short duration. Generally, these protocols can be utilized for safe production of nitriles and recycling and cleaning the sewage waters and other environmental pollutants.
Availability of data and materials
Not applicable; presented information is based on previously published data and our experimental results only.
Abbreviations
- NPs:
-
Nanoparticles
- NCs:
-
Nanocatalysts or nanocomposites
- SEM:
-
Scanning electron microscopy
- EDS:
-
Electron dispersive spectroscopy
- XRD:
-
X-ray diffractions
- BET:
-
Brunauer–Emmett–Teller
- UV–Vis:
-
Ultraviolet–visible
- Eco-NCs:
-
Ecosynthesized nanocatalyst
References
Mansoori GA, Vakili-Nezhaad GR, Ashrafi AR (2005) Some mathematical concepts applicable in nanothermodynamics. Int J Pure Appl Math Sci 2:58
Mansoori GA, Mohazzabi P, McCormack P, Jabbari S (2007) Nanotechnology in cancer prevention, detection and treatment: bright future lies ahead. World Rev Sci Technol Sustain Dev 4:226
Ghorbanpour M, Hatami M (2015) Changes in growth, antioxidant defense system and major essential oils constituents of Pelargonium graveolens plant exposed to nano-scale silver and thidiazuron. Indian J Plant Physiol 20:116
Lee J, Mahendra S, Alvarez PJ (2010) Nanomaterials in the construction industry: a review of their applications and environmental health and safety considerations. ACS Nano 4:3580
Mirsasaani SS, Hematia M, Tavasolid T, Dehkorda ES, Yazdia GT, Poshtiri DA (2013) Nanotechnology and nanobiomaterials in dentistry. Nanobiomaterials in clinical dentistry. Elsevier, New York
Ghorbanpour M, Khanuja M, Varma A (eds) (2017) Nanoscience and plant-soil systems. Springer, Berlin
Jiafu Qu DC, Najun L, Qingfeng X, Hua L, Jinghui H, Jianmei L (2019) Ternary photocatalyst of atomic-scale Pt coupled with MoS2 co-loaded on TiO2 surface for highly efficient degradation of gaseous toluene. Appl Catal B Environ 256:117877
Huang H, Song Y, Li N, Chen D, Xu Q, Li H, He J, Lu J (2019) One-step in situ preparation of N-doped TiO2@ C derived from Ti3C2 MXene for enhanced visible-light driven photodegradation. Appl Catal B Environ 251:154–161
Tian L, Wang J, Wang K, Wo H, Wang X, Zhuang W, Li T, Du X (2019) Carbon-quantum-dots-embedded MnO2 nanoflower as an efficient electrocatalyst for oxygen evolution in alkaline media. Carbon 1(143):457–466
Zhao L, Zizhan J, Wenyou Z, Changchun H, Peng W, Xiang W, Tongxiang L, Lin T (2019) Facile preparation of CoSe2 nano-vesicle derived from ZIF-67 and their application for efficient water oxidation. Appl Surf Sci. https://doi.org/10.1016/j.apsusc.2019.144368
Shilin X, Xinlei N, Zhaosheng H, Chunhong G (2020) A multifunctional gelatine–quaternary ammonium copolymer: an efficient material for reducing dye emission in leather tanning process by superior anionic dye adsorption. J Hazard Mater 383:121142
Lim B, Jiang M, Camargo PH, Cho EC, Tao J, Lu X, Zhu Y, Xia Y (2009) Pd–Pt bimetallic nanodendrites with high activity for oxygen reduction. Science 324:1302
Larock RC (1989) In comprehensive organic transformations. VCH, New York, p 819
Rappoport Z (1970) Chemistry of the Cyano Group. Wiley, London, p 121
Habibi D, Heydari S, Nasrollahzadeh M (2012) Synthesis of aryl nitriles using the stable aryl diazonium silica sulfates. J Chem Res 36:573
Sundermeier M, Mutyala S, Zapf A, Spannenberg A, Beller M (2003) A convenient and efficient procedure for the palladium-catalyzed cyanation of aryl halides using trimethylsilylcyanide. J Organomet Chem 684:50
Sundermeier M, Zapf A, Beller M (2003) A convenient procedure for the palladium-catalyzed cyanation of aryl halides. Angew Chem Int Ed 42:1661
Zanon J, Klapars A, Buchwald SL (2003) Copper-catalyzed domino halide exchange-cyanation of aryl bromides. J Am Chem Soc 125:2890
Chatani N, Hanafusa T (1986) Transition-metal-catalyzed reactions of trimethylsilyl cyanide 4 Palladium-catalyzed cyanation of aryl halides by trimethylsilyl cyanide. J Org Chem. 51:4714
