Abstract
Background
Peutz-Jeghers syndrome (PJS) is a rare autosomal dominantly inherited disease characterized by gastrointestinal hamartomatous polyposis and mucocutaneous pigmentation. The genetic predisposition for PJS has been shown to be associated with germline mutations in the STK11/LKB1 tumor suppressor gene. The aim of the present study was to characterize Hungarian PJS patients with respect to germline mutation in STK11/LKB1 and their association to disease phenotype.
Methods
Mutation screening of 21 patients from 13 PJS families were performed using direct DNA sequencing and multiplex ligation-dependent probe amplification (MLPA). Comparative semi-quantitative sequencing was applied to investigate the mRNA-level effects of nonsense and splice-affecting mutations.
Results
Thirteen different pathogenic mutations in STK11, including a high frequency of large genomic deletions (38%, 5/13), were identified in the 13 unrelated families studied. One of these deletions also affects two neighboring genes (SBNO2 and GPX4), located upstream of STK11, with a possible modifier effect. The majority of the point mutations (88%, 7/8) can be considered novel. Quantification of the STK11 transcript at the mRNA-level revealed that the expression of alleles carrying a nonsense or frameshift mutation was reduced to 30-70% of that of the wild type allele. Mutations affecting splice-sites around exon 2 displayed an mRNA processing pattern indicative of co-regulated splicing of exons 2 and 3.
Conclusions
A combination of sensitive techniques may assure a high (100%) STK11 mutation detection frequency in PJS families. Characterization of mutations at mRNA level may give a deeper insight into the molecular consequences of the pathogenic mutations than predictions made solely at the genomic level.
Similar content being viewed by others
Background
Peutz-Jeghers Syndrome (PJS) is a rare autosomal-dominant hereditary condition with incomplete penetrance, characterized by hamartomatous polyps of the gastrointestinal tract and pigmented lesions of the buccal mucosa, perioral region and other sites [1, 2]. PJS patients have an increased risk of cancer of multiple locations, predominantly the colon, small intestine, stomach, esophagus, pancreas, breast, ovary and uterine cervix [3–6].
This condition is primarily associated with germline mutations in the serine/threonine kinase 11 (STK11/LKB1) gene, localized on the chromosomal segment 19p13.3 [7, 8]. The gene spans 23 kb, consists of nine coding exons and a final noncoding exon [9, 10]. The protein it codes for plays a role in cellular energy metabolism, cell polarization, p53-dependent apoptosis, VEGF regulation and Wnt signal transduction [11–14].
The prevalence of germline pathogenic STK11 point mutations in PJS cases has been reported with very different frequencies, ranging from about 90% to only 10% [9, 10, 15], depending on both patient selection criteria and the screening methods used. The low point mutation rates reported in some studies raised the possibility of the existence of another PJS susceptibility gene, a notion investigated by genetic linkage analyses in PJS families [16, 17]. An alternative hypothesis, offering an explanation for the absence of STK11 mutations in PJS families in some studies, is the existence of alterations not detectable by the conventional mutation screening methods used. Multiplex Ligation-dependent Probe Amplification (MLPA) proved to be a powerful, robust and easy-to-perform approach to scan for large genomic deletions, and this type of mutation was indeed shown to have a significant contribution to the STK11 mutation pattern [18–20]. Taken together, STK11 aberrations seem to date to account for almost all familial PJS cases, bringing the existence of genetic heterogeneity into question.
We report here the clinicopathological manifestation and results of a comprehensive mutation analysis of the STK11 gene in 13 unrelated PJS families. We describe a number of novel mutations in PJS patients comprising the largest number of patients from the Central-Eastern European region reported so far.
Methods
Patients and samples
Individuals in this study were referred for genetic counseling and testing to the Department of Molecular Genetics at the National Institute of Oncology, Budapest, Hungary between 1995 and 2008. All investigations have been carried out in compliance with internationally recognized guidelines. Study protocols have been approved by the Institutional Ethical Board. Written informed consent was obtained from each patient. Included in this study were 21 patients from 13 Peutz-Jeghers families.
Mutation analysis
DNA was extracted from blood samples of all consenting subjects using the classic phenol-chloroform method. The entire coding region and splice junctions of the STK11 gene were amplified by PCR (primer sequences are available upon request). Systematic mutation screening was performed using direct bidirectional sequencing applying an ABI 3130 Genetic Analyzer (Applied Biosystems). The presence of all mutations was confirmed using a different blood sample.
