Abstract
Composite membranes were obtained by modification of heterogeneous polymer cation and anion-exchange membranes with nanoparticles of zirconium hydrophosphate and hydrated zirconium dioxide, respectively. The ion-exchange materials were investigated with the methods of electron microscopy, potentiometry, voltammetry, and impedance spectroscopy. Single nanoparticles, which were precipitated in aqueous media, form aggregates, when the composites are in a contact with polar organic solvent. Both single nanoparticles (up to 10 nm) and their aggregates (up to 200 nm) were precipitated in ion-exchange polymers in glycerol media. Non-aggregated nanoparticles improve electrical conductivity of the ion-exchange materials, the aggregates are barriers against fouling. The membranes were applied to NaCl removal from highly concentrated glycerine-water mixture containing organic additives (byproduct of biodiesel production). As opposite to pristine materials, the composites demonstrate stability against fouling.
Similar content being viewed by others
Background
Electrodialysis is applied as a solution of different problems: water treatment and water conditioning [1], particularly removal of toxic ionic components from ground water [2,3,4] or preparation of water from liquid wastes of dairy industry for washing of equipment [5], processing of reverse osmosis concentrate [6], or secondary wastes after sorbent regeneration [7], desalination of protein concentrate [8], production of organic acids [9], and many other practical tasks.
Very important practical problem is processing of non-aqueous solutions, for instance, glycerol, which is formed as a byproduct during biodiesel production [10]. Glycerol can be further used for synthesis of dihydroxyacetone, succinic, propionic, citric acid, pigments, etc. [11], for production of synthetic gas [12] and even as fuel [13]. However, preliminary deep desalination is necessary since glycerol produced by this manner contains high amount of mineral components (mainly NaCl). The most common purification method is extremely energy-intensive distillation [14]. Ion exchange [15] as well as reverse osmosis [16] could be applied only to slightly mineralized solution. Ultrafiltration, which has been proposed for removal of palm and oleic acid from glycerol [17], cannot be applied to desalination.
Electrodialysis is expected to be the most suitable method for glycerol desalination since the process can be used for removal of inorganic ions from solutions of wide concentration interval [18, 19]. Bipolar electrodialysis has been developed earlier for glycerol desalination: the demineralization degree above 80% was achieved with glycerol losses below 2% [18]. Traditionally, polymer ion-exchange membranes are used for electrodialysis [20]. In the case of crude glycerol, which contains high amount of organic additives, fouling of the polymer membranes is expected [21,22,23].
In the case of materials for baromembrane separation, modification of the membranes with inorganic nanoparticles (SiO2 [24], Fe2O3 [25], ZrO2 [26, 27], TiO2 [28], zirconium hydrophosphate [29]) provides their stability against fouling with organics. Similar approach was applied to ion-exchange membranes for fuel cells [30,31,32]. Functions of the inorganic modifier are to enhance proton conductivity of the membranes and to prevent their dehydration under high temperature. Last years, the organic-inorganic membranes for electrodialysis were investigated [33,34,35,36,37]. Nanoparticles of inorganic ion exchanger transform even inert polymer to ion-exchange membrane [35], it is similarly to ceramic membranes [38,39,40]. However, polymer ion-exchange membranes are poisoned with organic solvents [41, 42]: reorganization of their porous structure results in deterioration of functional properties, for instance, it enhances methanol crossover [42]. This undoubtedly influences location of inorganic particles, which have to be non-aggregated in order to provide high rate of ion transport [43, 44].
The aim of the work was to obtain organic-inorganic membranes for desalination of non-aqueous solutions, which would combine stable structure in these media, high charge selectivity, considerable electric conductivity, and stability against fouling with organics. The task of the work is the development of the modification methods using ion-exchange resins as model polymer matrices since these materials are used for preparation of heterogeneous membranes. Other problems are the application of the modification technique to membrane preparation, the investigation of morphology and functional properties of the composite materials, the testing of the membranes in the process of desalination of crude glycerol.
