Abstract
Dispersion stability of nanoparticles in the liquid media is of great importance to the utilization in practice. This study aims to investigate the effects of mechanical dispersion method on the dispersibility of functionalized TiO2 nanoparticles in the transformer oil. Dispersion methods, including stirring, ultrasonic bath, and probe processes, were systematically tested to verify their versatility for preparing stable nanofluid. The test results reveal that the combination of ultrasonic bath process and stirring method has the best dispersion efficiency and the obtained nanofluid possesses the highest AC breakdown strength. Specifically, after aging for 168 h, the size of nanoparticles in the nanofluid prepared by the combination method has no obvious change, while those obtained by the other three paths are increased obviously.
Similar content being viewed by others
Background
Nanofluids are a new type of engineering materials by dispersing nanoparticles into the base fluid, which have received considerable attention for many years due to their superior thermal and dielectric properties [1–4]. Transformer oil, as a cooling and insulating medium, is a major part of the electrical insulation system in many types of electrical equipment, such as transformers, cables, and bushings. The dielectric strength and thermal conductivity of transformer oil are of great importance to keep power transformers operating safely and optimize their structure design. Recently, it has been found that the presence of nanoparticles can greatly improve thermal conductivity and breakdown strength of transformer oil [5–12]. However, the nanoparticles tend to aggregate into bigger particles mainly by the attractive forces and external stresses [11–13], leading to the performance degradation of nanofluids. So, the long-term stability of nanoparticles dispersion in the host oil is still a key challenge in this field.
Much work has been done to improve the dispersion stability of the nanoparticles in the base fluid [14–16]. In comparison with mechanical method, surface functionalization has been proved to be a more useful approach to control the balance of Van der Waals attraction and electrostatic repulsion between nanoparticles through surface modification of nanoparticles [17, 18]. Dispersion stability of iron oxide nanoparticles in mineral oil was greatly improved by optimizing their surface functionalization state and the nanofluid had no visible sedimentation after aging for 24 months at room temperature [17]. The dispersion of TiO2 nanoparticles in the mineral oil can also be improved by adjusting the usage of modifying agents to functionalize the nanoparticles [18]. Meanwhile, the adsorption of functional groups on the surface of nanoparticles could be influenced by many factors, including temperature, the type of base liquid, and the interaction between functional group and nanoparticle. Although the mechanical dispersion process can provide energy to overcome the adhesion force between nanoparticles, it may affect the interaction between functional group and nanoparticle at the same time. However, no other studies have been found directly point out the effect of the mechanical dispersion method on the stability and breakdown strength of transformer oil-based nanofluids modified by functionalized nanoparticles.
In this paper, TiO2 nanoparticles functionalized by oleic acid were synthesized by a solvothermal method. Three kinds of mechanical dispersion methods were employed to prepare transformer oil-based TiO2 nanofluids. The dispersion stability, AC breakdown strength, and thermos-physical property of obtained nanofluids were measured and compared.
Methods
Nanoparticle Synthesis and Functionalization
TiO2 nanoparticles were prepared and functionalized by using titanium n-butoxide and DI water as reactants by a solvothermal method. In a typical procedure, reactants were first introduced into a mixed solution of cyclohexane and triethylamine under stirring. After stirring for 5 min, oleic acid was added into the above solution at room temperature with vigorous agitation. The resulting mixture was subsequently heated to the temperature of 150 °C. After heating for 24 h, the resulting product was cooled down naturally and washed with distilled water and absolute ethanol for several times to remove the ions possibly remaining in the product and finally dried in the vacuum at 70 °C.
Nanofluid Preparation
In order to study the effect of dispersion method on the stability of nanofluid, functionalized TiO2 nanoparticles with the same volume fraction of 0.075% were added into the mineral transformer oil (No. 25 Karamay). After treating for 3 min in ultrasonic bath, the obtained mixture was then divided into 16 parts. The three kinds of dispersion instruments are shown in Fig. 1. The six parts were stirred using a magnetic stirrer for a time range from 10 to 180 min at a rotate speed of 1800 r/m. The other six parts were placed in an ultrasonic bath and treated for the same times as that of stirring method at 20 kHz. Four parts were sonicated by a probe for 10, 20, 30, and 40 min at 20 kHz, respectively. To avoid overheating, the mixtures were ultra-sonicated for every 5 min by a break duration about 1 min.