Additionally, the coding region of STK11 was screened for genomic aberrations using the MLPA Kit P101 (MRC-Holland), according to the manufacturer's recommendations, and as described previously [21]. For the determination of the exact lengths of deletions having both breakpoints within the gene, we applied a combination of XL-PCR and sequencing. Since the various deletions detected all required individual approaches to determine the exact length of the deletion, a detailed description is outlined for each of them in the legends of the figures (Additional files 1 and 2: Figures S1 and S2).
To assess the approximate length of deletions extending over the 5' gene boundaries, the sequence copy number at multiple sites upstream of STK11 was determined. Gene dosage assays were performed in triplicates using the Power SYBR Green PCR Master Mix on an ABI Prism 7900 HT Sequence Detection System (Applied Biosystems). The conditions for thermal cycling were 50°C for 2 min and 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min. The regions used for copy number testing were 16.4 kb, 31.6 kb, 99.4 kb, 110.5 kb, and 120.1 kb upstream of the STK11 gene, all selected from non-repetitive regions. Primer sequences and localization data are given in Additional file 3: Table S1. The ratio changes between these 5' and known two-copy regions were calculated by the 2-ΔΔCt method [22] using mutation-negative samples for calibrations after ensuring that amplification efficiencies of the control and target amplicons were comparable.
The mutation nomenclature used here complies with the recommendations of den Dunnen and Antonarakis [23, 24], sequence variations are named in relation to the ATG codon in cDNA reference sequence NM_000455.4, and predicted changes at the protein level are given according to the protein reference sequence NP_000446.1, as detailed on the website of the Human Genome Variation Society [25].
Expression analysis
We performed RNA extraction from blood leukocytes of mutation carriers using the RNAqueous Kit, and carried out cDNA synthesis starting from 400 ng of total RNA using the High-Capacity cDNA Reverse Transcription Kit according to the manufacturer's instructions (Applied Biosystems). In order to assess the effects of the mutations at the mRNA level, we sequenced the cDNA of carrier individuals and estimated the expression levels of the mutant alleles by comparing the area-under-the-curve ratios at heterozygous mutation positions, using the genomic DNA sample as calibrator.
Statistical analysis
Differences between groups were calculated using a Student's t-test, with p values less than 0.05 considered significant.
Results
Patient characteristics
A total of 13 probands and 8 affected relatives with a history of Peutz-Jeghers syndrome (PJS) were included in this study. The disease was diagnosed at an average age of 21.9 years (within a range of 3-51 years). All but one of the probands showed the classical PJS phenotype of buccal freckling and hamartomatous polyps; one proband (HP09), diagnosed at 35 years of age, showed no typical mucocutaneous pigmentation. From the 13 probands only 8 had a family history of the disease, while the remaining 5 cases (38%) appeared to be the result of de novo mutations. These two groups showed no significant difference in their average age of disease onset (20.7 years for those with family history versus 22.2 years for the suggested de novo mutation carriers; p = 0.41). Clinical characteristics of the patients in this study are detailed in Table 1.
Germline mutations
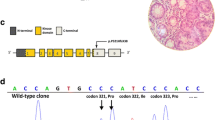
From the 13 unrelated families enrolled in this study, 13 distinct pathogenic mutations were indentified. These included three one-base deletions leading to frameshifts, three nonsense base changes, two substitutions affecting splice sites and five genomic deletions removing one to seven exons; three of this latter type of aberrations extended into the upstream genomic regions of the STK11 gene (Table 2), one of them also affecting two neighboring genes (SBNO2 and GPX4). A schematic representation of the mutations uncovered is presented in Figure 1, showing that all of them affect the kinase domain of the protein.
Germline mutations in the STK11 gene. Panel A shows the mutations within the gene: exon structure of the STK11 gene is at the top, the coding region is shown in blue. The introns are not drawn to scale. The locations of point mutations are marked by red arrowheads; the intragenic large deletions are depicted as red rectangles below the gene. The approximate localization of large deletions extending outwards STK11 is portrayed on Panel B. The known RefSeq genes of the region are shown as filled arrows. The minimal and maximal sizes of the large genomic deletions are indicated as red and pink bars, respectively. The loci where copy number analyses were done are marked by vertical green arrows, names reflecting their localization with respect to the STK11 gene. Panel C shows the data of the gene dosage experiments.
Reduced mRNA expression level of the mutant allele was observed for all cases carrying point mutations causing premature stop codons (small deletions and nonsense changes). This reduction of allelic expression showed a broad range (30-70% mutant allele level in cDNA, as compared to the corresponding genomic DNA) and was more prominent for the new acquired stop codons closer to the 3' end of the gene. Interestingly, the three mutations (c.540delG, c.550delC and c.801delC) giving rise to the same premature stop codon (in exon 6) also showed a comparatively wide spectrum of decrease in the expression of the mutant allele (30-60%) (Figure 2).