Hydrated zirconium dioxide (HZD) was used as a modifier of anion-exchange membrane. This ion-exchanger demonstrates anion-exchange ability in acidic and neutral media [45]. Amorphous zirconium hydrophosphate (ZHP) was applied to modification of cation-exchange membranes. This inorganic ion exchanger possesses high exchange capacity, it is chemically stable and requires no expensive chemical reagents for synthesis.
Experimental
Solutions for Electrodialysis
The effluent obtained during biodiesel production (Trostyanetz distillery plant of "Ukrspirt State Comrany", Ukraine) was applied to investigations. This glycerol-based solution contained water (10 mass %), organic impurities (8 mass %), and 1000 mol m−3 NaCl. Aqueous NaCl solutions were also used for potentiometric and impedance measurements.
Modification of Ion-Exchange Resins
Granulated polystyrene-divinylbenzene gel-like resins, namely, Dowex HCR-S (strongly acidic cation exchanger) and Dowex Marathon A (strongly basic anion exchanger), which had been produced by Dow Chemical company, were researched preliminary. It was necessary for investigations of the composites with transmission electronic microscopy (TEM) and for a choice of the most suitable modification method. The cation exchanger and anion exchanger were modified with ZHP and HZD, respectively.
The first series of the samples was prepared in accordance with following stages: (i) impregnation of the resin with water, (ii) impregnation of the wet resin with a 1 M ZrOCl2 solution for 24 h at 298 K (a ratio of volumes of the resin and solution was 1:20), (iii) washing of the resin with a HCl solution (10 mol m−3) up to constant pH of the effluent (about 2) to remove additionally sorbed electrolyte as completely as possible, (iv) treatment of the resin with a 1 M H3PO4 solution at 298 K (a ratio of volumes of the resin and solution was 1:10) followed by washing with deionized water up to neutral reaction of the effluent, (v) treatment with ultrasound at 30 kHz by means of a Bandelin device (Bandelin, Hungary) in order to clean outer surface of the granules, and (vi) treatment with glycerol followed by washing with deionized water and drying in a desiccator over CaCl2 at room temperature down to constant mass. After stage (v), a part of the resin was taken and dried in the desiccator.
Regarding the anion exchanger, the modification procedure was similar. However, a mixed solution (1 M ZrOCl2 and 7 M HCl) was used for resin impregnation (stage ii), 7 M HCl was employed for washing until disappearance of turbidity of the effluent after neutralization (stage iii). The inorganic constituent was precipitated with a 1 M NH4OH solution (stage iv).
The second series of the samples was prepared similarly; however, a 0.1 M ZrOCl2 solution in glycerol was used for resin impregnation. Solutions of H3PO4 or NH4OH in glycerol were used for precipitation of ZHP or HZD, respectively.
Modification of Ion-Exchange Membranes
CMI 7000 cation exchange (CM) and AMI 7000 anion exchange (AM) heterogeneous membranes (Membrane International), a thickness of which in a swelling state is about 600 μm, were investigated. The membranes were modified with HZP and HZD, respectively. The modification procedure was similar to that described above for the second series of the samples. After the last drying, the membranes were weighted.
SEM and TEM
Investigations of the membranes with a method of scanning electron microscopy (SEM) were provided by means of JEOL JSM 6700 F and JEOL JFC-1600 microscopes (JEOL, Japan). Preliminary, a platinum layer was deposited onto the sample at 3 Pa using an JEOL JFC-1600 Auto fine coater (JEOL, Japan). A JEOL JEM 1230 transmission electron microscope (JEOL, Japan) was applied to crushed ion-exchange resins. Before the investigations, both the membranes and resins were treated with ultrasound.
Investigation of Ion Transport
Two-compartment divided cell supplied by Ag/AgCl electrodes was used for potentiometric measurements, which were performed by means of a SCH-1312 voltmeter (Analitpribor, Ukraine). The cell compartments were filled with aqueous NaCl solutions (0.5 and 1 M) similarly to [46, 47].
