Abstract
Interleukin-11 (IL-11) is a pleiotropic cytokine that regulates the growth and development of hematopoietic stem cells and decreases the proinflammatory mediators of cytokine and nitric oxide production. In animal models of arthritis, treatment with recombinant human IL-11 (rhIL-11) reduces both the level of synovitis and the histologic lesion scores in the joints.
The goal of this phase-I/II study in adults with rheumatoid arthritis (RA) was to evaluate the safety and clinical activity of different doses and schedules of rhIL-11 in patients with active RA for whom treatment with at least one disease-modifying antirheumatic drug had failed.
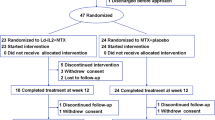
This was a multicenter, randomized, placebo-controlled trial that evaluated the safety and tolerability of rhIL-11 in 91 patients with active RA. rhIL-11 was administered subcutaneously; patients were randomized into one of five treatment groups (ratio of rhIL-11 to placebo, 4:1). Patients were treated for 12 weeks with either 2.5 or 7.5 μg/kg of rhIL-11 or placebo twice per week or 5 or 15 μg/kg of rhIL-11 or placebo once per week. The status of each subject's disease activity in accordance with the American College of Rheumatology (ACR) criteria was assessed before, during, and after completion of administration of the study drug.
Administration of rhIL-11 was well tolerated at all doses and schedules. The most frequent adverse event was a reaction at the injection site. The data suggest a statistically significant reduction in the number of tender joints (P < 0.008) at the 15 μg/kg once-weekly dose schedule but showed no overall significant benefit at the ACR criterion of a 20% response.
The trial showed rhIL-11 to be safe and well tolerated at a variety of doses and schedules over a 12-week treatment period in patients with active RA. The only adverse event clearly associated with rhIL-11 administration was reaction at the injection site.


Similar content being viewed by others
Abbreviations
- ACR:
-
American College of Rheumatology
- CRP:
-
C-reactive protein
- DMARD:
-
disease-modifying antirheumatic drug
- IL:
-
interleukin
- NF:
-
nuclear factor
- RA:
-
rheumatoid arthritis
- rhIL-11:
-
recombinant human IL-11
- TNF:
-
tumor necrosis factor.
References
Trepecchio WL, Bozza M, Pednault G, Dorner AJ: Recombinant human IL-11 attenuates the inflammatory response through down-regulation of proinflammatory cytokine release and nitric oxide production. J Immunol. 1996, 157: 3627-3634.
Leng SX, Elias JA: Interleukin-11 inhibits macrophage interleukin-12 production. J Immunol. 1997, 159: 2161-2168.
Hermann JA, Hall MA, Maini RN, Feldmann M, Brennan F: Important immunoregulatory role of interleukin-11 in the inflammatory process in rheumatoid arthritis. Arthritis Rheum. 1998, 41: 1388-97. 10.1002/1529-0131(199808)41:8<1388::AID-ART7>3.0.CO;2-F.
Trepecchio WL, Wang L, Bozza M, Dorner AJ: IL-11 regulates macrophange effector function through inhibition of nuclear factor-κB. J Immunol. 1997, 159: 5661-5670.
Walmsley M, Butler DM, Marinova-Mutafchieva L, Feldmann M: An anti-inflammatory role for interleukin-11 in established murine collagen-induced arthritis. Immunology. 1998, 95: 31-37. 10.1046/j.1365-2567.1998.00568.x.
Hochberg MC, Spector TD: Epidemiology of rheumatoid arthritis: update. Epidemiol Rev. 1990, 12: 247-252.
Ferraccioli G, Salaffi F, Nervetti A, Cavalieri F: Slow-acting drugs - outcome is no different than 15 years ago. J Rheumatol. 1990, 17: 1249-1250.
Moreland L, Heck L, Koopman W: Biologic agents for treating rheumatoid arthritis. Arthritis Rheum. 1997, 40: 397-409.
Thorton SC, Por SB, Penny R, Richter M, Shelley L, Breit SN: Identification of the major fibroblast growth factors released spontaneously in inflammatory arthritis as platelet derived growth factor and tumor necrosis factor-alpha. Clin Exp Immunol. 1991, 86: 79-86.
Beckham JC, Caldwell DS, Peterson BL, Pippen AM, Currie MS, Keefe FJ, Weinberg JB: Disease severity in rheumatoid arthritis: relationships of plasma tumor necrosis factor-alpha, soluble interleukin 2-receptor, soluble CD4/CD8 ratio, neopterin, and fibrin D-dimer to traditional severity and functional measures. J Clin Immunol. 1992, 12: 353-361.
Moreland LW, Schiff MH, Baumgartner SW, Tindall EA, Fleischmann RM, Bulpitt KJ, Weaver AL, Keystone EC, Furst DE, Mease PJ, Ruderman EM, Horwitz DA, Arkfeld DG, Garrison L, Burge DJ, Blosch CM, Lange ML, McDonnell ND, Weinblatt ME: Etanercept therapy in rheumatoid arthritis. A randomized, controlled trial. Ann Intern Med. 1999, 130: 478-486.
Lipsky PE, van der Heijde DMFM, St Clair EW, Furst DE, Breedveld FC, Kalden JR, Smolen JS, Weisman M, Emery P, Feldmann M, Harriman GR, Maini RN: Infliximab and methotrexate in the treatment of rheumatoid arthritis. N Engl J Med. 2000, 343: 1594-602. 10.1056/NEJM200011303432202.
Sands B, Bank S, Sninsky C, Robinson M, Katz S, Singleton J, Miner P, Safdi M, Galandiuk S, Hanauer S, Varilek G, Buchman A, Rogers V, Salzberg B, Cai B, Rogge H, Schwertschlag U: Safety and activity evaluation of rh-IL-11 in subjects with active Crohn's disease. Gastroenterology. 1999, 117: 59-64.
Sands B, Winston B, Salzberg B, Barish C, Safdi M, Wruble L, Varilek G, Singleton J, Katz S, Miner P, Shapiro M, Schwertschlag US: A randomized, double-masked, placebo-controlled study of rh-IL-11 in Crohn's disease subjects not receiving prednisone. American Gastrologists Association, Orlando,. 1999
Hochberg MC, Chang RW, Dwosh I, Lindsey S, Pincus T, Wolfe F: The American College of Rheumatology 1991 Revised Criteria for the Classification of Global Functional Status in Rheumatoid Arthritis. ArthritisRheum. 1992, 35: 498-502.
Felson DT, Anderson JJ, Boers M, Bombardier C, Furst D, Goldsmith C, Katz LM, Lightfoot R, Paulus H, Strand V: American College of Rheumatology. Preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum. 1995, 38: 727-735.
Acknowledgements
The secretarial assistance of Bettie Stone in preparing the manuscript and the expertise of the clinical coordinators involved with this study are greatly appreciated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moreland, L., Gugliotti, R., King, K. et al. Results of a phase-I/II randomized, masked, placebo-controlled trial of recombinant human interleukin-11 (rhIL-11) in the treatment of subjects with active rheumatoid arthritis. Arthritis Res Ther 3, 247 (2001). https://doi.org/10.1186/ar309
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/ar309