Demonstration of the decreased expression of alleles carrying nonsense and frameshift mutations. Panel A shows the results of semiquantitative sequencing of six STK11 mutations. The relative expression of the mutant allele (based on area-under-the-curve measurements, AuC) is shown in relation to the nucleotide position of the newly acquired stop codon. An example of AuC measurement is shown on Panel B. The genomic DNA and cDNA sequencing electrophoretograms of a c.801delC mutation carrier sample (HP02) is presented, with the AuC base ratio as well as the ratio change (cDNA compared to gDNA) given below for the nucleotide position marked by an asterisk.
Bioinformatics analysis predicted the two splice-site affecting mutations (c.291-2A>T and c.597 + 1G>A) to abrogate normal splicing (a decrease in splice site score from 9.4 to 1.6 for the 3' site in intron 1 and from 6.5 to 4.2 for the 5' site in intron 4 as calculated by the online tool hosted by the Zhang Lab [26]). Indeed, cDNA analysis revealed that the intron 4 nucleotide substitution results in skipping of exon 4, while the c.291-2A>T mutation resulted in a loss of both exons 2 and 3, demonstrated at the cDNA level (data not shown).
In the cases with genomic deletions where both 5' and 3' breakpoints were situated within the gene, junction determination revealed different sequence features at the boundaries. The deletion encompassing exons 2-3 in HP01 most probably occurred by homologous recombination between two Alu elements in the same strand (AluJr in intron 1/AluY in intron 3). The breakpoints of the other deletion (removing exons 3-7 in HP10) are not localized to Alu elements or any other repetitive sequences, but revealed the addition of one nucleotide at the junction site, (close to but not within a microhomology). See Additional files 1, 2, 4 and 5: Figures S1, S2, S3 and S4) for more details on the determination of genomic deletion breakpoints.
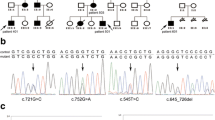
Interestingly, cDNA analysis of these cases with deletions showed a splicing pattern of exon 2-3 skipping on cDNA of HP01, confirming the alteration seen at the genomic (DNA) level. For the carrier of the genomic deletion of exons 3-7 we found that exons 2 to 7 were skipped from the cDNA, inconsistent with the initial prediction of exon 3-7 cDNA loss (Figure 3).
PCR analysis and sequencing results of the proband of family HP10 presenting with a large genomic deletion encompassing exons 3-7. Panel A shows the results of the PCR amplification of the cDNA sample of the mutation carrier along with samples from two negative controls (designated as 'neg'), using a sense primer in exon 1 and an antisense primer in exon 8. The extra band in the mutation carrier sample (marked by a red arrow) indicates the presence of a variant mRNA species resulting from fusion of exon 1 to exon 8. Faint bands on the gel are most likely the results of heteroduplex molecules. MW: molecular weight marker; neg: mutation negative samples. The sequencing result illustrating the skipping of exons 2-7 is shown on Panel B with the sequences of the normal as well as the mutant allele given below the sequenogram.
Discussion
In most previous studies germline STK11 mutations were found in 50-90% of PJS patients [20, 27]. In this study we identified deleterious sequence alterations in all 13 PJS families studied, including point mutations (8/13, 62%) as well as large genomic deletions removing from one to several exons of the gene (5/13, 38%). This high fraction of large genomic aberrations identified in Hungarian patients is in agreement with the proportions seen in several other populations [18, 19, 28, 29]. Only one of the point mutations uncovered in our cohort (c.180C>A; p.Tyr60X) can be found in the publicly available version of The Human Genome Mutation Database [30, 31], all other point mutations can be considered novel. The novel or recurrent status of the large genomic deletions cannot be safely assessed, since the exact breakpoint sequences of these are usually left unidentified in the literature except for in two very recent publications [32, 33]. According to these cited studies, Alu elements show a striking overrepresentation in the genomic region where STK11 resides, and play a major role in its instability, although deletion mechanisms not involving repetitive elements are also common [32, 33]. Our data are in line with these recent results, since out of the two deletions for which we were able to determine the breakpoints, one junction involved two Alu elements with a 26 bp perfect homology, suggesting the mechanism of homologous recombination. The other deletion showed the insertion of one nucleotide at the junction site, which is a common feature of NHEJ repair. Unfortunately, we could not determine the exact breakpoints of the other deletions due to the difficulties in amplifying the GC- and repeat-rich regions involved.