Electrical resistance of the membranes was measured using a two-compartment cell supplied with platinum electrodes. Aqueous NaCl solutions filled the cell. The measurements were performed using an Autolab impedance system at 1 × 10−2 − 1 × 106 Hz. The cell resistance was determined as a wide plateau of frequency dependence of the real part of impedance. The membrane resistance was calculated as a difference between resistances of the cell with and without membrane [47, 48]. For comparison, electrical conductivity of H–(OH) forms of the ion-exchange resins was measured similarly to [43, 44]. Deionized water was used as a non-conducting medium.
Voltammetric measurements were provided according to four-electrode scheme similarly to [46]. The scheme involved two-compartment divided cell, two platinum working electrodes, which were connected with a IPPT 65-49 power supplier (Ukrrospribor LTD, Ukraine) and a SCH-4311 ammeter (Analitprobor, Ukraine). Two Ag/AgCl electrodes were connected with a voltmeter. The reference electrodes were supplied with Luggin capillaries.
All experiments were carried out at 298 K.
Electrodialysis of Glycerol Solution
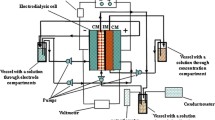
Experimental setup involved seven-compartment cell, three independent liquid lines, power supplier, and measuring instrumentation mentioned above (Fig. 1). The desalination chambers contained a grid for flow turbulization. An effective membrane area was 30 cm2 (30 cm × 1 cm), a distance between the membranes was 4 mm, a cross section area of each compartment was 0.4 cm2. The composite membranes were placed between the desalination and concentration compartments, other membranes were pristine. For comparison, the separation process was performed using only pristine membranes between all compartments.
A glycerol solution (200 cm3) was passed through the desalination compartments according to cyclic operation. A NaCl solution, initial concentration of which was 0.01 M (200 dm3) circulated through the concentration compartments. A 0.05 M Na2SO4 solution was passed through the electrode compartments.
Results
Aggregation of Nanoparticles Inside Polymer Matrix
Location of inorganic particles inside polymer ion-exchangers is determined by porous structure of these materials in a swollen state. The structure is known to include gel-like regions, where nanosized clusters (up to 20 nm [36, 43, 44, 46, 49,50,51]) and narrower channels between them are located (cluster-channel structure of polymer ion-exchange materials is described in detail in [49,50,51]). Clusters and channels, which contain functional groups, are considered as transport pores. Hydrophobic fields of hydrocarbonaceous chains are placed in voids between gel fields. Pores of micron size are related to structure defects and voids between ion exchanger and binder (for heterogeneous membranes).
Visualization of non-aggregated inorganic nanoparticles is possible only for resins: their grains can be crushed relatively easy down to size, which allows us to obtain TEM images. The photos of the organic-inorganic cation exchanger of the first series before and after treatment with glycerol (i.e., after modification stages v and vi) are given in Fig. 2. Non-aggregated globular ZHP nanoparticles (4–20 nm) can be seen, the aggregates were found to be practically absent. Non-aggregated HZD nanoparticles were also found in the anion exchanger. The nanoparticles are evidently placed inside clusters and channels and stabilized by their walls.
After treatment with organic solvent, no single nanoparticles were found. They form aggregates (≈100 nm), which are evidently located outside transport pores. The aggregation is probably due to cluster reorganization caused by adsorption of organic solvent [42]. Moreover, the reorganization can be caused by lower dielectric permittivity of glycerol in comparison with water. This enhances repulsion of counter ions of functional groups. As a result of reorganization, the nanoparticles leave the transport pores and form aggregates outside them.
Non-aggregated ZHP nanoparticles (2–10 nm), which were precipitated from glycerol solution, are seen in the sample of the second series (Fig. 3). Larger particles (up to 300 nm) are also visible on the image with smaller resolution. These particles are evidently related to aggregates, which are evidently places in voids between gel regions.
