Abstract
Background
Several studies have examined the association of biomarkers of iron metabolism with measures of carotid artery atherosclerosis, with inconsistent results. Few studies, however, have evaluated the association between biomarkers of iron metabolism and peripheral arterial disease (PAD). The purpose of this study is to examine the association of ferritin and transferrin saturation with PAD.
Methods
Serum ferritin, transferrin saturation, and PAD, defined as having an ankle-brachial blood pressure index <0.9, were measured in 1,631 men and 1,031 postmenopausal women participating in the 1999-2002 National Health and Nutrition Examination Survey (NHANES).
Results
The multivariable adjusted odds ratios (95% confidence interval) for PAD associated with a two-fold increase in serum ferritin and transferrin saturation were 1.18 (1.00-1.41) and 1.45 (0.83-2.51), respectively, for men and 1.04 (0.87-1.25) and 1.55 (0.98-2.45), respectively, for women. After stratifying by cholesterol levels, the multivariable adjusted odds ratios (95% confidence intervals) for PAD associated with a two-fold increase in ferritin and transferrin saturation was 1.04 (0.78-1.39) and 0.73 (0.35-1.50), respectively, for men with total cholesterol <200 mg/dL and 1.30 (0.99-1.72) and 2.59 (0.99-6.78), respectively, for men with total cholesterol ≥ 200 mg/dL (p-value for interaction was 0.58 for ferritin and 0.08 for transferrin saturation). After stratifying by cholesterol levels, the multivariable adjusted odds ratios (95% confidence intervals) for PAD associated with a two-fold increase in ferritin and transferrin saturation was 0.66 (0.41-1.05) and 0.75 (0.44-1.28), respectively, for women with total cholesterol <200 mg/dL, and 1.20 (0.95-1.51) and 2.07 (1.01-4.22), respectively, for women with total cholesterol ≥ 200 mg/dL (p-value for interaction was 0.05 for ferritin and 0.02 for transferrin saturation).
Conclusion
In this large nationally representative sample of men and postmenopausal women, we found a modest association of ferritin and transferrin saturation with PAD, particularly among those with high cholesterol levels.
Similar content being viewed by others
Background
Peripheral arterial disease (PAD), a condition characterized by advanced atherosclerosis in the lower extremities [1], affects 5% of US adults aged ≥ 40 years and 18% of US adults aged ≥ 75 years [2]. Individuals with atherosclerosis in the lower extremities are likely to have atherosclerosis in other arteries placing them at an increased risk of coronary heart disease, stroke, and all-cause mortality [3–6]. Iron has been hypothesized to promote atherosclerosis based on its ability to catalyze the generation of highly reactive oxygen species, such as hydroxyl radicals, through Fenton and Haber-Weiss reactions [7]. There is no consensus in the literature regarding the role of iron in the development of atherosclerosis [8]. Several studies have examined the association of biomarkers of iron metabolism with measures of carotid artery atherosclerosis, with inconsistent results [9–13]. Few studies, however, have evaluated the association between biomarkers of iron metabolism and PAD [8].
The purpose of the current analysis was to evaluate the association of serum ferritin and transferrin saturation with the prevalence of PAD, assessed through the ankle-brachial blood pressure index (ABI), using data from the 1999-2002 National Health and Nutrition Examination Survey (NHANES 1999-2002).
Methods
Study Population
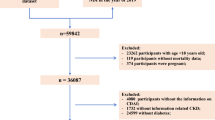
NHANES 1999-2002 was a stratified, multistage probability survey designed to select a representative sample of the civilian non-institutionalized US population [14]. NHANES 1999-2002 measured ABI in participants aged ≥ 40 years. Of 6,671 adults aged ≥ 40 years who participated in the interview and examination, we excluded 1,475 premenopausal women, 846 participants with anemia (non-Hispanic black men: hemoglobin <12.5 g/dL; other men: hemoglobin <13.5 g/dL; non-Hispanic black women: hemoglobin <11 g/dL; other women: hemoglobin <12 g/dL), 506 participants taking iron supplements, 10 participants with likely hemochromatosis (men: serum iron >190 μg/dL, serum ferritin >300 ng/mL, and transferrin saturation >60%; women: serum iron >175 μg/dL, serum ferritin >200 ng/mL, and transferrin saturation >60%), 123 participants with an ABI ≥ 1.3 (a sign of severe arterial stiffness) [4], 486 participants missing data for ABI, 50 participants missing data for ferritin, 23 participants missing data for transferrin saturation, and 490 participants missing other covariate information, the final sample included 2,662 participants (n = 1631 men and n = 1031 women). Using alternative cutoff points for excluding participants with anemia did not materially alter the results of the study. When participants excluded because of missing data were compared to those included in the study, excluded participants were more likely to be older, non-Hispanic black, and to have a higher body mass index, lower cholesterol levels, and a higher prevalence of diabetes. The protocol for NHANES 1999-2002 was approved by the National Center for Health Statistics of the Centers for Disease Control and Prevention Institutional Review Board. All participants gave written informed consent.
