Abstract
Background
Lung cancer is the leading cause of cancer death in China and around the world. Early detection is key to improving the survival rate of non-small cell lung cancer (NSCLC). Alteration in glycosylation has been observed in cancers, and glycans can be a source for the development of new biomarkers for NSCLC.
Methods
In this glycan biomarker discovery study, we measured serum N- and O-glycan profiles in NSCLC patients with different stages and healthy controls by performing lectin microarray analysis. The alterations of serum glycopatterns were compared between NSCLC patients and controls, and the stage-related changes in serum glycosylation were evaluated.
Results
There were 18 lectins (e.g., AAL, Jacalin, GSL-I and DBA) to give significantly alterations of serum glycopatterns in lung adenocarcinoma compared with control group. Meanwhile, 16 lectins (e.g., Jacalin, HHL, and PHA-E+L) exhibited significantly alterations of serum glycopatterns in squamous cell carcinoma (SCC) compared with control group. Importantly, most of the lectins showing altered signals exhibited significantly increased or decreased NFIs in patients with early stage adenocarcinoma and SCC.
Conclusions
The serum glycan profiles were significantly different between NSCLC and healthy control, and most of the glycosylation changes had occurred at early stage. Further evaluation is needed to examine the diagnostic value of the glycan markers identified in this study.
Similar content being viewed by others
Background
In China, lung cancer is the most common incident cancer and the leading cause of cancer-related death [1]. According to the Global Burden of Disease Study 2016 (GBD2016), lung cancer had been identified as the fourth most frequent cause of death, and the death rate of lung cancer increased by 24.4% from 2005 to 2016 in China [2]. Non-small cell lung cancer (NSCLC), which accounts for 85% of all lung cancer cases, is divided into 3 major pathologic subtypes: adenocarcinoma, squamous cell carcinoma (SCC) and large cell carcinoma. Early diagnosis is critical because the chance of survival is greater in early stage (Stage I and II) NSCLC [3]. It is estimated that 36–73% of patients will survive longer than 5 years if the cancer is detected early [4]. At late stage (Stage III and IV), however, less than 15% will survive longer than 5 years.
Low-dose computed tomography (LDCT) has been suggested as a screening tool for early diagnosis of lung cancer [5], however its clinical utility is limited by its limited ability of differentiating benign pulmonary nodules from lung cancer. A systematic review of randomized clinical trials indicated that the average nodule detection rate of LDCT was 24.2%, with 96.4% of nodules being benign [6]. The high false positive rate of LDCT scan results in multiple screening rounds and unnecessary radiation exposure. The development of non-invasive complementary biomarkers could be a useful strategy to decrease false positive rate of CT scans and the overdiagnosis rate. A number of serum-based biomarkers have been identified and are being used clinically for lung cancer detection. Currently, carcinoembryonic antigen (CEA) is the most common clinical serum marker for lung adenocarcinoma. Cytokeratin 19 (CYFRA21-1) and squamous cell carcinoma antigen (SCCA) are commonly used as SCC markers. However, because of their low sensitivity, these markers described above have not been generally recommended as a tool for the early detection of lung cancer. During the early stages of NSCLC, the sensitivity of these markers is less than 25% [7]. Therefore, it is necessary to investigate novel serum biomarkers for lung cancer detection.
Aberrant glycosylation is observed in many types of cancers, and glycan-based biomarker discovery has advanced significantly recently [8,9,10]. Protein glycosylation involves addition of an oligosaccharide to either a nitrogen atom (N-linked glycosylation) or an oxygen atom (O-linked glycosylation) of the polypeptide chain. Glycosylation is the most common post-translation modification of secreted proteins, and alterations in cellular pathways can affect glycan structures [11]. The cancer-associated changes in glycosylation, including sialylation, fucosylation, O-glycan truncation, and N- and O-linked glycan branching, have been reported in the occurrence and progression of different cancers [12]. Aberrant glycosylation changes in the serum glycome could provide promising biomarkers for NSCLC.
In this glycan biomarker exploratory study, changes in serum glycan profiles was investigated in patients with NSCLC. We analyzed serum N- and O-glycan profiles in patients with NSCLC and healthy controls using lectin microarrays, a high-throughput glycome technique that we have proven to be useful in the given complex biological sample [13, 14]. In addition, glycan profiles of serum proteins from NSCLC patients with various stages were also compared.