The extent of large genomic aberrations involving other tumor suppressor genes is also only rarely determined, although these deletions may affect other neighboring genes having a potential phenotype-modifying effect [33–35], sometimes even raising the possibility of novel mutation mechanisms [36]. In our PJS families, out of the 5 large deletions one extended into the coding region of other genes situated upstream of STK11, as determined by copy-number assays at specific genomic loci. In this case (HP13), the deletion affected the 5' genes SBNO2 and GPX4, but did not reach the next upstream gene POLR2E. The gene SBNO2, a component of the IL-10-mediated anti-inflammatory pathway [37] has currently no known association with gastrointestinal diseases [33], but it was affected together with STK11 in several cell lines investigated [38, 39]. Also, interleukin and JAK-STAT signaling, in which SBNO2 plays a role [37], is widely implicated in gastrointestinal cancers. Interestingly, a polymorphism at the 3' UTR of the glutathione peroxidase GPX4 was recently reported as a risk-modifier for colorectal cancer [40], the allele associated with lower GPX4 expression being linked to lower cancer risk. Taking these results into account, it is possible that the complete inactivation of one allele of this gene by a deletion may have a similar protective effect on the disease phenotype. Indeed, the age at disease onset was higher for both affected members of the HP13 family (28 and 51 years of age) as compared to the average age of onset of all other PJS patients (19.1 years), but no other specific feature was detected. Our results are comparable with those reported by Le Meur and co-workers [41], who characterized a large genomic deletion in one family completely removing one copy of the STK11 gene and several other genes upstream of it (including GPX4). In that study, two relatives of the proband were diagnosed with PJS at the relatively late age of 43 years, suggesting a potential modifier role for GPX4 in PJS.
In order to assess the potential of aberrant STK11 mRNA species to produce truncated proteins in blood leukocytes, we determined the allelic ratio of mutation carrier transcripts by semi-quantitative sequencing and found that their amount ranged from ~30-70% compared to their wild-type counterpart. Despite the semi-quantitative nature of this method, our results are similar to those obtained by other groups examining mutated alleles of several different genes [42, 43]. The reduction of the level of premature stop codon-containing transcripts is partially attributable to the mechanism of nonsense-mediated mRNA-decay, an evolutionarily conserved mechanism distinguishing normal from premature termination codons on the basis of their location with respect to exon-exon junctions, and targeting the latter for degradation [44]. Moreover, the fact that three different frameshift mutations giving rise to the same early stop codon presented with highly dissimilar levels of the mutant allele either reflects on the inter-individual variability in NMD efficiency [45–47], or it may imply that mechanisms other than nonsense-mediated mRNA decay play a role in determining the fate of these transcripts.
Another mechanism preventing the emergence of truncated Stk11 protein forms is that most known STK11 mutations, and all mutations mentioned in the present study, destroy the kinase domain of the protein, which in turn prevents the binding of Hsp90 and Cdc37, two proteins indispensable for stabilizing Stk11 [48, 49].
Aside from the decreased stability of the mutant transcripts, mRNA-level characterization of the carriers' samples revealed another interesting feature. The effect of the c.291-2A>T mutation destroying the consensus sequence of the intron 1 splice acceptor site was predicted to be the loss of exon 2, but both exons 2 and 3 were skipped in the mutant mRNA; likewise, the genomic deletion of exons 3-7 caused exon 2-7 skipping. This pattern of closely coupled splicing of two adjacent exons might be associated with the fact that the second intron of STK11 is processed by the minor U12-dependent spliceosome [50]. Moreover, the linked skipping of exon 2 and 3 seems to be an evolutionarily conserved phenomenon, since this isoform is the most prominent alternatively spliced STK11 mRNA species listed in The Alternative Splicing and Transcript Diversity Database for both men and mice [51, 52]. Its protein product has been shown to be catalytically inactive and to reside exclusively in the nucleus, in contrast to the diverse cellular localization of full-length Stk11 [48].
Conclusions
In summary, the high detection rate of mutations in our study underlies the importance of using a combination of techniques, preferably direct sequencing and MLPA, for STK11 germline mutation screening in PJS patients. Analysis of mRNA also seems to be crucial to appropriately assess the consequences of mutations which may not be straightforward from bioinformatics predictions only. Our results support the idea that elucidating the role of the GPX4 gene and potentially also the SBNO2 as modifiers in PJS or in PJS-associated tumors would be of high interest.
References
Tomlinson IP, Houlston RS: Peutz-Jeghers syndrome. J Med Genet. 1997, 34: 1007-1011. 10.1136/jmg.34.12.1007.