Zieger HE, Wo S (1994) Titanium(IV) chloride-catalyzed cyanation of benzylic halides with trimethylsilyl cyanide. J Org Chem 59:3838
Chobanian HR, Fors BP, Lin LS (2006) A facile microwave-assisted palladium-catalyzed cyanation of aryl chlorides. Tetrahedron Lett 47:3303
Sajadi SM, Kolo K, Hamad SM, Mahmud SA, Yasin AT (2019) Green synthesis of NiO NPs using the aqueous extract of Drimia maritima and investigation of its catalytic activity for removal of aromatics from the natural water sources of Soran city. Mor J Chem 7:24
Hamad SM, Mahmoud SA, Omar ZA, Sajadi SM (2019) Biosynthesis of Cu/ZrO2 nanocomposite using 7-hydroxy-4′-methoxy-isoflavon extracted from Commelina diffusa and evaluation of its catalytic activity. Surfaces and Interfaces 15:125
Sajadi SM, Kolo K, Abdullah ShM, Hamad SM, Khalid HS, Yassein AT (2018) Green synthesis of highly recyclable CuO/eggshell nanocomposite to efficient removal of aromatic containing compounds and reduction of 4-nitrophenol at room temperature. Surf Interf 13:205
Sajadi SM, Kolo K, Pirouei M, Mahmud SA, Ali JA, Hamad SM, Khalid KM (2018) Natural iron ore as a novel substrate for the biosynthesis of bioactive-stable ZnO@CuO@iron ore NCs: a magnetically recyclable and reusable superior nanocatalyst for the degradation of organic dyes, reduction of Cr(VI) and adsorption of crude oil aromatic compounds, including PAHs. RSC Adv 8:35557
Atarod M, Nasrollahzadeh M, Sajadi SM (2016) Euphorbia heterophylla leaf extract mediated green synthesis of Ag/TiO2 nanocomposite and investigation of its excellent catalytic activity for reduction of variety of dyes in water. J Colloid Interface Sci 462:272
Nasrollahzadeh M, Sajadi SM, Khalaj M (2014) Green synthesis of copper nanoparticles using aqueous extract of the leaves of Euphorbia esula L and their catalytic activity for ligand-free Ullmann-coupling reaction and reduction of 4-nitrophenol. RSC Adv. 4:47313
Issaabadi Z, Nasrollahzadeh M, Sajadi SM (2017) Green synthesis of the copper nanoparticles supported on bentonite and investigation of its catalytic activity. J Clean Prod. 142:3584
Ambashta RD, Sillanpaa M (2010) Water purification using magnetic assistance: a review. J Hazard Mater 180:38
Amin MT, Alazba AA, Manzoor U (2014) A review of removal of pollutants from water/wastewater using different types of nanomaterials. Adv Mater Sci Eng. 82:5910
Smail SA, Arai S, Ahmed AH, Shimizu Y (2009) Chromitite and peridotite from Rayat, northeastern Iraq, as fragments of a Tethyan ophiolite. Island Arc 18:175
Arai S, Shimizu Y, Ismail SA, Ahmed AH (2006) Low-T formation of high-Cr spinel with apparently primary chemical characteristics within podiform chromitite from Rayat, northeastern Iraq. Mineral Mag 70:499
Jackson ED, Thayer TP (1972) Some criteria for distinguishing between stratiform, concentric, and alpine peridotite-gabbro complexes. In: Proceedings of 24th international geological congress, Section 2. pp 289
Schareina T, Zapf A, Beller M (2004) Potassium hexacyanoferrate(ii)—a new cyanating agent for the palladium-catalyzed cyanation of aryl halides. Chem Commun 56:1388
Schareina T, Zapf A, Beller M (2004) Improving palladium-catalyzed cyanation of aryl halides: development of a state-of-the-art methodology using potassium hexacyanoferrate (II) as cyanating agent. J Organomet Chem 689:4576
Ren YL, Liu ZF, Zhao SA, Tian XZ, Wang JJ, Yin WP, He SB (2009) Ethylenediamine modified Co/SiO2 sol–gel catalysts for non-ASF FT synthesis of middle distillates. Catal Commun 10:768
Schareina T, Zapf A, Cotte A, Muller N, Beller M (2008) A bioinspired copper catalyst system for practical catalytic cyanation of aryl bromides. Synthesis 28:3351
Zhu YZ, Cai C (2007) CuI/1,10-phenanthroline: an efficient Catalyst System for the Cyanation of Aryl Halides. J Chem Res 13:484