Methods
Subject recruitment and sample collection
Serum samples were obtained from 62 newly diagnosed cases of primary NSCLC, including 28 adenocarcinoma and 34 SCC patients, at the First Affiliated Hospital of Xi’an Jiaotong University. The NSCLC cases should meet the following inclusion criteria: (1) newly diagnosed and confirmed histologically cases of primary NSCLC, (2) the patient had not received any treatment for NSCLC, (3) the patient had complete demographic and related clinical data. The study excluded the patients who did not meet the inclusion criteria and/or were diagnosed for any other primary cancer and lung cancer as their secondary primary cancer. Patients with NSCLC were confirmed by computed tomography guided fine needle aspiration cytology (FNAC) or fibre optic bronchoscopy (FOB) guided biopsy. Healthy control individuals were recruited from the health checkup center in the same hospital, and met the following inclusion criteria: (1) no clinical or radiological evidence of malignancy or other acute or chronic pathology, (2) the individual had complete demographic and related clinical data. Their cancer-free status was confirmed by testing for plasma levels of tumor biomarkers, including CEA, NSE, CYFRA21-1, SCCA, ProGRP, AFP, ferritin, PSA, CA-125, CA 15-3, CA 19-9 and CA 72-4, as well as computed tomography examination. Additionally, the subjects were chosen at the same or similar age stage. The samples of adenocarcinoma and control groups are gender-matched. Since SCC is rare in women compared with men in China, we only collected 1 female patient with SCC during the serum sample recruitment. Serum samples were kept at an − 80 °C freezer until processing.
Serum protein labelling
We segregated adenocarcinoma and SCC patients using their disease stages based on the American Joint Committee on Cancer staging system. Among the 28 adenocarcinoma samples used for the detection of serum protein glycosylation, 8 were at Stage I/II, 8 at Stage III and 12 at Stage IV. Of the 34 SCC samples, 9 were at Stage I/II, 12 at Stage III and 13 at Stage IV. To normalize the differences between subjects and to tolerate individual variation, 100 μL of each sample from Stage I/II adenocarcinoma, Stage III adenocarcinoma, Stage IV adenocarcinoma, Stage I/II SCC, Stage III SCC, Stage IV SCC and healthy controls was, respectively, pooled. The pooled serum proteins were labelled with Cy3 fluorescence dye (GE Healthcare, Buckinghamshire, UK), and then labelled proteins were separated from the excess free dye by Sephadex G-25 columns (GE Healthcare) according to the manufacturer’s instructions. The Cy3-labeled serum proteins were quantified and stored at − 20 °C in the dark until use.
Lectin microarrays and data analysis
A lectin microarray was produced using 37 lectins (Vector Laboratories, Sigma-Aldrich, and Calbiochem) with different specificities covering N- and O-linked glycans as previously described [13, 15]. The sugar-binding specificities of lectins and the layout of the lectin microarrays are summarized in Additional files, Additional file 1: Fig. S1 and Additional file 2: Table S1. Each lectin was printed in triplicate per block with triplicate blocks on one slide. BSA and BSA-conjugated with Cy3 were used to validate the feasibility of the lectin microarray. After immobilization, the slides were blocked with blocking buffer (2%, w/v, BSA in PBS, pH 7.4) for 1 h and rinsed twice with PBS. Next, 4 μg of Cy3-labeled serum proteins was diluted to 700 μL with hybridization buffer (2%, w/v, BSA, 500 mM glycine and 0.1%, v/v, Tween-20 in PBS, pH 7.4), and the resultant Cy3-labeled protein solution was applied to the lectin microarrays. After incubation and washing, the slides were scanned using a Genepix 4000B confocal scanner set at 70% photomultiplier tube and 100% laser power (Axon Instruments, Union City, CA).
The acquired images were analyzed at 532 nm by Genepix 3.0 software (Axon Instruments). The average background was subtracted, and values less than the average background ± 2 standard deviations (SD) were removed from each data point. The median of the effective values of each lectin was normalized by the sum of the medians of all effective data points for each lectin in one block. The pooled serum of each group was observed by 3 repeated microarrays and the normalized medians of each lectin from 9 repeated blocks were averaged and its SD was calculated.
