Abstract
Background
Survivin is an inhibitor of apoptosis and a regulator of mitotic progression. TP53 protein is a negative transcriptional regulator of survivin. The aim of our study was to evaluate the clinical significance of survivin expression in advanced stages ovarian cancer with respect to the TP53 status.
Methods
Survivin and TP53 expression was evaluated immunohistochemically in 435 archival samples of ovarian carcinomas (244 patients were treated with platinum/cyclophosphamide-PC/PAC; 191-with taxane-platinum (TP) agents). Univariate and multivariate statistical analyses were performed in patients groups divided according to the administered chemotherapeutic regimen, and in subgroups with and without TP53 accumulation (TP53+ and TP53-, respectively).
Results
Nuclear and cytoplasmic survivin expression was observed in 92% and 74% of the carcinomas, respectively. In patients treated with TP, high nuclear survivin expression decreased the risk of disease recurrence and death, and increased the probability of high platinum sensitivity (p < 0.01), but only in the TP53(+) group, and not in the TP53(-) group.
Conclusions
It appears that TP53 status determines the clinical importance of nuclear survivin expression in taxane-platinum treated ovarian cancer patients.
Similar content being viewed by others
Background
Recently molecular anticancer therapies have undergone rapid development. Survivin, the smallest member of the family of the protein inhibitors of apoptosis (IAP) [1], is considered to be a potential target for molecular therapy [2]. Target survivin arose from data obtained from cancer cell lines, showing that survivin inhibition contributes to increased tumour response to various anticancer agents [3]. The results of clinical analyses are less consistent, as high survivin expression had been associated with both favourable and unfavourable prognosis [4].
Ovarian cancer is the most lethal gynaecological malignancy. In the last decade, taxanes combined with cisplatin or its analogues (TP therapy) have been considered standard first-line treatment for ovarian cancer [5, 6]. Although the introduction of taxanes has significantly improved treatment results, still 20% to 30% of the patients fail to achieve complete remission [6–8].
Taxanes interact with β-tubulin and increase its polymerisation and stabilisation. In the presence of paclitaxel, cells form dysfunctional mitotic spindles and eventually die by apoptosis or necrosis (depending on drug concentration) [9, 10]. The mechanism of action of taxanes is linked to survivin, which is a member of the chromosomal passenger complex (CPC) [11, 12]. The CPC complex controls many aspects of mitosis, including regulation of the mitotic spindle checkpoint and mitotic progression [13]. It has been recently shown that, on treatment with taxol, survivin is involved in the spindle checkpoint activation and mitotic arrest [14, 15]. Survivin, expressed during foetal development [16], and undetectable in most adult tissues [17] has been found in many types of human cancers, including ovarian cancer. The clinical role of survivin in ovarian cancer patients is not clear [18–20].
TP53 dysfunction enhances ovarian cancer response to taxane-platinum treatment [8, 21, 22]. In addition, the results recently obtained by our group suggest that the TP53 status, as determined by TP53 accumulation, may influence the clinical importance of other molecular factors [23–25]. This may also be the case with survivin, which is down-regulated by the wild-type TP53 [26, 27]. Thus, there may be a synergistic clinical effect of TP53 dysfunction and high survivin expression in taxane/platinum-treated ovarian cancer patients.
We studied large groups of ovarian cancer patients in order to evaluate the clinical importance of survivin expression with respect to the TP53 status, and to the treatment regimen applied.
Materials and methods
Patients and tumours
The study was performed on 435 archival samples of ovarian carcinomas. Medical records were critically reviewed by at least two clinicians. The patients were treated with standard PC (cisplatin-cyclophosphamide or carboplatin-cyclophosphamide) or PAC chemotherapy (PC and doxorubicin) (244 patients), or with taxane-platinum chemotherapy (TP: paclitaxel or docetaxel with cisplatin or carboplatin) (191 patients). The material was carefully selected out of a total of 899 cases submitted to meet the following criteria: no chemotherapy before staging laparotomy, adequate staging procedure, International Federation of Gynaecologists and Obstetricians (FIGO) stage IIB to IV disease [28], tumour tissue from the first laparotomy available, moderate (G2) or poor tumour differentiation (G3 and G4), availability of clinical data, incl. residual tumour size and follow-up.
All tumours were uniformly reviewed histopathologically, classified according to the criteria of the World Health Organisation [29] and graded in a four-grade scale, according to the criteria given by Broders [30]. Clinicopathological characteristics have been presented in Table 1.