Similar regularities of nanoparticle formation are evidently characteristic for the membranes. As found, the mass content of ZHP and HZD in the membranes was 4.5 and 3.9%, respectively. After the treatment with glycerol, small aggregates (up to 300 nm) were found inside the ion-exchange constituent of the membranes (Fig. 4). These aggregates are evidently located in the voids between gel regions. No large particles, a size of which is comparable with pores between the ion-exchange polymer and binder, were found (Fig. 4).
Electrical Conductivity and Charge Selectivity of the Membranes
Logarithm of specific electrical conductivity of the membranes \( \left( \log \overline{\kappa}\right) \) is plotted in Fig. 5 vs conductivity of aqueous NaCl solution (κ). As seen, a reducing of the solution concentration causes a decrease of the \( \overline{\kappa} \) values due to diminution of a content of additionally sorbed electrolyte (both counter and co-ions). The electrolyte fills pores, which contain no functional groups. The \( \overline{\kappa} \) magnitude involves ion transport through clusters and channels. When the diffusion parts of electric double layers are not overlapped, this transport is due to surface and fluid conductivity.
In the case of cation-exchange membrane impregnated with a solution, its conductivity is determined as follows:
Here, F is the Faraday constant, z is the charge number, ū is the mobility and \( \overline{C} \) is the concentration, “+” and “−” subscripts correspond to cations and anions, respectively, “/” superscript is related to counter ions in clusters and channels, “//” index is attributed to counter and co-ions in pores, which are free from functional groups. Under the conditions of \( {z}_{+}{\overline{u}}_{+}^{/}{\overline{C}}_{+}^{/}<<{\overline{C}}^{//}\left({z}_{+}{\overline{u}}_{+}^{//}+{z}_{-}{\overline{u}}_{-}^{//}\right) \), the concentration of species outside clusters and channels can be determined as \( {\overline{C}}^{//}=\frac{\overline{\kappa}}{z_{+}{\overline{u}}_{+}^{//}+{z}_{-}{\overline{u}}_{-}^{//}} \). Here \( {\overline{u}}_{+}^{//} \) and \( {\overline{u}}_{-}^{//} \) are assumed to be equal to mobility of species in outer solution.
The dependencies of \( \frac{{\overline{C}}^{.//}}{C} \) on C (where C is the concentration of outer solution) are shown in Fig. 6. This ratio increases in the region of low concentration due to depression of surface conductivity through clusters. Further, the ratio reaches approximately constant values. The plateau corresponds to the concentration interval, at which the conductivity is determined mainly by the additionally sorbed electrolyte.
Extrapolation of the curve, which reflects the dependence of \( \lg \overline{\kappa} \) on κ , to κ = 0 gives a rather low magnitude. This value corresponds to ion transport only through the clusters and channels (Table 1). These \( \overline{\kappa} \) values are lower for the modified membranes, it is in agreement with data obtained for the ion-exchange resins (Table 2). Linear regions of the curves are related to the concentration diapason, where the conductivity of the membranes is determined mainly by additionally sorbed electrolyte. It is valid for this concentration interval:
where b 1 and b 2 are the empirical coefficients. They reflect the screening effect of polymer matrix (pristine membranes) or both matrix and aggregates (modified membranes).
Lower values of the coefficients for the modified membranes show that the aggregates perform a function of the barrier against additionally sorbed electrolyte. Since organics can be adsorbed on hydrophobic parts of polymer chains, the aggregates are assumed to protect the membranes from fouling.
Transport numbers of counter ions through the membrane (\( \overline{t} \)) were determined from measurements of membrane potential (E m ) followed by calculations from the formula [47]:
where a 1 and a 2 are the activity of less and more concentrated solutions, R is the gas constant, and T is the temperature.
Voltammetric curves obtained for aqueous NaCl solutions according to four-electrode scheme are given in Fig. 7. As seen, the values of limiting current density (i lim) are practically the same both for the pristine and modified cation-exchange membranes. The modified anion-exchange separator shows slightly lower current density than the pristine membrane indicating deterioration of charge selectivity.