Data Collection
Data were collected during an in-home interview and a subsequent visit to a mobile examination center. During the in-home interview, information on demographic, household income, education, smoking status, alcohol consumption, and medication use was collected using a standardized questionnaire.
Height and weight were measured and body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Systolic blood pressure was determined based on the average of 3 blood pressure measurements. Participants had a blood specimen drawn from their antecubital vein by a trained phlebotomist according to a standardized protocol. Serum C-reactive protein (CRP) was quantified using latex-enhanced nephelometry, a high sensitivity assay. Alanine aminotransferase (ALT) and total serum cholesterol was measured enzymatically. High-density lipoprotein (HDL) cholesterol was measured using a heparin-manganese precipitation method. Serum creatinine was measured by a kinetic rate Jaffe method. We calculated estimated glomerular filtration rate (eGFR) using the Modification of Diet and Renal Disease equation after aligning the serum creatinine concentrations with the assay employed in the development of the equation [15, 16]. Individuals were classified as having an eGFR ≥ 90, 60-89, or <60 ml/min/1.73 m2. We defined diabetes mellitus as a self-reported history of diabetes with concurrent use of anti-diabetes medication.
Markers of Iron Metabolism
A detailed description of the measurement of ferritin and transferrin saturation is available elsewhere [14]. Ferritin was measured using a single-incubation two-site immunoradiometric assay (Bio-Rad Laboratories, Hercules, CA). The software used was not able to quantify ferritin levels below 5 ng/mL; therefore, participants with ferritin levels below 5 ng/mL (n = 2) were assigned a value of 3 ng/mL. The inter-assay coefficient of variation ranged from 2.4% to 5.7% in NHANES 1999-2000 and 3.8% to 5.1% in NHANES 2001-2002. Serum iron and total iron-binding capacity were measured using a modification of the automated AAII-25 colorimetric method on an Alpkem Flow Solutions IV system (Alpkem, Inc., College Station, TX). Transferrin saturation was calculated as (iron/total iron-binding capacity)*100%.
Peripheral Arterial Disease
Systolic blood pressure measurements for ABI determination were taken via blood pressure cuff on the right brachial artery and both posterior tibial arteries with the participant lying supine on the exam table. These measurements were taken separately from the measurements to determine blood pressure. Participants with conditions preventing measurement in the right arm had the left brachial artery systolic blood pressure measured instead (n = 8). ABI measurements were taken twice for participants aged 40-59 years and once for participants aged ≥ 60 years. Right and left ABI were calculated as the ratio of the mean systolic blood pressure in the respective ankle to the mean brachial systolic blood pressure in the arm. Participants with an ABI <0.9 in either leg were considered to have PAD. The 0.9 cutoff value for ABI has 95% sensitivity for detecting angiogram positive disease and almost 100% specificity in excluding healthy individuals [17].
Statistical Analysis
All analyses were conducted separately for men and postmenopausal women. Participants were categorized into gender-specific tertiles of ferritin and transferrin saturation based on the weighted population distribution. Age and race-ethnicity adjusted means and percentages were calculated by linear regression for continuous covariates and logistic regression for dichotomous covariates.
We calculated the odds ratios of PAD associated with tertile of ferritin and transferrin saturation after multivariable adjustment using logistic regression. Initial models adjusted for age (continuous) and race-ethnicity (non-Hispanic white, non-Hispanic black, Mexican-American, other). Subsequent models further adjusted for household income (<$20,000 and ≥ $20,000), high school education, smoking status (current, former, never), alcohol consumption (<1 and ≥ 1 drink per week), and body mass index (<25, 25-29, and ≥ 30 kg/m2). Additional models further adjusted for systolic blood pressure (continuous), high sensitivity C-reactive protein (log-transformed, continuous), ALT (log-transformed, continuous), total cholesterol (continuous), high-density lipoprotein-cholesterol (continuous), estimated glomerular filtration rate (<60, 60-89, and ≥ 90 ml/min/1.73 m2), diabetes mellitus, use of blood pressure medication, diabetes medication, aspirin, and hormone replacement therapy (among women). Tests for linear trend across tertiles of ferritin and transferrin saturation were computed by including an ordinal variable with the median of each tertile of ferritin or transferrin in the regression models. Further analyses included ferritin and transferrin saturation as continuous, log-transformed variables. For these analyses, we present odds ratios for PAD associated with a two-fold increase in ferritin or transferrin saturation. The analysis of ferritin and transferrin saturation as continuous, log-transformed variables was repeated after stratifying by total cholesterol levels above and below 200 mg/dL to investigate the potential interaction with cholesterol levels as has been reported in previous studies [11, 18–20].