For the PC/PAC-treated group, the follow-up time ranged from 4.4 to 198.3 months (median: 27.5); the respective values for the TP-treated group were: 4.8 to 100.6 months (median: 32.2). Short follow-up time resulted from early patients death. All surviving patients had at least a 6-month follow-up. Response to chemotherapy was evaluated retrospectively according to the World Health Organisation response evaluation criteria [31]. The evaluation was based on data from medical records describing patient's clinical condition and CA125 levels in 3-4 week intervals. Complete remission (CR) was defined as disappearance of all clinical and biochemical symptoms of ovarian cancer evaluated after completion of first-line chemotherapy and confirmed at four weeks. Within the CR group, we identified a platinum-sensitive group (PS, disease-free survival longer than six months) and a highly platinum-sensitive group (HPS, disease-free survival longer than 24 months). Other tumours were described as platinum resistant [32].
The study was approved by the bioethics committee of the Institute of Oncology (ref.no. 39/2007).
Immunohistochemical analysis
Immunohistochemical stainings were performed on paraffin-embedded material after heat-induced epitope retrieval (HIER). We used a rabbit polyclonal anti-survivin antibody (1/1000, Novus Biologicals, Littleton, USA). TP53 protein was detected with the use of PAb1801 monoclonal antibody (1/3000, Sigma-Genosys, Cambridge, UK), as described previously [23]. The antigens were retrieved by heating the sections in 0.01 M citrate buffer (pH 6.0): 6 × 5 min. for survivin and 2 × 5 min. for TP53, at 700 W in a microwave oven. Non-specific tissue and endogenous peroxidase reactivities were blocked with 10% BSA and 3% H2O2, respectively. The sections were incubated with primary antibodies overnight, at 4°C. Biotinylated secondary goat anti-rabbit IgG (for survivin) (1/1500) and anti-mouse IgG (for TP53) (1/1500), peroxidase-conjugated streptavidin (1/500) (all from Immunotech, Marseille, France), and DAB were used as a detection system. As a positive control for TP53 accumulation, we used a tumour with a defined TP53 gene missense mutation [33]. Normal rabbit IgG or normal mouse IgG of the same subclasses and at the concentrations of the relevant primary antibodies served as negative controls.
The evaluation of immunohistochemical stainings
Survivin expression was scored independently for nuclear and cytoplasmic staining. Light microscopic evaluation at 400× magnification was used to count at least 200 tumour cells within the areas of the strongest staining. Each nucleus in a given field was categorised according to the staining intensity (0 or weak to strong: 1 to 3), and counted. The nuclear survivin expression was further described as an ID score. It was calculated according to the following formula: ID = [(N0 × 0) + (N1 × 1) + (N2 × 2) + (N3 × 3)]/100, where N0, N1, N2 and N3 stands for the percentage of cells in each intensity category (0, 1, 2 or 3) [34]. A cytoplasmic staining was scored 0 (absent) or 1 to 3 (weak to strong). For the cytoplasmic expression, tumours with scores 0 and 1 were described as low expression, and those scoring 2 or 3, as high expression. Two independent assessors (A.R. and A.F-G.) concurred in 80% of the cases, and reached consensus in the remaining cases. TP53 protein accumulation was assessed as present (more than 10% positive cells) or absent, as previously described [8].
Statistical analysis
The associations between survivin expression and clinicopathological variables were assessed using the chi-square test. Probability of survival and disease-free survival (DFS) were estimated using the Kaplan-Meier method and a log-rank test for censored survival data. Overall and disease-free survival time analyses were performed using multivariate Cox's proportional hazard models. Tumour response to chemotherapy (probability of CR, probability of PS or HPS) was evaluated using a multivariate logistic regression model.
Statistical analyses included the following independent variables: age of patients (median: 53 years), the FIGO stage, histopathological type and grade, residual tumour size and TP53 accumulation status. The survivin ID score was analysed as a continuous variable, and alternatively, as a categorical variable (cut-off point at median-1.5). Important factors were selected using a backward selection technique, where factors not significant at 0.1 were removed stepwise from the model.
The analyses were performed in the two groups of patients treated with different chemotherapeutic regimens, and additionally in the TP53(-) and TP53(+) subgroups. All tests were two-sided. P < 0.05 was considered significant. All calculations were performed using STATA 5 software.