Merg A, Lynch HT, Lynch JF, Howe JR: Hereditary colorectal cancer - part II. Curr Probl Surg. 2005, 42: 267-333. 10.1067/j.cpsurg.2005.02.003.
Giardiello FM, Welsh SB, Hamilton SR, Offerhaus GJ, Gittelsohn AM, Booker SV, Krush AJ, Yardley JH, Luk GD: Increased risk of cancer in the Peutz-Jeghers syndrome. N Engl J Med. 1987, 316: 1511-1514. 10.1056/NEJM198706113162404.
Boardman LA, Thibodeau SN, Schaid DJ, Lindor NM, McDonnell SK, Burgart LJ, Ahlquist DA, Podratz KC, Pittelkow M, Hartmann LC: Increased risk for cancer in patients with the Peutz-Jeghers syndrome. Ann Intern Med. 1998, 128: 896-899.
Giardiello FM, Brensinger JD, Tersmette AC, Goodman SN, Petersen GM, Booker SV, Cruz-Correa M, Offerhaus JA: Very high risk of cancer in familial Peutz-Jeghers syndrome. Gastroenterology. 2000, 119: 1447-1453. 10.1053/gast.2000.20228.
van Lier MG, Wagner A, Mathus-Vliegen EM, Kuipers EJ, Steyerberg EW, van Leerdam ME: High Cancer Risk in Peutz-Jeghers Syndrome: A Systematic Review and Surveillance Recommendations. Am J Gastroenterol. 2010,
Amos CI, Bali D, Thiel TJ, Anderson JP, Gourley I, Frazier ML, Lynch PM, Luchtefeld MA, Young A, McGarrity TJ, Seldin MF: Fine mapping of a genetic locus for Peutz-Jeghers syndrome on chromosome 19p. Cancer Res. 1997, 57: 3653-3656.
Hemminki A, Tomlinson I, Markie D, Järvinen H, Sistonen P, Björkqvist AM, Knuutila S, Salovaara R, Bodmer W, Shibata D, de la Chapelle A, Aaltonen LA: Localization of a susceptibility locus for Peutz-Jeghers syndrome to 19p using comparative genomic hybridization and targeted linkage analysis. Nat Genet. 1997, 15: 87-90. 10.1038/ng0197-87.
Hemminki A, Markie D, Tomlinson I, Avizienyte E, Roth S, Loukola A, Bignell G, Warren W, Aminoff M, Höglund P, Järvinen H, Kristo P, Pelin K, Ridanpää M, Salovaara R, Toro T, Bodmer W, Olschwang S, Olsen AS, Stratton MR, de la Chapelle A, Aaltonen LA: A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature. 1998, 391: 184-187. 10.1038/34432.
Jenne DE, Reimann H, Nezu J, Friedel W, Loff S, Jeschke R, Müller O, Back W, Zimmer M: Peutz-Jeghers syndrome is caused by mutations in a novel serine threonine kinase. Nat Genet. 1998, 18: 38-43. 10.1038/ng0198-38.
Karuman P, Gozani O, Odze RD, Zhou XC, Zhu H, Shaw R, Brien TP, Bozzuto CD, Ooi D, Cantley LC, Yuan J: The Peutz-Jegher gene product LKB1 is a mediator of p53-dependent cell death. Mol Cell. 2001, 7: 1307-1319. 10.1016/S1097-2765(01)00258-1.
Ylikorkala A, Rossi DJ, Korsisaari N, Luukko K, Alitalo K, Henkemeyer M, Mäkelä TP: Vascular abnormalities and deregulation of VEGF in Lkb1-deficient mice. Science. 2001, 293: 1323-1326. 10.1126/science.1062074.
Yoo LI, Chung DC, Yuan J: LKB1 - a master tumour suppressor of the small intestine and beyond. Nat Rev Cancer. 2002, 2: 529-535. 10.1038/nrc843.
Alessi DR, Sakamoto K, Bayascas JR: LKB1-dependent signaling pathways. Annu Rev Biochem. 2006, 75: 137-163. 10.1146/annurev.biochem.75.103004.142702.
Jiang CY, Esufali S, Berk T, Gallinger S, Cohen Z, Tobi M, Redston M, Bapat B: STK11/LKB1 germline mutations are not identified in most Peutz-Jeghers syndrome patients. Clin Genet. 1999, 56: 136-141. 10.1034/j.1399-0004.1999.560207.x.
Mehenni H, Gehrig C, Nezu J, Oku A, Shimane M, Rossier C, Guex N, Blouin JL, Scott HS, Antonarakis SE: Loss of LKB1 kinase activity in Peutz-Jeghers syndrome, and evidence for allelic and locus heterogeneity. Am J Hum Genet. 1998, 63: 1641-1650. 10.1086/302159.