Characterization Method
The morphology of the as-prepared nanoparticles was characterized by high-resolution transmission electron microscopy (HRTEM: JEM-2100F). Fourier transform infrared spectra (FT-IR) was used to analyze the surface functionalization state of TiO2 nanoparticles scanned from 400 to 4000 cm-1 with a resolution of 4 cm-1. A dynamic light scattering device (Malvern Nano ZS90) was used to determine the average size of nanoparticles in the fresh and aged nanofluids. The polydispersity index (PdI) describes the width of the particle size distribution. The viscosity of pure oil and nanofluid was measured at the temperature of 29 °C with the rotational viscometer Brookfield DVII, and their thermal conductivity was characterized by a Netzsch LFA447 tester. A portable Jian-tong Oil Tester 6801 was used to measure AC breakdown voltages of pure oil and nanofluids according to IEC standard 60156 using brass spherically capped electrodes set at 2 mm gap.
Results and Discussion
Morphology and Surface Functionalization of Nanoparticles
The morphology of the as-prepared TiO2 nanoparticles is shown in Fig. 2. It can be clearly seen that the as-prepared nanoparticles have a small average diameter of 6 nm and exhibit a uniform particle size distribution. No obvious aggregation was observed among the nanoparticles. Moreover, the clear lattice fringes of single nanoparticles in Fig. 2b demonstrate the single-crystalline nature of the nanoparticles.
The FT-IR spectrum of as-prepared TiO2 nanoparticles is depicted in Fig. 3. The IR peak around 500 cm-1 is attributed to the TiO2, whereas the absorption peaks related with the functional group of oleic acid are at higher bands [19, 20]. The transmission bands at 3301 and 1060 cm-1 are due to the presence of hydroxyl group (–OH). The bands in the 2919 and 2850 cm-1 region are associated with the asymmetric and symmetric –CH2– and –CH3 modes of the oleic acid-saturated chain fragments. The extra peaks around 902 and 1168 cm-1 can be assigned to stretching vibration of –C–O– groups [21]. It should be note that the peak at 1717 cm-1 associated with the C=O stretching mode is not observed in the spectrum [22]. This means that no free physically absorbed oleic acid exists in the nanoparticles.
The C=O stretch from oleic acid is replaced by the appearance of two new peaks at 1554 and 1431 cm-1, which correspond to the asymmetric and symmetric carboxylate (–COO–) stretching modes [23, 24]. Studies have shown that these two peaks could be utilized to predict the types of binding interaction between the carboxylate head and metal oxide surface [25]. Depending on the wave number separation between asymmetric and symmetric peaks, it is indicated that the functional group of oleic acid is covalently bonded with the titanium sites at the nanoparticles surface mainly by bidentate linkages [17, 26]. These results confirm that the carboxylate group is chemically bonded with the surface titanium ion, and the as-prepared TiO2 nanoparticles are well functionalized by oleic acid.
Dispersion Stability of Nanofluids
The effect of the stirring time on the average size of TiO2 nanoparticles in the nanofluids are shown in Fig. 4. It can be seen that the nanoparticle size in the fresh nanofluid is dropped down with the increasing of stirring time.
For the nanofluid prepared by stirring for 10 min, the nanoparticle size is abruptly enlarged from 30.63 to 72.52 nm with the prolonging of aging time and then achieves a constant value of 81.24 nm after aging for 192 h. With the increasing of stirring time from 30 to 180 min, the stability of as-prepared nanofluids is greatly improved. The size of nanoparticles in nanofluid stirring for 180 min is smaller than those in other nanofluids, which is in the range from 18.3 to 20.0 nm during the aging time of 336 h. In addition, the PdI of particles in this nanofluid is 0.16, indicating the uniform size distribution of nanoparticles. These test results demonstrate that for the functionalized TiO2 nanoparticles, the shear force at a high agitation speed can decrease the tendency of particle agglomeration and the nanofluid with a good dispersion stability can be observed by stirring for 180 min.