Results
Cellular distribution of survivin expression
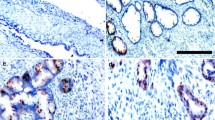
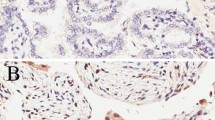
Survivin expression was observed both in the nuclei and the cytoplasm of ovarian cancer cells, predominantly in the former (Figure 1). Nuclear and cytoplasmic survivin expression of any intensity was observed in 92% and 74% of the tumours, respectively. Distribution of the nuclear and cytoplasmic survivin expression in all patients and in the subgroups treated with either chemotherapeutic regimen has been presented in Table 2.
Various patterns of survivin expression in four different ovarian carcinomas (400×, hematoxylin counterstain): a) negative survivin expression (clear cell carcinoma; FIGO IIIC), b) survivin expression absent in the nucleus but present in the cytoplasm (serous carcinoma; FIGO IIIB), c) survivin expression present in the nucleus only (serous carcinoma; FIGO IV), d) survivin expression present in the nucleus and cytoplasm (serous carcinoma; FIGO IV).
There were no associations between the cytoplasmic and nuclear survivin expression, nor between survivin expression and the clinicopathological variables studied nor TP53 accumulation.
Survivin expression in the taxane/platinum-treated group
We analysed both cytoplasmic and nuclear survivin expression; only the nuclear expression related to the clinical endpoints. This was observed in the TP53(+) subgroup and, to a lesser degree, in the group comprising all patients, but not in the TP53(-) subgroup.
Analysis of survivin expression as a continuous variable
In the univariate analysis, high nuclear survivin expression positively influenced disease-free survival (HR 0.48, 95% CI 0.30-0.76, p = 0.002 for the TP53(+) group; HR 0.66, 95% CI 0.48-0.91, p = 0.013 for the entire group), overall survival (HR 0.63, 95% CI 0.43-0.91, p = 0.016 for the TP53(+) group only) and high platinum sensitivity (OR 4.25, 95% CI 1.57-11.51, p = 0.004 for the TP53(+) group; OR 2.40, 95% CI 1.27-4.53, p = 0.007 for the entire group). This was confirmed by the multivariate analyses (Table 3), in which the associations between survivin expression and the clinical endpoints were stronger and more significant in the TP53(+) group than in the entire group. In the TP53(+) group, high nuclear survivin expression apparently correlated with a lesser risk of recurrence (HR 0.44, p = 0.000) and death (HR 0.64, p = 0.010), and enhanced the odds of high platinum sensitivity (OR 5.04, p = 0.010) (Table 3).
In the TP53(+) group, the clinical importance of the survivin expression appeared stronger and more statistically significant than that of some clinicopathological factors (Table 3).
The residual tumour size was the clinicopathological factor most constantly associated with the clinical endpoints (Table 3). Complete remission and platinum sensitivity did not associate with nuclear survivin expression.
Analysis of survivin expression as a categorical variable
In both univariate and multivariate analyses of the median survivin score (ID ≥ 1.5 vs < 1.5), the only association of survivin expression we observed was that with disease-free survival (univariate analysis: HR 0.57, 95% CI 0.33-0.98, p = 0.043 for the TP53(+) group [Figure 2]; multivariate analysis: HR 0.44, 95% CI 0.25-0.79, p = 0.005 for the TP53(+) group; HR 0.63, 95% CI 0.42-0.95, p = 0.029 for the entire group).
Survivin expression in the platinum/cyclophosphamide-treated group
Neither nuclear nor cytoplasmic survivin expression has been found to correlate with clinical endpoints or clinicopathological factors in the PC/PAC patient group.
Discussion
Survivin is regarded as a potential target of molecular therapy due to its strong antiapoptotic activity. Nevertheless, the results of numerous studies on the clinical importance of survivin in cancer patients are inconsistent. We present the first multivariate analysis which shows the positive prognostic significance of survivin expression in ovarian cancer patients. This was clearly demonstrated in patients treated with taxane-platinum agents, but not in those treated with platinum-cyclophosphamide regimens. In addition, the highest clinical significance of survivin was observed in patients with TP53 dysfunctional tumours.
A number of authors have reported, that the expression of survivin in cancer cell nuclei was associated with poor survival [35–37], while only a few studies have reported a reverse correlation [38–41]. Two studies on breast and lung cancer, have also shown the influence of nuclear survivin expression on DFS, similar to that observed in our analysis [39, 41]. In case of ovarian cancer, the issue of survivin expression and tumour response to chemotherapy has been addressed by two groups [18, 42]. One has not observed any correlation between nuclear or cytoplasmic survivin expression and the response to platinum/cyclophosphamide, but contrary to our results, they have not found any correlation with the response to TP therapy, either [18]. The other group has reported significantly higher rates of complete remission after taxol-based therapy in patients with low survivin-expressing tumours. However the latter group studied cytoplasmic survivin expression only [42].