In the region of i < 0.75 i lim, no linearity of the voltammetric dependencies is observed for the pristine membranes. The non-linearity indicates concentration polarization, which occurs in the largest pores between the ion-exchange polymer and binder. This phenomenon is typical for heterogeneous membranes [50]. However, the voltammetric dependence is linear for the modified membranes at i < 0.75 i lim. This indicates an exclusion of ion transport through the largest pores of the composites membranes evidently due to reduction of amount of additionally sorbed electrolyte.
Desalination of Waste Glycerol
Electrodialysis was performed under the constant voltage, which provided i = 0.75i lim, where i and i lim are the current density and limiting current, respectively. This was necessary to avoid precipitation of organic additives inside the membrane system. The current gradually decreased in accordance to reduction of NaCl concentration in the desalination compartments. Configuration of the membrane system provided stability of the pH (about 6) both of the concentrate and solution being purified.
When the modified membranes separated the desalination and concentration compartments, the salt content in the glycerol solution diminished gradually. This reflects a dependence of electrical conductivity of the solution through the desalination compartment on time plotted in semi-logarithmic coordinates (Fig. 8).
Linear dependence in these coordinates is due to diffusion limitations. No organic impurities, which were present in crude glycerol, were found in the desalination compartment. The current efficiency reached 95–98%. The process was stopped, when the residual salt concentration was in 1000 times lower than that in the initial solution. After finishing the process, the membranes were removed, washed with deionized water, and their conductivity was measured using aqueous NaCl solution (40 mol dm−3) as described in “Investigation of Ion Transport.” A decrease of conductivity was about 2% for the cation-exchange membrane in a comparison with the value obtained before the process. Regarding the anion-exchange separator, the conductivity was even slightly higher after electrodialysis (about 5%). However, these deviations are practically within experimental error indicating stability of the modified membranes against fouling.
In the case of the pristine membranes, the rate of desalination is much slower evidently due to their blockage with organics. The cell voltage increased dramatically. Moreover, the solution through the desalination compartment was acidified indicating preferable blocking of the anion-exchange membrane. Indeed, after cleaning, its conductivity was 15 times lower. In the case of cation-exchange membrane, a decrease of conductivity was about 50%. This shows formation of precipitate inside pores of the pristine membranes.
Discussion
Modification improves charge selectivity of the cation-exchange membrane (see Table 1), this is probably due to screening of pores with inorganic particles. The aggregated nanoparticles form secondary porous structures inside the membranes. Small pores between the nanoparticles as well as high surface charge density, which is realized in neutral media due to dissociation of phosphorus-containing functional groups [45], prevent transport of co-ions. At the same time, lower transport number of counter ions was found for the modified anion-exchange membrane. Indeed, HZD sorbs anions (An−) mainly in acidic media:
and cations (Cat+) from alkaline solutions:
Normally, isoelectric point of HZD is reached in neutral media: under these conditions cation- and anion-exchange capacities are equal. Thus, the HZD aggregates permit both counter (Cl−) and co- (Na+) ions. However, the aggregates protect the ion-exchange materials against fouling with organics.
Thus, ZHP increases the transport number of counter-ions in the cation-exchange membrane. At the same time, HZD slightly deteriorates charge selectivity of the anion-exchange membrane. Improvement of anion transport is expected in acidic media. However, the possibility of glycerol desalination is realized even in neutral media.
Conclusions
As shown, the nanoparticles are aggregated in ion exchangers during their treatment with glycerol. In order to reach stability of incorporated nanoparticles and functional properties of the materials, the modification procedure was performed in glycerol media. Under these conditions, both non-aggregated nanoparticles and their small aggregates (up to 300 nm) are formed. They are evidently located inside voids between gel regions and perform a function of a barrier against adsorption of organic impurities on hydrophobic fragments of hydrocarbonaceous chains. No sufficient influence of ZHP on semi-permittivity of the cation-exchange membrane was found in aqueous NaCl solutions. At the same time, HZD slightly deteriorates charge selectivity of the anion-exchange membranes in neutral media due to amphoteric properties of the modifier. They are evidently a barrier against not only additionally sorbed electrolyte but also adsorption of organics.