Data were analyzed using SUDAAN (version 9.0; Research Triangle Institute, Research Triangle Park, NC) to account for the complex NHANES sampling design, including unequal probabilities of selection, over-sampling, and non-response.
Results
The geometric mean ferritin and transferrin saturation levels were 148 ng/mL and 25.8%, respectively, for men and 81 ng/mL and 21.9%, respectively, for women. The prevalence of PAD was 4% for men and 8% for post-menopausal women. Among men, PAD was associated with being older, being non-Hispanic black, having low income, being a current smoker, having higher CRP, having lower ALT, and having an eGFR <60 ml/min/1.73 m2 (Table 1). Among women, PAD was associated with being older, being non-Hispanic black, being a former smoker, having a lower HDL-cholesterol level, and having an eGFR <60 ml/min/1.73 m2.
Among men, the multivariable adjusted odds ratios (95% confidence intervals) for PAD comparing the highest to the lowest tertiles of ferritin and transferrin saturation were 1.87 (0.96-3.63) and 1.48 (0.82-2.64), respectively (Table 2). The multivariable adjusted odds ratios (95% confidence intervals) for PAD associated with a two-fold increase in ferritin and transferrin saturation were 1.18 (1.00-1.41) and 1.45 (0.83-2.51), respectively. After stratifying by cholesterol levels, the multivariable adjusted odds ratios (95% confidence intervals) for PAD associated with a two-fold increase in ferritin and transferrin saturation was 1.04 (0.78-1.39) and 0.73 (0.35-1.50), respectively, for men with total cholesterol <200 mg/dL and 1.30 (0.99-1.72) and 2.59 (0.99-6.78), respectively, for men with total cholesterol ≥ 200 mg/dL (p-value for interaction was 0.58 for ferritin and 0.08 for transferrin saturation).
Among women the multivariable adjusted odds ratios (95% confidence intervals) for PAD comparing the highest to the lowest tertiles of ferritin and transferrin saturation were 0.77 (0.46-1.30) and 1.29 (0.79-2.10), respectively (Table 2). The multivariable adjusted odds ratios (95% confidence intervals) for PAD associated with a two-fold increase in ferritin and transferrin saturation were 1.04 (0.87-1.25) and 1.55 (0.98-2.45), respectively. After stratifying by cholesterol levels, the multivariable adjusted odds ratios (95% confidence intervals) for PAD associated with a two-fold increase in ferritin and transferrin saturation were 0.66 (0.41-1.05) and 0.75 (0.44-1.28), respectively, for women with total cholesterol <200 mg/dL and 1.20 (0.95-1.51) and 2.07 (1.01-4.22), respectively, for women with total cholesterol ≥ 200 mg/dL (p-value for interaction was 0.05 for ferritin and 0.02 for transferrin saturation).
Discussion
In this representative sample of US adult men and postmenopausal women, ferritin and transferrin saturation were not statistically significantly associated with PAD. After stratifying the analysis by cholesterol levels, the observed associations were consistent with a synergistic interaction between ferritin and transferrin saturation with cholesterol levels among men and women. Our findings were consistent with a modest association of ferritin and transferrin saturation with PAD, particularly among those with high cholesterol levels, but we cannot exclude a chance association.
In 1981 Sullivan [21] introduced the hypothesis that elevated iron levels, below the levels found in hemochromatosis, may increase cardiovascular risk. This hypothesis was based on the observation that premenopausal women had lower rates of heart disease compared to men and this difference narrowed in concert with increasing iron levels as women went through menopause. The human body carefully maintains iron homeostasis, as both iron deficiency and iron overload are harmful [8]. Nearly all iron in the human body is bound to other molecules since free ferrous iron is highly reactive and may catalyze free-radical oxidative reactions. Free ferrous iron may be involved in the Fenton or Haber-Weiss reactions and catalyze the formation of hydroxyl radicals, which have been implicated in low-density lipoprotein (LDL) cholesterol oxidation. As a consequence, it has also been hypothesized that excess iron may be more harmful in those with elevated LDL cholesterol than those with lower LDL cholesterol [8].