Statistical analysis
All statistical analysis was done with the Statistical Package for the Social Sciences (SPSS) version 20.0 (SPSS Inc., Chicago, IL). Differences between two arbitrary datasets were evaluated using one-way analysis of variance (ANOVA) or Student’s t tests. Hierarchical clustering analysis (HCA) was performed by Expander 7.1 (http://acgt.cs.tau.ac.il/expander/). P values of < 0.05 were considered statistically significant.
Results
Serum glycopatterns in patients with NSCLC and healthy controls
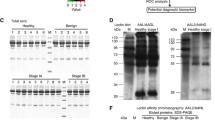
The characteristics of study participants are summarized in Table 1. The glycopatterns of Cy3-labeled serum proteins from Stage I/II adenocarcinoma, Stage III adenocarcinoma, Stage IV adenocarcinoma, Stage I/II SCC, Stage III SCC, Stage IV SCC and control subjects bound to the lectin microarrays are shown in Fig. 1a. Their normalized fluorescent intensities (NFIs) for each lectin are summarized in Additional file, Additional file 3: Table S2. The normalized data of the 7 groups were imported into Expander 7.1 to perform a hierarchical clustering analysis (Fig. 1b). Lectin signal patterns were classified into 3 categories to evaluate whether the glycan profiles of serum glycoproteins were altered in NSCLC: (1) those showing significant increases in NFIs (fold change > 1.5, P < 0.05), (2) those showing significant decreases in NFIs (fold change < 0.67, P < 0.05), and (3) those showing almost even level in NFIs (fold change range from 0.67 to 1.5, no significant difference) [14, 15]. Those lectins showing the difference of glycan expression between NSCLC and healthy control were marked with frames in Fig. 1a.
The glycopatterns of serum glycoproteins from NSCLC patients and healthy controls using the lectin microarrays. a The glycopatterns of Cy3-labeled serum proteins from Stage I/II adenocarcinoma, Stage III adenocarcinoma, Stage IV adenocarcinoma, Stage I/II SCC, Stage III SCC, Stage IV SCC and healthy controls bound to the lectin microarrays. A block of the slide with 3 replicates of 37 lectins was shown. Lectins showing the difference between adenocarcinoma and control were marked with white frames; lectins showing the difference between SCC and control were marked with yellow frames; and compared with control group, lectins showing the difference in both adenocarcinoma and SCC groups were marked with red frames. b Heat map and hierarchical clustering analysis of the normalized data of 37 lectins in the 7 groups. The groups were listed in columns, and the lectins were listed in rows. The color and intensity of each square indicated lectin signals relative to other groups in the row. Red, high; green, low; black, medium
Alterations of serum glycopatterns between adenocarcinoma patients and control individuals
Eighteen lectins that showed significant differences in glycan expression of serum glycoproteins between the adenocarcinoma and healthy control groups are shown in Table 2. Among the 14 lectins exhibiting increased NFIs in adenocarcinoma groups compared to control group, 11 lectins, e.g. the Fucα1-6GlcNAc (core fucose) binder AAL, the GlcNAc and αGal binder GSL-I, and the terminal GalNAc binder SBA, showed significantly increased NFIs in Stage I/II, Stage III and Stage IV adenocarcinoma groups. Compared with control group, the Galβ1-3GalNAcα-Ser/Thr (T antigen) binder Jacalin and the Fucα1-2Galβ1-4GlcNAc binder UEA-I showed increased NFIs in Stage III and Stage IV adenocarcinoma, while the GalNAcα-Ser/Thr (Tn antigen) and GalNAcα1-3Gal binder DBA only showed increased NFIs in Stage I/II adenocarcinoma. Moreover, compared with control group, the Fucα1-2Galβ1-4GlcNAc, Fucα1-3(Galβ1-4)GlcNAc binder LTL, the β-Gal, Galβ-1,4GlcNAc (type II) and Galβ1-3GlcNAc (type I) binder RCA-120, the (GlcNAc)n binder STL, the high-Mannose, Manα1-6(Manα1-3)Man binder ConA showed significantly decreased NFIs in adenocarcinoma groups. The results indicated that the significant differences in the signals of 12 lectins showing increased NFIs and 4 lectins showing decreased NFIs had occurred in patients with early stage lung adenocarcinoma.