The composite membranes were applied to desalination of glycerol-water mixture containing organic additives (byproduct of biodiesel production). In opposite to pristine membranes, the composite materials were shown to demonstrate stability against fouling. It is possible to decrease the salt concentration in 100 times, organic additives remain in the desalinated solutions. Acceleration of the desalination process requires improvement of the electrodialysis stack. Due to the problem of limiting current, deeper desalination can be carried out using ion exchange. Organic-inorganic ion exchangers modified in non-aqueous media could be probably used for this purpose.
References
Bernardes A, Rodrigues MAS, Ferreira JZ (2014) Electrodialysis and water reuse: novel approaches. Springer-Verlag, Berlin, Heidelberg
Melnyk LA (2015) Removal of Mn(II) compounds from water in electrodialysis desalination. J Water Chem Technol 37(3):122–127
Abou-Shady A, Peng C, Xu H (2012) Effect of pH on separation of Pb(II) and NO3 − from aqueous solutions using electrodialysis. Desalination 285:46–53
Osipenko VO, Balakina MN, Kucheruk DD, Goncharuk VV (2014) Water purification of nitrates with their deep concentration by the method of electrodialysis. J Water Chem Technol 35(2):75–79
Zmievskii YG, Kirichuk II, Mironchuk VG (2014) Membrane treatment of wastewater obtained after the whey processing. J Water Chem Technol 36(6):309–316
Güler E, Kaya C, Kabay N, Arda M (2015) Boron removal from seawater: state-of-the-art review. Desalination 356:85–93
Białek R, Mitko K, Dydo P, Turek M (2014) Electrodialytic separation of boric and hydrochloric acids. Desalination 342:29–34
Baldasso C, Lazzari LK, Scopel BS, Marczak LDF, Tessaro IC (2016) Whey fractionation through membranes separation process. Separ Sci Technol 41(11):1862–1871
Huang C, Xu T, Zhang Y, Xue Y, Chen G (2007) Application of electrodialysis to the production of organic acids: state-of-the-art and recent developments. J Membr Sci 288(1–2):1–12
Thompson JC, He BB (2006) Characterization of crude glycerol from biodiesel production from multiple feedstocks. Appl Eng Agriculture 22(2):261–265
Silva GP, Mack M, Contiero J (2009) Glycerol: a promising and abundant carbon source for industrial microbiology. Biotechnol Adv 27:30–39
Anger S, Trimis D, Stelzner B, Makhynya Y, Peil S (2011) Development of a porous burner unit for glycerin utilization from biodiesel production by Supercritical Water Reforming. Int J Hydrogen Energy 36(13):7877–7883
Day P, McNeil J, Sirovski F (2012) Glycerine from biodiesel: the perfect diesel fuel. Process Safety and Environmental Protection 37(3):180–188
Yong KC, Dzulkefly K, Wanyunus WMZ, Hazamah AH (2001) Refining of crude glycerin recovered from glycerol residue by simple vacuum distillation. J Oil Palm Res 13(2):39–44
Ziels NW (1956) Recovery and purification of glycerol. J Amer Oil Chem Soc 33(11):556–565
Mah SK, Chang CCH, Wu TY, Chai SP (2014) The study of reverse osmosis on glycerin solution filtration: dead-end and crossflow filtrations, transport mechanism, rejection and permeability investigations. Desalination 352:66–81
Mah SK, Leo CP, Wu TY, Chai SP (2012) A feasibility investigation on ultrafiltration of palm oil and oleic acid removal from glycerin solutions: flux decline, fouling pattern, rejection and membrane characterizations. J Membr Sci 389:245–256
Schaffner F, Pontalier PY, Sanchez V, Lutin F (2003) Bipolar electrodialysis for glycerin production from diester wastes. Filtration and Separation 40(10):35–39