Schareina T, Zapf A, Mägerlein W, Müller N, Beller M (2007) Copper-catalyzed cyanation of heteroaryl bromides: a novel and versatile catalyst system inspired by nature. Synlett 13:555
Nandurkar NS, Bhanage BM (2008) Palladium bis(2,2,6,6-tetramethyl-3,5-heptanedionate) catalyzed Suzuki. Heck, Sonogashira, and cyanation reactions, Tetrahedron 64:3655
Cheng Y-N, Duan Z, Yu L, Li Z, Zhu Y, Wu Y (2008) Palladium-catalyzed three-component arylcyanation of internal alkynes with aryl bromides and K4[Fe(CN)6]. Org Lett 10:901
Zhu Y-Z, Cai C (2008) Palladium-catalyzed cyanation of aryl triflates. Synth Commun 38:2753
Zhu YZ, Cai C (2007) Pd/C: a recyclable catalyst for cyanation of aryl bromides. Eur J Org Chem 36:2401
Cheng YN, Duan Z, Li T, Wu Y (2007) Cyanation of aryl chlorides with potassium hexacyanoferrate(II) catalyzed by cyclopalladated ferrocenylimine tricyclohexylphosphine complexes. Synlett 33:543
Li LH, Pan ZL, Duan XH, Liang YM (2006) An environmentally benign procedure for the synthesis of aryl and arylvinyl nitriles assisted by microwave in ionic liquid. Synlett 22:2094
Sajadi SM, Maham M (2014) An efficient and new method for the copper-catalyzed cyanation of aryl halides is reported using a new oxazole ligand and K4Fe(CN)6 as a non-toxic source of cyanide. Lett Org Chem 11:136
Schareina T, Zapf A, Beller M (2005) An environmentally benign procedure for the Cu-catalyzed cyanation of aryl bromides. Tetrahedron Lett 46:2585
Balaji VR, Sanjeev KM, Kandikere RP (2012) A novel oxidative transformation of alcohols to nitriles: an efficient utility of azides as a nitrogen source. Chem Commun 48:5506
Khemnar AB, Bhanage BM (2014) Copper catalyzed nitrile synthesis from aryl halides using formamide as a nitrile source. RSC Adv 4:13405
Tao C, Liu F, Zhu Y, Liu W, Cao Z (2013) Copper-catalyzed aerobic oxidative synthesis of aryl nitriles from benzylic alcohols and aqueous ammonia. Org Biomol Chem 11:3349
Saha D, Adak L, Mukherjee M, Ranu BC (2012) Hydroxyapatite-supported Cu(i)-catalysed cyanation of styrenyl bromides with K4[Fe(CN)6]: an easy access to cinnamonitriles. Org Biomol Chem 10:952
Leggio A, Belsito EL, Gallo S, Liguori A (2017) One-pot conversion of aldehydes to nitriles mediated by TiCl4. Tetrahedron Lett 58:1512
Zhuang YJ, Liu J, Kang YB (2016) Tin or gallium-catalyzed cyanide-transition metal-free synthesis of nitriles from aldehydes or oximes. Tetrahedron Lett 57:5700
Khemnar AB, Sawant DN, Bhanage BM (2013) Rhodium catalyzed cyanide-free cyanation of aryl halide by using formamide as a cyanide source. Tetrahedron Lett 54:2682
Chatterjee T, Dey R, Ranu BC (2014) ZnO-supported pd nanoparticle-catalyzed ligand- and additive-free cyanation of unactivated aryl halides using K4[Fe(CN)6]. J Org Chem 79:5875
Wen Q, Jin J, Zhang L, Luo Y, Lu P, Wang Y (2014) Copper-mediated cyanation reactions. Tetrahedron Lett 55:1271
Shargi H, Sarvari MH (2003) Graphite as an efficient catalyst for one-step conversion of aldehydes into nitriles in dry media. Synthesis 11:243
Acknowledgements
We truly appreciate Scientific Research Center of Soran University for instrumental support of the work.
We are thankful to scientific research center of Soran University for providing research facilities.
Declarations
The authors report no applicable declarations during this manuscript.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
MP prepared and identified the geosubstrate, NAS and KK prepared the organic reactions and manuscript draft, respectively. SMH helped to provide the reviewers answer. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sajadi, S.M., Pirouei, M., Salih, N.A. et al. Rapid ecosynthesis of TiO2@CuO@Chromite nanocatalyst for environmentally friendly applications: solventless cyanation of aldehydes and high efficient treatment of sewage waters. Environ Sci Eur 32, 14 (2020). https://doi.org/10.1186/s12302-020-0293-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12302-020-0293-y