Buchet-Poyau K, Mehenni H, Radhakrishna U, Antonarakis SE: Search for the second Peutz-Jeghers syndrome locus: exclusion of the STK13, PRKCG, KLK10, and PSCD2 genes on chromosome 19 and the STK11IP gene on chromosome 2. Cytogenet Genome Res. 2002, 97: 171-178. 10.1159/000066620.
Aretz S, Stienen D, Uhlhaas S, Loff S, Back W, Pagenstecher C, McLeod DR, Graham GE, Mangold E, Santer R, Propping P, Friedl W: High proportion of large genomic STK11 deletions in Peutz-Jeghers syndrome. Hum Mutat. 2005, 26: 513-519. 10.1002/humu.20253.
Hearle NC, Rudd MF, Lim W, Murday V, Lim AG, Phillips RK, Lee PW, O'donohue J, Morrison PJ, Norman A, Hodgson SV, Lucassen A, Houlston RS: Exonic STK11 deletions are not a rare cause of Peutz-Jeghers syndrome. J Med Genet. 2006, 43: e15-10.1136/jmg.2005.036830.
Volikos E, Robinson J, Aittomäki K, Mecklin JP, Järvinen H, Westerman AM, de Rooji FW, Vogel T, Moeslein G, Launonen V, Tomlinson IP, Silver AR, Aaltonen LA: LKB1 exonic and whole gene deletions are a common cause of Peutz-Jeghers syndrome. J Med Genet. 2006, 43: e18-10.1136/jmg.2005.039875.
Papp J, Kovacs ME, Olah E: Germline MLH1 and MSH2 mutational spectrum including frequent large genomic aberrations in Hungarian hereditary non-polyposis colorectal cancer families: implications for genetic testing. World J Gastroenterol. 2007, 13: 2727-2732.
Livak KJ, Schmittgen TD: Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001, 25: 402-408. 10.1006/meth.2001.1262.
den Dunnen JT, Antonarakis SE: Nomenclature for the description of human sequence variations. Hum Genet. 2001, 109: 121-124. 10.1007/s004390100505.
den Dunnen JT, Antonarakis SE: Mutation nomenclature. Curr Protoc Hum Genet. 2003, Ch7: U7.13-
Human Genome Variation Society: Nomenclature for the description of sequence variations. [http://www.hgvs.org/mutnomen/]
Zhang Lab: Splice Site Score Calculation. [http://rulai.cshl.edu/new_alt_exon_db2/HTML/score.html]
Hearle N, Schumacher V, Menko FH, Olschwang S, Boardman LA, Gille JJ, Keller JJ, Westerman AM, Scott RJ, Lim W, Trimbath JD, Giardiello FM, Gruber SB, Offerhaus GJ, de Rooij FW, Wilson JH, Hansmann A, Möslein G, Royer-Pokora B, Vogel T, Phillips RK, Spigelman AD, Houlston RS: Frequency and spectrum of cancers in the Peutz-Jeghers syndrome. Clin Cancer Res. 2006, 12: 3209-3215. 10.1158/1078-0432.CCR-06-0083.
Chow E, Meldrum CJ, Crooks R, Macrae F, Spigelman AD, Scott RJ: An updated mutation spectrum in an Australian series of PJS patients provides further evidence for only one gene locus. Clin Genet. 2006, 70: 409-414. 10.1111/j.1399-0004.2006.00704.x.
Vasovcák P, Puchmajerová A, Roubalík J, Krepelová A: Mutations in STK11 gene in Czech Peutz-Jeghers patients. BMC Med Genet. 2009, 10: 69-10.1186/1471-2350-10-69.
Wang ZJ, Churchman M, Avizienyte E, McKeown C, Davies S, Evans DG, Ferguson A, Ellis I, Xu WH, Yan ZY, Aaltonen LA, Tomlinson IP: Germline mutations of the LKB1 (STK11) gene in Peutz-Jeghers patients. J Med Genet. 1999, 36: 365-368.
The Human Gene Mutation Database. [http://www.hgmd.cf.ac.uk/ac/index.php]
De Rosa M, Galatola M, Quaglietta L, Miele E, De Palma G, Rossi GB, Staiano A, Izzo P: Alu-mediated genomic deletion of the serine/threonine protein kinase 11 (STK11) gene in Peutz-Jeghers syndrome. Gastroenterology. 2010, 138: 2558-2560. 10.1053/j.gastro.2010.03.061.