Vadthya P, Kumari A, Sumana C, Sridha S (2015) Electrodialysis aided desalination of crude glycerol in the production of biodiesel from oil feedstock. Desalination 362:133–140
Mulder M (1996) Basic principles of membrane technology. Kluwer Academic Publisher, Dordrecht, Boston, London
Ruiz B, Sistat P, Huguet P, Pourcelly G, Araya-Farias M, Bazinet L (2007) Application of relaxation periods during electrodialysis of a casein solution: impact on anion-exchange membrane fouling. J Membr Sci 287(1):41–50
Lee H-J, Hong M-K, Han S-D, Cho S-H, Moon S-H (2009) Fouling of an anion exchange membrane in the electrodialysis desalination process in the presence of organic foulants. Desalination 238:60–69
Mikhaylin S, Bazinet L (2016) Fouling on ion-exchange membranes: classification, characterization and strategies of prevention and control. Adv Colloid Interface Sci 229:34–56
Chen J, Ruan H, Wu L, Gao C (2011) Preparation and characterization of PES-SiO2 organic-inorganic composite ultrafiltration membrane for raw water pre-treatment. Chem Eng J 168(3):1272–1278
Rahimi Z, Zinatizadeh AA, Zinadini S (2014) Preparation and characterization of a high antibiofouling ultrafiltration PES membrane using OCMCS-Fe3O4 for application in MBR treating wastewater. J Appl Res Water Wastewater 1:13–17
Pang R, Li X, Li J, Zh L, Sun X, Wang L (2014) Preparation and characterization of ZrO2/PES hybrid ultrafiltration membrane with uniform ZrO2 nanoparticles. Desalination 332(1):60–66
Myronchuk VG, Dzyazko YS, Zmievskii YG, Ukrainets AI, Bildukevich AV, Kornienko LV, Rozhdestvenskaya LM, Palchik AV (2016) Organic-inorganic membranes for filtration of corn distillery. Acta Period Technol 47:153–165
Kwak SY, Kim SH, Kim SS (2001) Hybrid organic/inorganic reverse osmosis (RO) membrane for bactericidal anti-fouling. 1. Preparation and characterization of TiO2 nanoparticle self-assembled aromatic polyamide thin-film-composite (TFC) membrane. Environ Sci Technol 35(11):2388–2394
Dzyazko YS, Rozhdestvenskaya LM, Zmievskii YG, Vilenskii AI, Myronchuk VG, Kornienko LV, Vasilyuk SL, Tsyba NN (2015) Organic-inorganic materials containing nanoparticles of zirconium hydrophosphate for baromembrane separation. Nanoscale Res Let 10:64
Bakangura E, Wu L, Ge E, Yang L, Xu T (2016) Mixed matrix proton exchange membranes for fuel cells: state of the art and perspectives. Progr Polym Sci 57:103–152
Moradi M, Moheb A, Javanbakht M, Hooshyari MK (2016) Experimental study and modeling of proton conductivity of phosphoric acid doped PBI-Fe2TiO5 nanocomposite membranes for using in high temperature proton exchange membrane fuel cell (HT-PEMFC). Int J Hydrogen Energy 41(4):2896–2910
Feng T, Lin B, Zhang S, Yuan N, Chu F, Hickner MA, Wang C, Zhu L, Ding J (2016) Imidazolium-based organic–inorganic hybrid anion exchange membranes for fuel cell applications. J Membr Sci 508:7–14
Pandey RP, Thakur AK, Shahi VK (2014) Stable and efficient composite anion-exchange membranes based on silica modified poly(ethyleneimine)–poly(vinyl alcohol) for electrodialysis. J Membr Sci 469:478–487
Zabolotskii VI, Protasov KV, Sharafan MV (2010) Sodium chloride concentration by electrodialysis with hybrid organic-inorganic ion-exchange membranes: an investigation of the process. Russ J Electrochem 6(9):979–986
Kumar M, Khan MA, Alothman ZA, Siddiqui MR (2013) Polyaniline modified organic–inorganic hybrid cation-exchange membranes for the separation of monovalent and multivalent ions. Desalination 325:95–103