Further statistical analysis was performed to evaluate the stage-related changes in NFIs of the 18 lectins showing altered signals in adenocarcinoma patients. The NFIs of each lectin from 3 stage groups were compared based upon fold changes and P value in pairs (Fig. 2). The NFIs of AAL had an increasing trend with stage in lung adenocarcinoma and was significantly higher in Stage IV adenocarcinoma than in Stage I/II adenocarcinoma. The NFIs of SJA, GSL-I and MAL-I were increased in lung adenocarcinoma compared to healthy control, but they had a decreasing trend with stage in adenocarcinoma patients. The NFIs of SJA and MAL-I showed significantly lower NFIs in Stage IV adenocarcinoma compared with those in Stage I/II and Stage III adenocarcinoma. The NFIs of GSL-I showed significant differences between each pair of the 3 stage groups.
Alterations of serum glycopatterns between patients with SCC and control individuals
The NFIs of 16 lectins were significantly different between SSC and control groups. As shown in Table 3, there were 9 lectins to show stronger signal with the fold change greater than 1.5 in SCC groups compared with control group, for example, the high-Mannose, Manα1-3Man, and Manα1-6Man binder HHL, the bisecting GlcNAc and biantennary N-glycans binder PHA-E+L, etc. Meanwhile, 7 lectins, including the T antigen binder Jacalin, the Galα1-3(Fucα1-2)Gal binder EEL, the Fucα1-2Galβ1-4GlcNAc and Fucα1-3(Galβ1-4)GlcNAc binder LTL, and the GalNAcα-Ser/Thr and GalNAcα1-3Gal binder DBA, showed weak signal with the fold change lower than 0.67 in SSC groups compared with control group. All the lectins showing different signals in SCC groups, except EEL, exhibited significantly increased or decreased NFIs in patients with early stage SCC compared with healthy controls. In addition, further analysis on stage-related alterations indicated that the NFIs of these lectins were not significantly different among Stage I/II, Stage III, and Stage IV SCC groups.
Discussion
Protein modifications, which occur as translational, post-translational, regulatory, and/or degradation products, play important roles in biological processes and increase the amount of useful cancer-related information obtained from the proteins [16]. Glycosylation is involved in various aspects of cancer development, including cell–cell interactions, cell adhesion, malignant transformation and metastasis. Driven by technology advancements in the field of analytical glycoscience, protein glycosylation has become increasingly important in cancer research and hold considerable promise to provide new candidate glycan markers for cancer. Aberrant glycosylation of serum proteins has been found in patients with various types of cancer, including breast, colon, ovarian, and pancreatic cancer [17, 18]. Therefore, glycan-based serological assays may be useful to detect serum biomarkers for cancer. Kyselova et al. performed a MALDI mass spectrometry (MS)-based glycomic profile analysis, and found increases in sialylation and fucosylation of serum N-glycans in breast cancer appeared to be indicative of cancer progression [19]. In another study, Akimoto et al. found that 9 glycans were highly expressed in the sera of patients with invasive intraductal papillary mucinous neoplasms (IPMNs), and fucosylated tri-antennary glycan could distinguish invasive IPMNs from non-invasive IPMNs [10]. In this study, a lectin microarray was used firstly to investigate the glycan profiles of serum proteins in NSCLC patients at different stages, and systematically compare the alterations of serum glycopatterns between NSCLC patients and healthy controls. There were 18 lectins (e.g., AAL, Jacalin, GSL-I and DBA) to give significantly alterations of serum glycopatterns in lung adenocarcinoma patients compared with healthy control. Meanwhile, compared to control group, 16 lectins (e.g., Jacalin, HHL, and PHA-E+L) exhibited significantly alterations of serum glycopatterns in SCC patients.