Resta N, Giorda R, Bagnulo R, Beri S, Della Mina E, Stella A, Piglionica M, Susca FC, Guanti G, Zuffardi O, Ciccone R: Breakpoint determination of 15 large deletions in Peutz-Jeghers subjects. Hum Genet. 2010, 128: 373-382. 10.1007/s00439-010-0859-7.
van der Klift H, Wijnen J, Wagner A, Verkuilen P, Tops C, Otway R, Kohonen-Corish M, Vasen H, Oliani C, Barana D, Moller P, Delozier-Blanchet C, Hutter P, Foulkes W, Lynch H, Burn J, Möslein G, Fodde R: Molecular characterization of the spectrum of genomic deletions in the mismatch repair genes MSH2, MLH1, MSH6, and PMS2 responsible for hereditary nonpolyposis colorectal cancer (HNPCC). Genes Chromosomes Cancer. 2005, 44: 123-138. 10.1002/gcc.20219.
Takahashi M, Kikuchi M, Ohkura N, Yaguchi H, Nagamura Y, Ohnami S, Ushiama M, Yoshida T, Sugano K, Iwama T, Kosugi S, Tsukada T: Detection of APC gene deletion by double competitive polymerase chain reaction in patients with familial adenomatous polyposis. Int J Oncol. 2006, 29: 413-421.
Kovacs ME, Papp J, Szentirmay Z, Otto S, Olah E: Deletions removing the last exon of TACSTD1 constitute a distinct class of mutations predisposing to Lynch syndrome. Hum Mutat. 2009, 30: 197-203. 10.1002/humu.20942.
El Kasmi KC, Smith AM, Williams L, Neale G, Panopoulos AD, Watowich SS, Häcker H, Foxwell BM, Murray PJ: Cutting edge: A transcriptional repressor and corepressor induced by the STAT3-regulated anti-inflammatory signaling pathway. J Immunol. 2007, 179: 7215-7219.
Wingo SN, Gallardo TD, Akbay EA, Liang MC, Contreras CM, Boren T, Shimamura T, Miller DS, Sharpless NE, Bardeesy N, Kwiatkowski DJ, Schorge JO, Wong KK, Castrillon DH: Somatic LKB1 mutations promote cervical cancer progression. PLoS One. 2009, 4: e5137-10.1371/journal.pone.0005137.
McCabe MT, Powell DR, Zhou W, Vertino PM: Homozygous deletion of the STK11/LKB1 locus and the generation of novel fusion transcripts in cervical cancer cells. Cancer Genet Cytogenet. 2010, 197: 130-141. 10.1016/j.cancergencyto.2009.11.017.
Bermano G, Pagmantidis V, Holloway N, Kadri S, Mowat NA, Shiel RS, Arthur JR, Mathers JC, Daly AK, Broom J, Hesketh JE: Evidence that a polymorphism within the 3'UTR of glutathione peroxidase 4 is functional and is associated with susceptibility to colorectal cancer. Genes Nutr. 2007, 2: 225-232. 10.1007/s12263-007-0052-3.
Le Meur N, Martin C, Saugier-Veber P, Joly G, Lemoine F, Moirot H, Rossi A, Bachy B, Cabot A, Joly P, Frébourg T: Complete germline deletion of the STK11 gene in a family with Peutz-Jeghers syndrome. Eur J Hum Genet. 2004, 12: 415-418. 10.1038/sj.ejhg.5201155.
Anczuków O, Ware MD, Buisson M, Zetoune AB, Stoppa-Lyonnet D, Sinilnikova OM, Mazoyer S: Does the nonsense-mediated mRNA decay mechanism prevent the synthesis of truncated BRCA1, CHK2, and p53 proteins?. Hum Mutat. 2008, 29: 65-73. 10.1002/humu.20590.
Magyar I, Colman D, Arnold E, Baumgartner D, Bottani A, Fokstuen S, Addor MC, Berger W, Carrel T, Steinmann B, Mátyás G: Quantitative sequence analysis of FBN1 premature termination codons provides evidence for incomplete NMD in leukocytes. Hum Mutat. 2009, 30: 1355-1364. 10.1002/humu.21058.
Conti E, Izaurralde E: Nonsense-mediated mRNA decay: molecular insights and mechanistic variations across species. Curr Opin Cell Biol. 2005, 17: 316-325. 10.1016/j.ceb.2005.04.005.
Resta N, Susca FC, Di Giacomo MC, Stella A, Bukvic N, Bagnulo R, Simone C, Guanti G: A homozygous frameshift mutation in the ESCO2 gene: evidence of intertissue and interindividual variation in Nmd efficiency. J Cell Physiol. 2006, 209: 67-73. 10.1002/jcp.20708.