Survivin may play a double role depending on its cellular localisation. In the cytoplasm, it exerts an antiapoptotic function by caspase inhibition. There is evidence to prove that nuclear survivin is a cell-proliferation promoting factor [43]. Studies conducted on different cell lines have shown, that survivin inhibition causes defects in cell division and suppresses proliferation. Some clinicopathological studies have identified positive correlations between nuclear survivin expression and various parameters of growth fraction (MIB-1, PCNA and mitotic indices) in hepatocellular carcinoma [reviewed in 4]. These findings point to survivin expression as an unfavourable prognostic factor. However, the majority of research groups evaluating the clinical importance of survivin expression have failed to consider the specific anti-tumour therapy applied. It should be stressed that in this study the positive prognostic significance of survivin expression was found only in patients treated with taxane-platinum agents. This relationship may be explained by a functional link between survivin and taxanes during mitosis [14, 15].
Many studies regarding cell lines have revealed the influence of survivin expression on cancer sensitivity to taxanes. The studies that describe and/or explain the role of survivin in the mitotic checkpoint regulation are of particular interest. The data obtained from HeLa cells has shown that survivin is required for the maintenance of the spindle assembly checkpoint arrest in the presence of taxol, and this mechanism has been shown to be essential for taxol sensitivity [14, 44]. In the presence of taxol survivin-depleted cells were unable to maintain the BubR1 protein at the kinetochores (BubR1 delays the transition to anaphase until all chromosomes are properly aligned). Exogenous expression of the wild-type survivin was able to restore the mitotic arrest-response of taxol-resistant cells [15]. Nevertheless, some of the studies analysing the biological effect of survivin suppression, or its overexpression in cell lines, have shown that survivin inhibited taxol-induced apoptosis [45, 46]. Some authors have observed, that survivin overexpression (apparently the cytoplasmic survivin phosphorylated at Thr34) significantly decreased the sensitivity of human ovarian carcinoma cell lines to taxanes [42].
In our study patients with high survivin expression were at a lower risk of recurrence and death, but only in the TP53-positive group. As we have previously shown, the TP53 status may determine the clinical significance of the expression of other proteins, particularly of those regulated by, or interfering with TP53 in the control of tumour cell proliferation or apoptosis [23–25, 47, 48].
On the other hand, TP53 dysfunction positively influences cancer sensitivity to taxane-platinum therapy [8, 22, 49–51]. An increase in G2/M arrest or a loss of the TP53-dependent post-mitotic spindle checkpoint in the TP53 dysfunctional cells have been proposed as possible explanations of this phenomenon [52, 53]. Thus, in view of the literature reports, the effects of TP53-dysfunction and high survivin expression may possibly show synergism, enhancing the response of cancer cells to taxol. The positive prognostic importance of survivin appears as a paradox. However, this may result from the survivin-dependent control of the mitotic response to taxol, rather than from antiapoptotic and proliferation-stimulating survivin activity.
TP53 may play a part in negative survivin regulation. Studies on cancer cell lines have shown that the wild-type TP53 repressed survivin at both mRNA and protein levels, by binding to its promoter [26, 27]. Several authors have reported an association between dysfunctional TP53 status and high survivin expression. This has been shown in ovarian (high nuclear survivin in the TP53 mutant tumours), pancreatic, breast and gastric carcinomas [54–57]. We have failed to observe this correlation in our large group of 435 tumours; however, we evaluated TP53 accumulation only and it occurs with a frequency approximately 30% lower than TP53 mutations, thus the rate of TP53 dysfunctional tumours in our group may be much higher [33].
Conclusions
In conclusion, our results show that the nuclear survivin expression status, in combination with the TP53 status, may be of prognostic value in ovarian cancer patients treated with taxane-platinum agents. The present study confirms our previous observations that analyses of carcinomas with and without TP53 accumulation, may play a pivotal role in the identification of cancer biomarkers.