Although fucosylation is essential for normal biological functions, a change in fucosylation has been strongly implicated in tumor development and progression. In the present study, we found that the levels of core fucosylation identified by AAL was elevated in sera from lung adenocarcinoma patients. Importantly, since the serum levels of core fucosylation were significantly increased in Stage I/II adenocarcinoma compared with control group, the core fucosylation might be a potential diagnostic biomarker for early detection of lung adenocarcinoma. Core fucosylation is the addition of α1-6-linked fucose to the innermost GlcNAc residue of N-linked glycans, which is catalyzd by fucosyltransferase 8 (FUT8) [20]. The elevated core fucosylation has been also observed in sera of hepatocellular carcinoma (HCC), prostate and ovarian cancer patients [8, 21, 22]. In addition to the increase in core fucosylation, the transcription of FUT8 was enhanced in HCC and prostate cancer tissues [23, 24]. It should also be noted that a recent study by Mehta et al. observed a similar level of core fucosylation in HCC tissue compared to the adjacent nontumor tissue, after measuring increased expression level of FUT8 in HCC tissue compared to nontumor tissue, suggesting that there might be an abnormal secretion of core fucosylated proteins into the serum of HCC patients [25]. Two studies by Chen et al. and Honma et al. showed that the expression of FUT8 was up-regulated in NSCLC tissues, and it was significantly more prevalent in adenocarcinoma than in SCC [26, 27]. In line with these results mentioned above, the elevated level of core fucosylation was observed in the sera of adenocarcinoma patients, but not in SCC patients in this study. Moreover, according to the lectin microarray results, the serum levels of core fucosylation had an increasing trend with stage in adenocarcinoma. The increased serum level of core fucosylation may reflect the tumor burden and serve as a prognostic marker for lung adenocarcinoma.
In this study, we found that the signals of T/Tn antigen binders Jacalin, GSL-I and DBA was increased in the sera of patients with adenocarcinoma, while Jacalin and DBA showed decreased NFIs in the sera of SCC patients. Tn antigen is a common precursor of O-linked glycans. With the aid of T-synthase, Tn antigen can receive a galactose unit (from UDP-Gal) to form T antigen (Thomsen-Friedenreich antigen). It has been reported that Tn antigen was highly expressed in 10–90% of breast, colon, cervix, stomach, and prostate tumors, which indicated the relationship between Tn antigen and cancer [28]. T antigen was detected as a cancer-associated carbohydrate antigen, and involved in tumor cell adhesion and tissue invasion [29]. Consistent with our results, Lopez-Ferrer et al. found that T and Tn antigen were detected more frequently in lung adenocarcinomas than in lung squamous cell carcinomas by immumohistochemical staining [30]. In another study, the levels of T antigen and its respective binding site were significantly lower in the head and neck squamous cell carcinoma samples compared with the normal samples [31]. Given the differential expressions of T and Tn antigen in adenocarcinoma and SCC, these markers may have the potential to distinguish the histological subtypes of NSCLC. A recent study on breast cancer cell lines indicated that in the highly metastatic cell line MDA-MB435, enhanced level of T antigen was found, but the T antigen level was much lower in the non-metastatic cell line MDA-MB468 [28]. The present study also found that the serum level of T antigen was only elevated in the patients with Stage III and Stage IV adenocarcinoma compared with healthy controls. Hence, the expression of T antigen might be associated with enhanced metastatic potential and poor prognosis in caners, and its detection is probably useful in serologic diagnosis.
Previous studies have indicated that elevated high-mannose N-linked glycans were associated with various cancers including ovarian, breast and colorectal cancer [9, 32, 33]. De Leoz et al. reported an elevation of high-mannose glycans in the sera of patients with metastatic breast cancer compared with healthy controls [9]. In glycan biosynthesis, the high-mannose structures are synthesized early in the process. The mannose residues of the N-glycans are then cleaved sequentially and are replaced by N-acetylglucosamine and glucose residues to form complex and hybrid glycans. The elevated levels of secreted high-mannose glycans suggested that the incomplete maturation of N-glycosylation in the secretory pathway, which could result from higher protein secretion and/or lower abundance/activity of the glycosylation enzymes [34]. In this study, we found that the level of high-mannose glycans identified by HHL was significantly increased in the sera of SSC patients compared with control group. The increased high mannose structures could influence the function of glycoproteins in serum through altering the stability, adhesion and communication properties of the protein [9].