The variation of nanoparticle size in the nanofluids with the aging time by the ultrasonic bath processing is studied and shown in Fig. 5. The nanoparticle size in the fresh nanofluids keeps in the range of 18.1 to 21.4 nm with the increasing of ultrasonic treating time, which are much smaller than those obtained by the stirring method. This indicates that the ultrasonic bath dispersion is more efficient to prepare well-dispersed nanofluid due to its uniform higher intensity energy input. With the prolonging of treatment time from 30 to 60 min, the nanoparticle size is obviously increased and then decreased when the treatment time is prolonged from 120 to 180 min. This changing tendency is totally different with that observed in stirring dispersion process. It is well-known that the collapse of cavitation bubbles under ultrasonic treatment can release and transfer a good deal of energy into the nanofluid, which greatly decrease the agglomeration of nanoparticles. Meanwhile, the temperature of the nanofluid can be remarkably increased with a long-time treatment. This rise of the temperature in nanofluid will influence the adsorption equilibrium of functional groups on the surface of nanoparticles. By treating for 60 min, the rise of temperature probably makes the functional group begin to detach from the nanoparticles and has no enough time to re-attach, finally leading to the agglomeration of nanoparticles. This desorption tendency of functional group is inhibited due to the functional group reacts with the surface of nanoparticles when the treating time is prolonged to 120 and 180 min. In all, the nanofluid obtained by ultrasonic bath treatment for 10 min has the best dispersion stability and its nanoparticle size keeps in the range of about 18.1 nm with a PdI value of 0.26 even after aging for 240 h.
The average size of nanoparticles in the nanofluids ultra-sonicated with the probe is shown in Fig. 6. It can be clearly seen that the sizes of nanoparticles in fresh nanofluids are decreased from 23.8 (10 min) to 18.4 nm (20 and 30 min) first and then increased to 26.5 nm after treated for 40 min, which is 5.7 nm larger than that obtained by the ultrasonic bath process after treating the same time of 10 min. The nanoparticle size in nanofluid treated for 40 min is abruptly increased to 85 nm and maintained this value after aging for 192 h, while those treated for 20 and 30 min maintain below 23 nm and exhibit good stability with a PdI value of 0.31. This value is still 4.9 nm higher than that obtained by the ultrasonic bath method, and the uniformity of size distribution is also lowered. Although the ultrasonic probe process provides the higher energy to the suspension, this high-intensity energy is limited around the tip due to its small diameter. With the prolonging of the treatment time, the temperature of the nanofluid tends to greatly increase and the molecules of functional group on the surface of nanoparticles have a tendency to decompose into the oil. Then nanoparticles are much easier to agglomerate with each other, and the stability of nanofluid is getting worse. During the ultrasonic bath process, a more uniform high-intensity sonication energy is provided and its heating rate is much slower than that in the ultrasonic probe process. Therefore, the average sizes of nanoparticles treated with the bath are significantly smaller than those of the probe treated at the same treating time.
Based on the obtained results, we can see that the stirring and ultrasonic bath processes show better dispersion efficiency than ultrasonic probe process. So, the combination of these two methods under their optimum condition was used to prepare nanofluid with the same loading of functionalized nanoparticles. The average size of nanoparticle in the obtained fresh nanofluid and aged nanofluid for 168 h is shown in Fig. 7 and compared with those obtained by other three methods.
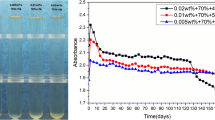
After stirring for 180 min and ultra-sonicated for 10 min in bath, the size of nanoparticles in the nanofluid is 17.6 nm, smaller than those obtained by each method individually. Specifically, after aging for 168 h, the size of nanoparticles in the nanofluid prepared by the combination method has no obvious change, while those obtained by the other three paths are increased obviously. The AC breakdown voltages of four kinds of fresh nanofluids were tested and compared with that of pure oil. As shown in Fig. 8, all the AC breakdown strength of nanofluids are higher than that of the pure oil and the nanofluid obtained by the combination dispersion method is improved by 32.8%, possessing the highest breakdown performance.