Abbreviations
- DFS:
-
disease-free survival
- HPS:
-
high platinum sensitivity
- ID:
-
ID score
- OS:
-
overall survival
- PC/PAC:
-
platinum/cyclophosphamide chemotherapy
- PS:
-
platinum sensitivity
- TP:
-
taxane-platinum chemotherapy
References
Ambrosini G, Adida C, Altieri DC: A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med 1997, 3: 917–921. 10.1038/nm0897-917
Ryan BM, O'Donovan N, Duffy MJ: Survivin: a new target for anti-cancer therapy. Cancer Treat Rev 2009, 35: 553–562. 10.1016/j.ctrv.2009.05.003
Duffy MJ, O'Donovan N, Brennan DJ, Gallagher WM, Ryan BM: Survivin: a promising tumour biomarker. Cancer Lett 2007, 249: 49–60. 10.1016/j.canlet.2006.12.020
Li F, Yang J, Ramnath N, Javle MM, Tan D: Nuclear or cytoplasmic expression of survivin: what is the significance? Int J Cancer 2005, 114: 509–512. 10.1002/ijc.20768
McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE: Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med 1996, 334: 1–6. 10.1056/NEJM199601043340101
Trimble EL, Wright J, Christian MC: Treatment of platinum-resistant ovarian cancer. Expert Opin Pharmacother 2001, 2: 1299–1306. 10.1517/14656566.2.8.1299
Stuart GCE: First-line treatment regimens and the role of consolidation therapy in advanced ovarian cancer. Gynecol Oncol 2003, 90: 8–15.
Kupryjanczyk J, Kraszewska E, Ziolkowska-Seta I, Madry R, Timorek A, Markowska J, Stelmachow J, Bidzinski M: TP53 status and taxane-platinum versus platinum-based therapy in ovarian cancer patients: a non-randomized retrospective study. BMC Cancer 2008, 8: 1–13. 10.1186/1471-2407-8-1
Fan W: Possible mechanisms of paclitaxel-induced apoptosis. Biochem Pharmacol 1999, 57: 1215–1221.
Kelling J, Sullivan K, Wilson L, Jordan MA: Suppression of centromere dynamics by taxol in living osteosarcoma cells. Cancer Res 2003, 63: 2794–2801.
Skoufias DA, Mollinari C, Lacroix FB, Margolis RL: Human survivin is a kinetochore-associated passenger protein. J Cell Biol 2000, 151: 1575–1581. 10.1083/jcb.151.7.1575
Bergstralh DT, Ting JP-Y: Microtubule stabilizing agents: their molecular signaling consequences and the potential for enhancement by drug combination. Cancer Treat Rev 2006, 32: 166–179. 10.1016/j.ctrv.2006.01.004
Vagnarelli P, Earnshaw WC: Chromosomal passengers: the four-dimensional regulation of mitotic events. Chromosoma 2004, 113: 211–222. 10.1007/s00412-004-0307-3
Carvalho A, Carmena M, Sambade C, Earnshaw WC, Wheatley SP: Survivin is required for stable checkpoint activation in taxol-treated HeLa cells. J Cell Sci 2003, 116: 2987–2998. 10.1242/jcs.00612
Zhou J, O'Brate A, Zelnak A, Giannakakou P: Survivin deregulation in beta-tubulin mutant ovarian cancer cells underlies their compromised mitotic response to taxol. Cancer Res 2004, 64: 8708–8714. 10.1158/0008-5472.CAN-04-2538
Adida C, Crotty PL, McGrath J, Berrebi D, Diebold J, Altieri DC: Developmentally regulated expression of the novel cancer anti-apoptosis gene survivin in human and mouse differentiation. Am J Pathol 1998, 152: 43–49.
Fukuda S, Pelus LM: Survivin, a cancer target with an emerging role in normal adult tissues. Mol Cancer Ther 2006, 5: 1087–1098. 10.1158/1535-7163.MCT-05-0375
Ferrandina G, Legge F, Martinelli E, Ranelletti FO, Zannoni GF, Lauriola L, Gessi M, Gallotta V, Scambia G: Survivin expression in ovarian cancer and its correlation with clinico-pathological, surgical and apoptosis-related parameters. Br J Cancer 2005, 92: 271–277.