While there is limited information in the literature on the association of iron with PAD, several studies have evaluated the association of iron with carotid artery atherosclerosis. In the Bruneck Study of 826 men and women, high ferritin levels were associated with greater progression of carotid artery atherosclerosis after 5 years [11]. In the Bruneck Study, the ferritin-carotid artery atherosclerosis relationship was significantly stronger at higher levels of LDL cholesterol. Conversely, a cross-sectional study of 206 Finnish men [13] and a case-control analysis of 730 men and women participating in the Atherosclerosis Risk in Communities (ARIC) study found no association between ferritin and carotid artery atherosclerosis [12]. In the ARIC study, there was no evidence to support a synergistic interaction between ferritin and cholesterol; in fact, the cholesterol stratified results indicated a lower odds ratio of carotid atherosclerosis among those with higher cholesterol levels.
The association of biomarkers of iron metabolism with coronary heart disease or stroke has been a source of substantial controversy [18–20, 22–28]. A meta-analysis of 12 prospective studies found no evidence to support an association between biomarkers of iron metabolism and coronary heart disease [29]. Three prospective studies investigating the association of measures of iron metabolism and myocardial infarction have found evidence of synergistic interaction with cholesterol levels [18–20], but other similar studies have failed to find evidence of interaction [22, 24]. Recently, a multicenter randomized controlled trial was conducted with 1,277 men and postmenopausal women randomized to either a control group or to receive phlebotomy at six-month intervals to reduce body iron stores; at the end of the six year follow-up, all-cause mortality and the secondary endpoint of all-cause mortality plus nonfatal myocardial infarction and stroke were nonsignificantly lower in the phlebotomy group [30].
The findings from the present study need to be considered in the context of its limitations. Ferritin and transferrin saturation are acute-phase reactants and may not accurately reflect body iron stores. To minimize this potential source of confounding we adjusted for CRP. NHANES 1999-2002 is a cross-sectional study susceptible to reverse causation and selection bias and, therefore, we cannot determine causality in any observed associations. There were relatively few cases of PAD, limiting our ability to detect modest associations and subjecting our findings to substantial random variability. Also, when investigating a possible interaction between ferritin and cholesterol levels, the use of LDL cholesterol would have been preferable to total cholesterol, but fasting LDL cholesterol was only available in 47% of our study participants. Despite these limitations, the present study maintains a number of strengths. NHANES data were collected using a rigorous study protocol with extensive quality control procedures and with technicians trained and certified in all data collection procedures. Additionally, the results are representative of the US noninstitutionalized civilian population.
Conclusion
In a large nationally representative sample of US adults, we found a modest association of ferritin and transferrin saturation with PAD, particularly among those with high cholesterol levels, but we cannot exclude a chance association. After stratifying the analysis by cholesterol levels, the associations were also consistent with a synergistic interaction of cholesterol levels with ferritin and transferrin saturation. Prospective cohort studies with adequate power to investigate ferritin-cholesterol interaction are needed to fully characterize the relationship between biomarkers of iron status, cholesterol, and measures of atherosclerosis.
Abbreviations
- PAD:
-
peripheral arterial disease
- ABI:
-
ankle-brachial blood pressure index
- NHANES:
-
National Health and Nutrition Examination Survey
- BMI:
-
body mass index
- CRP:
-
C-reactive protein
- HDL:
-
high-density lipoprotein
- eGFR:
-
estimate glomerular filtration rate
- LDL:
-
low-density lipoprotein
- ARIC:
-
Atherosclerosis Risk in Communities.
References
DeLoach SS, Mohler ER: Peripheral arterial disease: a guide for nephrologists. Clin J Am Soc Nephrol. 2007, 2: 839-846. 10.2215/CJN.04101206.
Menke A, Muntner P, Wildman RP, Dreisbach AW, Raggi P: Relation of borderline peripheral arterial disease to cardiovascular disease risk. Am J Cardiol. 2006, 98: 1226-1230. 10.1016/j.amjcard.2006.05.056.
Murabito JM, Evans JC, Larson MG, Nieto K, Levy D, Wilson PW: The ankle-brachial index in the elderly and risk of stroke, coronary disease, and death: the Framingham Study. Arch Intern Med. 2003, 163: 1939-1942. 10.1001/archinte.163.16.1939.