Zetoune AB, Fontanière S, Magnin D, Anczuków O, Buisson M, Zhang CX, Mazoyer S: Comparison of nonsense-mediated mRNA decay efficiency in various murine tissues. BMC Genet. 2008, 9: 83-10.1186/1471-2156-9-83.
Seoighe C, Gehring C: Heritability in the efficiency of nonsense-mediated mRNA decay in humans. PLoS One. 2010, 5: e11657-10.1371/journal.pone.0011657.
Boudeau J, Deak M, Lawlor MA, Morrice NA, Alessi DR: Heat-shock protein 90 and Cdc37 interact with LKB1 and regulate its stability. Biochem J. 2003, 370: 849-857. 10.1042/BJ20021813.
Nony P, Gaude H, Rossel M, Fournier L, Rouault JP, Billaud M: Stability of the Peutz-Jeghers syndrome kinase LKB1 requires its binding to the molecular chaperones Hsp90/Cdc37. Oncogene. 2003, 22: 9165-9175. 10.1038/sj.onc.1207179.
Hastings ML, Resta N, Traum D, Stella A, Guanti G, Krainer AR: An LKB1 AT-AC intron mutation causes Peutz-Jeghers syndrome via splicing at noncanonical cryptic splice sites. Nat Struct Mol Biol. 2005, 12: 54-59. 10.1038/nsmb873.
The Alternative Splicing and Transcript Diversity Database. [http://www.ebi.ac.uk/astd/]
Stamm S, Riethoven JJ, Le Texier V, Gopalakrishnan C, Kumanduri V, Tang Y, Barbosa-Morais NL, Thanaraj TA: ASD: a bioinformatics resource on alternative splicing. Nucleic Acids Res. 2006, 34: D46-D55. 10.1093/nar/gkj031.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2350/11/169/prepub
Acknowledgements
We are grateful to all the patients for agreeing to participate in the present study. We thank Baloghné Kovács Mária and Ferencziné Rab Judit for expert technical assistance.
This work was supported by Hungarian Research Grant NKTH-OTKA K-80745 (given to EO) and the Norwegian EEA Financial Mechanism Hu0115/NA/2008-3/ÖP-9 (given to MK and ALBD).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
JP carried out most of the molecular genetics studies and drafted the manuscript, participated in study conception and design, data acquisition and interpretation. MEK carried out the characterization of two genomic aberrations, including sequencing the deletion breakpoints; SS uncovered one of the germline mutations, participated in the expression studies and helped in critical revision of the manuscript. MK participated in the analysis of clinical data. EO participated in the conception, design and coordination of the study; recruited patients and samples for the study; participated in the collection, management, analysis and interpretation of the data. ALBD provided useful discussion and criticism. All authors read and approved the final version of the manuscript.
Marietta Eva Kovacs, Szilvia Solyom contributed equally to this work.
Electronic supplementary material
12881_2010_730_MOESM1_ESM.PPT
Additional file 1: XL-PCR analysis of two samples carrying the genomic deletion of exons 2-3 of the STK11 gene. The results of the XL-PCR amplification of the genomic deletion is shown. (PPT 286 KB)
12881_2010_730_MOESM2_ESM.PPT
Additional file 2: Determination of the length of the genomic deletion removing exons 3-7 of the STK11 gene. The results of the MLPA and PCR analyses of the genomic deletion is shown. (PPT 408 KB)
12881_2010_730_MOESM3_ESM.DOC
Additional file 3: Primers used for dosage assays. Primer sequences and localization information is given for all amplicons used for dosage assays of chromosome regions upstream of the STK11 gene. (DOC 33 KB)
12881_2010_730_MOESM4_ESM.PPT
Additional file 4: Breakpoint sequence of the genomic deletion removing exons 2-3 of the STK11 gene. The genomic deletion breakpoint is shown on a sequencing chromatogram with additional information on the repetitive elements involved in the deletion. (PPT 428 KB)
12881_2010_730_MOESM5_ESM.PPT
Additional file 5: Breakpoint sequence of the genomic deletion removing exons 3-7 of the STK11 gene. The genomic deletion breakpoint is shown on a sequencing chromatogram with additional information on the sequence elements involved in the deletion. (PPT 246 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Papp, J., Kovacs, M.E., Solyom, S. et al. High prevalence of germline STK11mutations in Hungarian Peutz-Jeghers Syndrome patients. BMC Med Genet 11, 169 (2010). https://doi.org/10.1186/1471-2350-11-169
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2350-11-169