Athanassiadou P, Grapsa D, Athanassiades P, Gonidi M, Athanassiadou A-M, Tsipis A, Patsouris E: The prognostic significance of COX-2 and survivin expression in ovarian cancer. Pathol Res Pract 2008, 204: 241–249. 10.1016/j.prp.2007.11.004
Engels K, Knauer SK, Loibl S, Fetz V, Harter P, Schweitzer A, Fisseler-Eckhoff A, Kommoss F, Hanker L, Nekljudova V, Hermanns I, Kleinert H, Mann W, du Bois A, Stauber RH: NO signalling confers cytoprotectivity through the survivin network in ovarian carcinomas. Cancer Res 2008, 68: 5159–5166. 10.1158/0008-5472.CAN-08-0406
Smith-Sorensen B, Kaern J, Holm R, Dorum A, Trope C, Borresen-Dale A-L: Therapy effect of either paclitaxel or cyclophosphamide combination treatment in patients with epithelial ovarian cancer and relation to TP53 gene status. Br J Cancer 1998, 78: 375–381. 10.1038/bjc.1998.502
Ueno Y, Enomoto T, Otsuki Y, Sugita N, Nakashima R, Yoshino K, Kuragaki C, Ueda Y, Aki T, Ikegami H, Yamazaki M, Ito K, Nagamatsu M, Nishizaki T, Asada M, Kameda T, Wakimoto A, Mizutani T, Yamada T, Murata Y: Prognostic significance of p53 mutation in suboptimally resected advanced ovarian carcinoma treated with the combination chemotherapy of paclitaxel and carboplatin. Cancer Lett 2006, 241: 289–300. 10.1016/j.canlet.2005.10.035
Kupryjanczyk J, Szymanska T, Madry R, Timorek A, Stelmachow J, Karpinska G, Rembiszewska A, Ziolkowska I, Kraszewska E, Debniak J, Emerich J, Ulanska M, Pluzanska A, Jedryka M, Goluda M, Chudecka-Glaz A, Rzepka-Gorska I, Klimek M, Urbanski K, Breborowicz J, Zielinski J, Markowska J: Evaluation of clinical significance of TP53, BCL-2, BAX and MEK 1 expression in 229 ovarian carcinoma treated with platinum-based regimen. Br J Cancer 2003, 88: 848–854. 10.1038/sj.bjc.6600789
Kupryjanczyk J, Madry R, Plisiecka-Halasa J, Bar J, Kraszewska E, Ziolkowska I, Timorek A, Stelmachow J, Emerich J, Jedryka M, Pluzanska A, Rzepka-Gorska I, Urbanski K, Zielinski J, Markowska J: TP53 status determines clinical significance of ERBB2 expression in ovarian cancer. Br J Cancer 2004, 91: 1916–1923. 10.1038/sj.bjc.6602238
Ziolkowska-Seta I, Madry R, Kraszewska E, Szymanska T, Timorek A, Rembiszewska A, Kupryjanczyk J: TP53, BCL-2 and BAX analysis in 199 ovarian cancer patients treated with taxane-platinum regimens. Gynecol Oncol 2009, 112: 179–184. 10.1016/j.ygyno.2008.09.008
Hoffman WH, Biade S, Zilfou JT, Chen J, Murphy M: Transcriptional repression of the anti-apoptotic survivin gene by wild type p53. J Biol Chem 2002, 277: 3247–3257. 10.1074/jbc.M106643200
Mirza A, McGuirk M, Hockenberry TN, Wu Q, Ashar H, Black S, Wen SF, Wang L, Kirschmeier P, Bishop WR, Nielsen LL, Pickett CB, Liu S: Human survivin is negatively regulated by wild-type p53 and participates in p53-dependent apoptotic pathway. Oncogene 2002, 21: 2613–2622. 10.1038/sj.onc.1205353
Creasman WJ: Announcement, FIGO stages 1988, revisions. Gynecol Oncol 1989, 35: 125–127.
Tavassoli FA, Devilee P: pathology and genetics of tumours of the breast and female genital organs World Health Organisation classification of tumours. IARC Press Lyon; 2003.
Barber HR, Sommers SC, Snyder R, Kwon TH: Histologic and nuclear grading and stromal reactions as indices for prognosis in ovarian cancer. Am J Obstet Gynecol 1975, 121: 795–807.
Miller AB, Hoogstraten B, Staquet M, Winkler A: Reporting results of cancer treatment. Cancer 1981, 47: 207–214. 10.1002/1097-0142(19810101)47:1<207::AID-CNCR2820470134>3.0.CO;2-6
Christian MC, Trimble EL: Salvage chemotherapy for epithelial ovarian carcinoma. Gynecol Oncol 1994, 55: 143–150.