There are still some limitations in this study. Lectin microarray is a high-cost and time-consuming procedure and not suitable for large number of samples. Therefore, a sample pooling strategy was employed to overcome this drawback. However, the pooled samples are unable to provide individual information of glycan expression. The data of pooled samples cannot be used to perform receiver operating characteristic curve analysis and calculate the cut-off value, sensitivity and specificity of the differentially expressed glycopatterns for NSCLC detection. Moreover, we pooled serum samples with an equal volume, which can globally reveal the differences of glycan expression in serum glycoproteins between patients with NSCLC and controls. And meanwhile, this pooling method can keep the concentration differences of both glycoproteins and glycans in each serum sample better than pooling samples with an equal total amount of serum proteins. However, the total protein content of sera depends individually on the nutrient, body fluid alteration, age, and physical and disease conditions. If the total serum protein concentration was significantly lower than the normal level in some patients, which influenced low-abundance proteins, pooling equal volumes of samples might give rise to some biases. Future studies are required to detect these aberrant glycopatterns in individual serum samples to verify their diagnostic value (sensitivity and specificity) as potential biomarkers for NSCLC. We will also perform studies to determine the diagnostic values of these glycan biomarkers for distinguishing NSCLC from benign pulmonary diseases and other types of cancers in the future.
Although this study requires further testing and validation, it suggests that the glycan profiles of serum proteins are significantly different between NSCLC and healthy control. Analysis of serum glycans by lectin microarray represents a new paradigm in cancer biomarker studies, i.e. a focus on glycosylation modification of proteins rather than protein expression. Most of the glycosylation changes in the sera of NSCLC patients were observed at early stage of this disease. Further investigation should be performed to evaluate the early diagnostic value of these serum markers for NSCLC in the future.
Abbreviations
- NSCLC:
-
non-small cell lung cancer
- SCC:
-
squamous cell carcinoma
- LDCT:
-
low-dose computed tomography
- CEA:
-
carcinoembryonic antigen
- SCCA:
-
squamous cell carcinoma antigen
- NSE:
-
neuron specific enolase
- ProGRP:
-
pro-gastrin-releasing peptide
- AFP:
-
α-fetoprotein
- PSA:
-
prostate-specific antigen
- SD:
-
standard deviations
- HCA:
-
hierarchical clustering analysis
- NFI:
-
normalized fluorescent intensity
- IPMN:
-
intraductal papillary mucinous neoplasm
- FUT8:
-
fucosyltransferase 8
- HCC:
-
hepatocellular carcinoma
- T antigen:
-
Thomsen–Friedenreich antigen
References
Chen W, Zheng R, Zhang S, Zeng H, Zuo T, Xia C, et al. Cancer incidence and mortality in China in 2013: an analysis based on urbanization level. Chin J Cancer Res. 2017;29(1):1–10.
Abajobir AA, Abbafati C, Abbas KM, Abd-Allah F, Abera SF, Aboyans V, et al. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the global burden of disease study 2016. Lancet. 2017;390(10100):1151–210.
Cruz CSD, Tanoue LT, Matthay RA. Lung cancer: epidemiology, etiology, and prevention. Clin Chest Med. 2011;32(4):605–44.
Padda SK, Burt BM, Trakul N, Wakelee HA. Early-stage non-small cell lung cancer: surgery, stereotactic radiosurgery, and individualized adjuvant therapy. Sem Oncol. 2014;41(1):40–56.
Smith RA, Andrews KS, Brooks D, Fedewa SA, Manassaram-Baptiste D, Saslow D, et al. Cancer screening in the United States, 2018: a review of current American Cancer Society guidelines and current issues in cancer screening. Cancer J Clin. 2018;68(4):297–316.
Team NLSTR. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;11(365):395–409.
Okamura K, Takayama K, Izumi M, Harada T, Furuyama K, Nakanishi Y. Diagnostic value of CEA and CYFRA 21-1 tumor markers in primary lung cancer. Lung Cancer. 2013;80(1):45–9.