Dzyazko Y, Rozhdestveskaya L, Zmievskii Y, Volfkovich Y, Sosenkin V, Nikolskaya N, Vasilyuk S, Myronchuk V, Belyakov V (2015) Heterogeneous membranes modified with nanoparticles of inorganic ion-exchangers for whey demineralization. Mater Today: Proceedings 2(6):3864–3873
Kumar M, Tripathi BP, Shahi VK (2009) Ionic transport phenomenon across sol–gel derived organic–inorganic composite mono-valent cation selective membranes. J Membr Sci 340(1–2):52–61
Dzyazko YS, Belyakov VN, Vasilyuk SL, Stefanyak NV (2006) Anion-exchange properties of composite ceramic membranes containing hydrated zirconium dioxide. Russ J Appl Chem 79(5):769–773
Dzyazko YS, Volfkovich YM, Sosenkin VE, Nikolskaya NF, Gomza YP (2014) Composite inorganic membranes containing nanoparticles of hydrated zirconium dioxide for electrodialytic separation. Nanoscale Res Let 9(1):271
Dzyazko YS, Rudenko AS, Yukhin YM, Palchik AV, Belyakov VN (2014) Modification of ceramic membranes with inorganic sorbents. Application to electrodialytic recovery of Cr(VI) anions from multicomponent solution. Desalination 342:52–60
Helfferich F (1995) Ion exchange. Dover, New York
Yang B, Manthiram A (2006) Comparison of the small angle X-ray scattering study of sulfonated poly(etheretherketone) and Nafion membranes for direct methanol fuel cells. J Power Sources 153(1):29–35
Dzyazko YS, Ponomareva LN, Volfkovich YM, Sosenkin VE, Belyakov VN (2013) Conducting properties of a gel ionite modified with zirconium hydrophosphate nanoparticles. Russ J Electrochem 49(3):209–215
Dzyazko YS, Ponomaryova LN, Volfkovich YM, Sosenkin VE, Belyakov VN (2013) Polymer ion-exchangers modified with zirconium hydrophosphate for removal of Cd2+ ions from diluted solutions. Separ Sci Technol 48(14):2140–2149
Amphlett CB (1964) Inorganic ion exchangers. Elsevier, Amsterdam
Berezina NP, Kononenko NA, Dyomina OA, Gnusin NP (2008) Characterization of ion-exchange membrane materials: properties vs structure. Adv Colloid Interface Sci 139(1-2):3–28
Sata T (2004) Ion exchange membranes. Preparation, characterization, modification and application. RSC Publisher, Cambridge
Hale DK, McGauley DJ (1961) Structure and properties of heterogeneous cation-exchange membranes. Trans Faraday Soc 57:135–149
Berezina NP, Kononenko NA, Vol'fkovich YM (1994) Hydrophilic properties of heterogeneous ion-exchange membranes. Russ J Electrochem 30(3):329–335
Hsu WY, Gierke TD (1983) Ion transport and clustering in Nafion perfluorinated membranes. J Membr Sci 13(3):307–326
Yaroslavtsev AB, Nikonenko VV (2009) Ion-exchange membrane materials: properties, modification, and practical application. Nanotechnol in Russia 4(3):137–159
Acknowledgements
The work was supported by projects within the framework of programme supported by the National Academy of Science of Ukraine (entitled “Fundamental problems of creation of new matters and materials for chemical industry”, grant N 21-13) and also by the Aquafuel Research Ltd. company (UK).
Author information
Authors and Affiliations
Contributions
YD carried out investigations of morphology of ion-exchange materials and drafted the manuscript. LR provided investigations of functional properties of the membranes. SV synthesized the composite materials. KK carried out electrodialysis. VB contributed to the valuable discussions on experimental results. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Dzyazko, Y.S., Rozhdestvenska, L.M., Vasilyuk, S.L. et al. Composite Membranes Containing Nanoparticles of Inorganic Ion Exchangers for Electrodialytic Desalination of Glycerol. Nanoscale Res Lett 12, 438 (2017). https://doi.org/10.1186/s11671-017-2208-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s11671-017-2208-4