Dansonka-Mieszkowska A, Ludwig AH, Kraszewska E, Kupryjanczyk J: Geographical variations in TP53 mutational spectrum in ovarian carcinomas. Ann Hum Genet 2006, 70: 594–604. 10.1111/j.1469-1809.2006.00257.x
Ball E, Bond J, Franc B, DeMicco C, Wynford-Thomas D: An immunohistochemical study of p16INK4a expression in multistep thyroid tumourigenesis. Eur J Cancer 2007, 43: 194–201. 10.1016/j.ejca.2006.08.025
Grabowski P, Kuhnel T, Muhr-Wilkenshoff F, Heine B, Stein H, Hopfner M, Germer CT, Scherubl H: Prognostic value of nuclear survivin expression in oesophageal squamous cell carcinoma. Br J Cancer 2003, 88: 115–119. 10.1038/sj.bjc.6600696
Lu B, Gonzalez A, Massion PP, Shyr Y, Shaktour B, Carbone DP, Hallahan DE: Nuclear survivin as a biomarker for non-small-cell lung cancer. Br J Cancer 2004, 91: 537–540. 10.1038/sj.bjc.6602027
Martinez A, Bellosillo B, Bosch F, Ferrer A, Marce S, Villamor N, Ott G, Montserrat E, Campo E, Colomer D: Nuclear survivin expression in mantle cell lymphoma is associated with cell proliferation and survival. Am J Pathol 2004, 164: 501–510. 10.1016/S0002-9440(10)63140-9
Trieb K, Lehner R, Stulnig T, Sulzbacher I, Shroyer KR: Survivin expression in human osteosarcoma is a marker for survival. Eur J Surg Oncol 2002, 29: 379–382.
Kennedy SM, O'Driscoll L, Purcell R, Fitz-simons N, McDermott EW, Hill AD, O'Higgins NJ, Parkinson M, Linehan R, Clynes M: Prognostic importance of survivin in breast cancer. Br J Cancer 2003, 88: 1077–1083. 10.1038/sj.bjc.6600776
Knauer SK, Kramer OH, Knosel T, Engels K, Rodel F, Kovacs AF, Dietmaier W, Klein-Hitpass L, Habtemichael N, Schweitzer A, Brieger J, Rodel C, Mann W, Petersen I, Heinzel T, Stauber RH: Nuclear export is essential for the tumour-promoting activity of survivin. FASEB J 2007, 21: 207–216.
Vischioni B, van der Valk P, Span SW, Kruyt FAE, Rodriguez JA, Giaccone G: Nuclear localisation of survivin is a positive prognostic factor for survival in advanced non-small-cell lung cancer. Ann Oncol 2004, 15: 1654–1660. 10.1093/annonc/mdh436
Zaffaroni N, Pennati M, Colella G, Perego P, Supino R, Gatti L, Pilotti S, Zunino F, Daidone MG: Expression of the anti-apoptotic gene survivin correlates with taxol resistance in human ovarian cancer. CMLS 2002, 59: 1406–1412. 10.1007/s00018-002-8518-3
Fortugno P, Wall NR, Giodini A, O'Connor DS, Plescia J, Padgett KM, Tognin S, Marchisio PC, Altieri DC: Survivin exists in immunochemically distinct subcellular pools and is involved in spindle microtubule function. J Cell Sci 2002, 115: 575–585.
Sudo T, Nitta M, Saya H, Ueno NT: Dependence of paclitaxel sensitivity on a functional spindle assembly checkpoint. Cancer Res 2004, 64: 2502–2508. 10.1158/0008-5472.CAN-03-2013
Li F, Ambrosini G, Chu EY, Plescia J, Tognin S, Marchisio PC, Altieri DC: Control of apoptosis and mitotic spindle checkpoint by survivin. Nature 1998, 396: 580–584. 10.1038/25141
Giodini A, Kallio MJ, Wall NR, Gorbsky GJ, Tognin S, Marchisio PC, Symons M, Altieri DC: Regulation of microtubule stability and mitotic progression by survivin. Cancer Res 2002, 62: 2426–2467.
Kupryjanczyk J: Why TP53 status does not associate with clinical endpoints in ovarian cancer: facts and hypotheses. Gynecol Oncol 2006, 100: 5–7. 10.1016/j.ygyno.2005.08.003
Ludwig AH, Murawska M, Panek G, Timorek A, Kupryjanczyk J: Androgen, progesterone, and FSH receptor polymorphisms in ovarian cancer risk and outcome. Endocr Relat Cancer 2009, 16: 1005–1016. 10.1677/ERC-08-0135
Jones NA, Turner J, McIlwrath AJ, Brown R, Dive C: Cisplatin- and paclitaxel-induced apoptosis of ovarian carcinoma cells and the relationship between bax and bak up-regulation and the functional status of p53. Mol Pharmacol 1998, 53: 819–826.
Lavarino C, Pilotti S, Oggionni M, Gatti L, Perego P, Bresciani G, Pierotti MA, Scambia G, Ferrandina G, Fagotti A, Mangioni C, Lucchini V, Vecchione F, Bolis G, Scarfone G, Zunino F: p53 gene status and response to platinum/paclitaxel-based chemotherapy in advanced ovarian carcinoma. J Clin Oncol 2000, 18: 3936–3945.