The thermal conductivity and viscosity of pure oil and nanofluid prepared by the combination method is measured and shown in Table 1. As can be seen, the TiO2 nanofluid shows no obvious improvement compared to the pure oil. The thermal conductivity of nanofluid is only increased by 1.2%. It is considered that thermal conductivity of nanofluids is mainly related with the physical nature of base liquid and nanoparticles, the diameter and concentration of nanoparticles. About 10% enhancement in thermal conductivity of lubricant-based nanofluids has been reported by the presence of 0.25 wt% TiO2 nanoparticles [6]. It is considered that the enhancement on the thermal conductivity is due to the formation of clusters by agglomerated nanoparticles, which provide channels for thermal waves and transport of heat [6, 7]. However, the volume fraction of TiO2 nanoparticles in our nanofluid is only 0.075% and the size of nanoparticles keeps uniform around 18 nm. The effects of concentration of TiO2 nanoparticles and their agglomeration state on the thermos-physical property of transformer oil-based nanofluids are under study.
Conclusions
This study investigated the dispersion stability of functionalized TiO2 nanoparticles in the transformer oil-based nanofluids, their AC breakdown strength and thermos-physical property, which were prepared through stirring, ultrasonic bath, and probe processes. The test results show that the dispersion stability of functionalized nanoparticles is clearly dependent on the dispersion method. The stirring and ultrasonic bath processes exhibit better dispersion efficiency than the ultrasonic probe process, which may disrupt the adsorption balance of functional group on the surface of nanoparticles due to the limitation of the high-intensity sonication energy around the probe tip. The combination method of stirring and ultrasonic bath can effectively reduce the tendency for nanoparticles to agglomerate and prepare the nanofluid with the best dispersion stability and breakdown performance.
References
Pastoriza-Gallego MJ, Casanova C, Paramo R, Barbes B, Legido JL, Pineiro MM (2009) A study on stability and thermophysical properties (density and viscosity) of Al2O3 in water nanofluid. J Appl Phys 106:064301-1–064301-3
Ghadimi A, Saidur R, Metselaar HSC (2011) A review of nanofluid stability properties and characterization in stationary conditions. Int J Heat Mass Transfer 54:4051–4068
Lv YZ, Zhou Y, Li CR, Wang Q, Qi B (2014) Recent progress in nanofluids based on transformer oil: preparation and electrical insulation properties. IEEE Electr Insul Mag 30:23–32
Teng TP, Wang WP, Hsu YC (2016) Fabrication and characterization of nanocarbon-based nanofluids by using an oxygen-acetylene flame synthesis system. Nanoscale Res Lett 11:288–300
Tijerina JT, Narayanan TN, Gao GH, Rohde M, Tsentalovich DA, Pasquali M, Ajayan PM (2012) Electrically insulating thermal nano-oils using 2D fillers. ACS Nano 6:1214–1220
Mohamed KAA, Hou XJ, Richard FT, Zhan P, Chen XD (2016) Enhancing the thermophysical properties and tribological behaviour of engine oils using nano-lubricant additives. RSC Adv 6:77913–77924
Sadegh A, Amin J, Mojtaba M, Hossein A, Kourosh J (2016) Experimental study on the rheological behavior of silver-heat transfer oil nanofluid and suggesting two empirical based correlations for thermal conductivity and viscosity of oil based nanofluids. Appl Therm Eng 101:362–372
Zhou W, Zhou CH, Zhang LL, Guo L (2010) Seed-mediated synthesis and characterization of Ni flower-like nanomaterials. J Nanosci & Nanotech 10:5004–5007
Hwang JG, Zahn M, O’Sullivan FM, Pettersson LAA, Hjortstam O, Liu RS (2010) Effects of nanoparticle charging on streamer development in transformer oil-based nanofluids. J Appl Phys 107:014310-1–014310-17