Saldova R, Fan Y, Fitzpatrick JM, Watson RWG, Rudd PM. Core fucosylation and α2-3 sialylation in serum N-glycome is significantly increased in prostate cancer comparing to benign prostate hyperplasia. Glycobiology. 2010;21(2):195–205.
de Leoz ML, Young LJ, An HJ, Kronewitter SR, Kim J, Miyamoto S, et al. High-mannose glycans are elevated during breast cancer progression. Mol Cell Proteom. 2011;10(1):M110-002717.
Akimoto Y, Nouso K, Kato H, Miyahara K, Dohi C, Morimoto Y, et al. Serum N-glycan profiles in patients with intraductal papillary mucinous neoplasms of the pancreas. Pancreatology. 2015;15(4):432–8.
Taniguchi N, Kizuka Y. Glycans and cancer: role of N-glycans in cancer biomarker, progression and metastasis, and therapeutics. Adv Cancer Res. 2015;126:11–51.
Pinho SS, Reis CA. Glycosylation in cancer: mechanisms and clinical implications. Nat Rev Cancer. 2015;15(9):540–55.
Qin Y, Zhong Y, Dang L, Zhu M, Yu H, Chen W, et al. Alteration of protein glycosylation in human hepatic stellate cells activated with transforming growth factor-β1. J Proteom. 2012;75(13):4114–23.
Liang Y, Ma T, Thakur A, Yu H, Gao L, Shi P, et al. Differentially expressed glycosylated patterns of alpha-1-antitrypsin as serum biomarkers for the diagnosis of lung cancer. Glycobiology. 2015;25(3):331–40.
Qin Y, Zhong Y, Zhu M, Dang L, Yu H, Chen Z, et al. Age- and sex-associated differences in the glycopatterns of human salivary glycoproteins and their roles against influenza A virus. J Proteome Res. 2013;12(6):2742–54.
Kailemia MJ, Park D, Lebrilla CB. Glycans and glycoproteins as specific biomarkers for cancer. Anal Bioanal Chem. 2017;409(2):395–410.
Wu J, Xie X, Nie S, Buckanovich RJ, Lubman DM. Altered expression of sialylated glycoproteins in ovarian cancer sera using lectin-based ELISA assay and quantitative glycoproteomics analysis. J Proteome Res. 2013;12(7):3342–52.
Rho J-H, Ladd JJ, Li CI, Potter JD, Zhang Y, Shelley D, et al. Protein and glycomic plasma markers for early detection of adenoma and colon cancer. Gut. 2016. https://doi.org/10.1136/gutjnl-2016-312794.
Kyselova Z, Mechref Y, Kang P, Goetz JA, Dobrolecki LE, Sledge GW, et al. Breast cancer diagnosis and prognosis through quantitative measurements of serum glycan profiles. Clin Chem. 2008;54(7):1166–75.
Christiansen MN, Chik J, Lee L, Anugraham M, Abrahams JL, Packer NH. Cell surface protein glycosylation in cancer. Proteomics. 2014;14(4–5):525–46.
Alley WR Jr, Vasseur JA, Goetz JA, Svoboda M, Mann BF, Matei DE, et al. N-linked glycan structures and their expressions change in the blood sera of ovarian cancer patients. J Proteome Res. 2012;11(4):2282–300.
Comunale MA, Lowman M, Long RE, Krakover J, Philip R, Seeholzer S, et al. Proteomic analysis of serum associated fucosylated glycoproteins in the development of primary hepatocellular carcinoma. J Proteome Res. 2006;5(2):308–15.
Ji J, Gu X, Fang M, Zhao Y, Yi C, Wang A, et al. Expression of alpha 1, 6-fucosyltransferase 8 in hepatitis B virus-related hepatocellular carcinoma influences tumour progression. Dig Liver Dis. 2013;45(5):414–21.
Wang X, Chen J, Li QK, Peskoe SB, Zhang B, Choi C, et al. Overexpression of alpha (1,6) fucosyltransferase associated with aggressive prostate cancer. Glycobiology. 2014;24(10):935–44.
Mehta A, Norton P, Liang H, Comunale MA, Wang M, Rodemich-Betesh L, et al. Increased levels of tetra-antennary N-linked glycan but not core fucosylation are associated with hepatocellular carcinoma tissue. Cancer Epidemiol Biomark Prev. 2012;21(6):925–33.