Gadducci A, Di Cristofano C, Zavaglia M, Giusti L, Menicagli M, Cosio S, Naccarato AG, Genazzani AR, Bevilacqua G, Cavazzana AO: P53 gene status in patients with advanced serous epithelial ovarian cancer in relation to response to paclitaxel-plus platinum-based chemotherapy and long-term clinical outcome. Anticancer Res 2006, 26: 687–693.
Wahl AF, Donaldson KL, Fairchild C, Lee FY, Foster SA, Demers GW, Galloway DA: Loss of normal p53 function confers sensitisation to taxol by increasing G2/M arrest and apoptosis. Nat Med 1996, 2: 72–79. 10.1038/nm0196-72
Cassinelli G, Supino R, Perego P, Polizzi D, Lanzi C, Pratesi G, Zunino F: A role for loss of p53 function in sensitivity of ovarian carcinoma cells to taxanes. Int J Canacer 2001, 92: 738–747. 10.1002/1097-0215(20010601)92:5<738::AID-IJC1249>3.0.CO;2-2
Cohen C, Lohmann ChM, Cotsonis G, Lawson D, Santoianni R: Survivin expression in ovarian carcinoma: correlation with apoptotic markers and prognosis. Mod Pathol 2003, 16: 574–583. 10.1097/01.MP.0000073868.31297.B0
Lu C-D, Altieri DC, Tanigawa N: Expression of a novel antiapoptosis gene, survivin, correlated with tumour cell apoptosis and p53 accumulation in gastric carcinomas. Cancer Res 1998, 58: 1808–1812.
Jinfeng M, Kimura W, Sakurai F, Moriya T, Takeshita A, Hirai I: Histopathological study of intraductal papillary mucinous tumour of the pancreas: special reference to the roles of Survivin and p53 in tumourigenesis of IPMT. Int J Gastrointest Cancer 2002, 32: 73–81. 10.1385/IJGC:32:2-3:73
Vegran F, Boidot R, Oudin C, Defrain C, Rebucci M, Lizard-Nacol S: Association of p53 gene alternations with the expression of antiapoptotic survivin splice variants in breast cancer. Oncogene 2007, 26: 290–297. 10.1038/sj.onc.1209784
Acknowledgements
We would like to thank other members of the POCSG: J. Markowska (Chair of Gynaecologic Oncology, Medical University, Poznan, Poland), J. Debniak, J. Emerich (Department of Gynaecologic Oncology, Medical University, Gdansk, Poland); M. Jedryka, M. Goluda (Department of Gynaecologic Oncology, Medical University, Wroclaw, Poland); M. Ulanska, A. Pluzanska (Department of Gynaecologic Oncology, Medical University, Lodz, Poland); M. Klimek, K. Urbanski (Department of Gynaecologic Oncology, Institute of Oncology, Krakow, Poland); A. Chudecka-Glaz, I. Rzepka-Gorska (Department of Gynaecologic Oncology, Medical University, Szczecin, Poland); I. Ziolkowska-Seta, M. Bidzinski (Department of Gynaecologic Oncology, The Maria Sklodowska-Curie Memorial Cancer Centre and Institute of Oncology, Warsaw, Poland); A. Timorek, B. Spiewankiewicz (Chair and Department of Obstetrics, Gynaecology and Oncology, IInd Faculty of Medicine, Warsaw Medical University and Brodnowski Hospital, Warsaw, Poland). We also thank Dr Magdalena Chechlinska and Dr Malgorzata Symonides for linguistic correction.
This study was supported by the grant no. 2 PO5A 068 27 and N N401 236134 of the Polish Ministry of Science and Higher Education.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
AFG designed and coordinated the study, participated in immunohistochemical analyses, drafted the manuscript. AR carried out immunohistochemical analyses. IKRz and LSz helped to draft the manuscript. MM carried out statistical analyses. TN, PS and BO, as well as the members of the POCSG collected and characterized the clinical material. JK participated in the design and coordination of the study, drafted and critically reviewed the manuscript. All authors have read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Felisiak-Golabek, A., Rembiszewska, A., Rzepecka, I.K. et al. Nuclear survivin expression is a positive prognostic factor in taxane-platinum-treated ovarian cancer patients. J Ovarian Res 4, 20 (2011). https://doi.org/10.1186/1757-2215-4-20
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1757-2215-4-20