Du YF, Lv YZ, Li CR, Chen MT, Zhou JQ, Li XX, Zhou Y, Tu YP (2011) Effect of electron shallow trap on breakdown performance of transformer oil-based nanofluids. J Appl Phys 110:104104-1–104104-4
Choi C, Yoo H, Oh J (2008) Preparation and heat transfer properties of nanoparticle-in-transformer oil dispersions as advanced energy-efficient coolants. Curr Appl Phys 8:710–712
Józefczak A (2009) Study of low concentrated ionic ferrrofluid stability in magnetic field by ultrasound spectroscopy. J Magn Magn Mater 321:2225–2231
Lee JC, Lee WH, Lee SH, Lee S (2012) Positive and negative effects of dielectric breakdown in transformer oil based magnetic fluids. Mater Res Bull 47:2984–2987
Thorat ND, Khot VM, Salunkhe AB, Prasad AI, Ningthoujam RS, Pawar SH (2013) Surface functionalized lsmo nanoparticles with improved colloidal stability for hyperthermia applications. J Phys D Appl Phys 46:105003–105013
Veriansyah B, Chun MS, Kim J (2011) Surface-modified cerium oxide nanoparticles synthesized continuously in supercritical methanol: study of dispersion stability in ethylene glycol medium. Chem Eng J 168:1346–1351
Zhou J, Song B, Zhao G, Han G (2012) Effects of acid on the microstructures and properties of three-dimensional TiO2, hierarchical structures by solvothermal method. Nanoscale Res Lett 7:1–10
Viali WR, Alcantara GB, Sartoratto PPC, Soler MAG, Mosiniewicz-szablewska E, Andrzejewski B, Morais PC (2010) Investigation of the molecular surface coating on the stability of insulating magnetic oils. J Phys Chem C 114:179–188
Lv YZ, Zhang SN, Du YF, Chen MT, Li CR (2013) Effect of oleic acid surface modification on dispersibility of TiO2 nanoparticles in transformer oils. J Inorg Mater 28:594–598
Su WG, Zhang J, Feng ZC, Chen T, Ying PL, Li C (2008) Surface phases of TiO2 nanoparticles studied by uv raman spectroscopy and FT-IRspectroscopy. J Phys Chem C 112:7710–7716
Alamgir KW, Ahmad S, Hassan MM, Naqvi AH (2014) Structural phase analysis, band gap tuning and fluorescence properties of Co doped TiO2 nanoparticles. Optic Mater 38:278–285
Liu MH, Yang YL, Zhu T, Liu ZF (2005) Chemical modification of single-walled carbon nanotubes with peroxytrifluoroacetic acid. Carbon 43:1470–1478
Khalil M, Yu J, Liu N, Lee RL (2014) Non-aqueous modification of synthesized hematite nanoparticles with oleic acid. Colloids Surf A 453:7–12
Zhang L, He R, Gu HC (2006) Oleic acid coating on the monodisperse magnetite nanoparticles. Appl Surf Sci 253:2611–2617
Yu TT (1993) Structural comparison of self-assembled monolayers of n-alkanoic acids on the surfaces of silver, copper, and aluminum. J Am Chem Soc 115:4350–4358
Baalousha M, Manciulea A, Cumberland S, Kendall K, Lead JR (2008) Aggregation and surface properties of iron oxide nanoparticles: influence of pH and natural organic matter. Environ Toxicol Chem 27:1875–1882
Nakamoto K (1978) Infrared and Raman spectra of inorganic and coordination compounds. Wiley, New York
Acknowledgements
The authors would like to thank the National Natural Science Foundation of China for supporting this research under Contract Nos. 51337003, 51472084, and 51477052 and the Fundamental Research Funds for the Central Universities (JB2015019).
Authors’ Contributions
The experiments were guided by YL. CL and QS prepared the TiO2 nanofluids. CL and MH characterized the nanofluids. RL and BQ participated in the discussion and gave valuable suggestions. The manuscript was composed by YL. All authors approved the final manuscript.
Competing Interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Lv, Yz., Li, C., Sun, Q. et al. Effect of Dispersion Method on Stability and Dielectric Strength of Transformer Oil-Based TiO2 Nanofluids. Nanoscale Res Lett 11, 515 (2016). https://doi.org/10.1186/s11671-016-1738-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s11671-016-1738-5