Chen CY, Jan YH, Juan YH, Yang CJ, Huang MS, Yu CJ, et al. Fucosyltransferase 8 as a functional regulator of nonsmall cell lung cancer. Proc Natl Acad Sci USA. 2013;110(2):630–5.
Honma R, Kinoshita I, Miyoshi E, Tomaru U, Matsuno Y, Shimizu Y, et al. Expression of fucosyltransferase 8 is associated with an unfavorable clinical outcome in non-small cell lung cancers. Oncology. 2015;88(5):298–308.
Fu C, Zhao H, Wang Y, Cai H, Xiao Y, Zeng Y, et al. Tumor-associated antigens: Tn antigen, sTn antigen, and T antigen. Hla. 2016;88(6):275–86.
Glinsky VV, Huflejt ME, Glinsky GV, Deutscher SL, Quinn TP. Effects of Thomsen–Friedenreich antigen-specific peptide P-30 on β-galactoside-mediated homotypic aggregation and adhesion to the endothelium of MDA-MB-435 human breast carcinoma cells. Cancer Res. 2000;60(10):2584–8.
Lopez-Ferrer A, Barranco C, de Bolos C. Differences in the O-glycosylation patterns between lung squamous cell carcinoma and adenocarcinoma. Am J Clin Pathol. 2002;118(5):749–55.
Choufani G, Nagy N, Saussez S, Marchant H, Bisschop P, Burchert M, et al. The levels of expression of galectin-1, galectin-3, and the Thomsen–Friedenreich antigen and their binding sites decrease as clinical aggressiveness increases in head and neck cancers. Cancer. 1999;86(11):2353–63.
Anugraham M, Jacob F, Nixdorf S, Everest-Dass AV, Heinzelmann-Schwarz V, Packer NH. Specific glycosylation of membrane proteins in epithelial ovarian cancer cell lines: glycan structures reflect gene expression and DNA methylation status. Mol Cell Proteom. 2014;13(9):2213–32.
Sethi MK, Kim H, Park CK, Baker MS, Paik YK, Packer NH, et al. In-depth N-glycome profiling of paired colorectal cancer and non-tumorigenic tissues reveals cancer-, stage- and EGFR-specific protein N-glycosylation. Glycobiology. 2015;25(10):1064–78.
Lee LY, Lin C-H, Fanayan S, Packer NH, Thaysen-Andersen M. Differential site accessibility mechanistically explains subcellular-specific N-glycosylation determinants. Front Immunol. 2014;5:404.
Authors’ contributions
YL, MC, ZL and AY designed the study, obtained the fund and conducted the microarrays; TW, LG, HR, PS, PH, SZ were involved with sample collection and processing; PH and YL performed the protein labelling and lectin microarrays; YL interpreted data and edited the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare no financial competing interests.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
All authors consent to the publication of this manuscript.
Ethics approval and consent to participate
The protocol for this study was in accordance with the Declaration of Helsinki and its subsequent revisions, and it was approved by the Institutional Review Boards of the First Affiliated Hospital of Xi’an Jiaotong University and Northwest University. All the subjects signed informed consent documents.
Funding sources
This study was supported by Collaboration Project of International Science and Technology of Shaanxi Province (No. 2018KW-039), Key Research and Development Program of Shaanxi Province (No. 2018SF-219), and the Fundamental Research Funds for the Central Universities in Xi’an Jiaotong University (No. xjj2018094).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding authors
Additional files
Additional file 1: Figure S1.
The layout of the lectin microarrays. Each lectin was spotted in triplicate per block, with triplicate blocks on one slide. Cy3-labeled BSA was spotted as a location marker and BSA as a negative control.
Additional file 2: Table S1.
Sugar-binding specificities of the 37 lectins used in the lectin microarray.
Additional file 3: Table S2.
The normalized fluorescent intensities (NFIs) of each lectin for the serum proteins from NSCLC groups and control group.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Liang, Y., Han, P., Wang, T. et al. Stage-associated differences in the serum N- and O-glycan profiles of patients with non-small cell lung cancer. Clin Proteom 16, 20 (2019). https://doi.org/10.1186/s12014-019-9240-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12014-019-9240-6