Newman AB, Siscovick DS, Manolio TA, Polak J, Fried LP, Borhani NO, et al: Ankle-arm index as a marker of atherosclerosis in the Cardiovascular Health Study. Cardiovascular Heart Study (CHS) Collaborative Research Group. Circulation. 1993, 88: 837-845.
Tsai AW, Folsom AR, Rosamond WD, Jones DW: Ankle-brachial index and 7-year ischemic stroke incidence: the ARIC study. Stroke. 2001, 32: 1721-1724.
Vogt MT, McKenna M, Wolfson SK, Kuller LH: The relationship between ankle brachial index, other atherosclerotic disease, diabetes, smoking and mortality in older men and women. Atherosclerosis. 1993, 101: 191-202. 10.1016/0021-9150(93)90116-C.
Halliwell B, Gutteridge JM: Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol. 1990, 186: 1-85. full_text.
Ramakrishna G, Rooke TW, Cooper LT: Iron and peripheral arterial disease: revisiting the iron hypothesis in a different light. Vasc Med. 2003, 8: 203-210. 10.1191/1358863x03vm493ra.
Drueke T, Witko-Sarsat V, Massy Z, scamps-Latscha B, Guerin AP, Marchais SJ, et al: Iron therapy, advanced oxidation protein products, and carotid artery intima-media thickness in end-stage renal disease. Circulation. 2002, 106: 2212-2217. 10.1161/01.CIR.0000035250.66458.67.
Kiechl S, Aichner F, Gerstenbrand F, Egger G, Mair A, Rungger G, et al: Body iron stores and presence of carotid atherosclerosis. Results from the Bruneck Study. Arterioscler Thromb. 1994, 14: 1625-1630.
Kiechl S, Willeit J, Egger G, Poewe W, Oberhollenzer F: Body iron stores and the risk of carotid atherosclerosis: prospective results from the Bruneck study. Circulation. 1997, 96: 3300-3307.
Moore M, Folsom AR, Barnes RW, Eckfeldt JH: No association between serum ferritin and asymptomatic carotid atherosclerosis. The Atherosclerosis Risk in Communities (ARIC) Study. Am J Epidemiol. 1995, 141: 719-723.
Rauramaa R, Vaisanen S, Mercuri M, Rankinen T, Penttila I, Bond MG: Association of risk factors and body iron status to carotid atherosclerosis in middle-aged eastern Finnish men. Eur Heart J. 1994, 15: 1020-1027.
Survey operation manuals, brochures, and consent documents: 1999-current NHANES. National Center for Health Statistics, Centers for Disease Control. 2008
Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al: Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006, 145: 247-254. 10.1053/j.ajkd.2007.08.020.
Selvin E, Manzi J, Stevens LA, Van LF, Lacher DA, Levey AS, et al: Calibration of serum creatinine in the National Health and Nutrition Examination Surveys (NHANES) 1988-1994, 1999-2004. Am J Kidney Dis. 2007, 50: 918-926. 10.1001/archinte.163.8.884.
Belch JJ, Topol EJ, Agnelli G, Bertrand M, Califf RM, Clement DL, et al: Critical issues in peripheral arterial disease detection and management: a call to action. Arch Intern Med. 2003, 163: 884-892. 10.1001/archinte.163.8.884.
Klipstein-Grobusch K, Koster JF, Grobbee DE, Lindemans J, Boeing H, Hofman A, et al: Serum ferritin and risk of myocardial infarction in the elderly: the Rotterdam Study. Am J Clin Nutr. 1999, 69: 1231-1236. 10.1097/00001648-199403000-00015.
Morrison HI, Semenciw RM, Mao Y, Wigle DT: Serum iron and risk of fatal acute myocardial infarction. Epidemiology. 1994, 5: 243-246. 10.1097/00001648-199403000-00015.
Salonen JT, Nyyssonen K, Korpela H, Tuomilehto J, Seppanen R, Salonen R: High stored iron levels are associated with excess risk of myocardial infarction in eastern Finnish men. Circulation. 1992, 86: 803-811. 10.1016/S0140-6736(81)92463-6.
Sullivan JL: Iron and the sex difference in heart disease risk. Lancet. 1981, 1: 1293-1294. 10.1016/S0140-6736(81)92463-6.
Ascherio A, Rimm EB, Giovannucci E, Willett WC, Stampfer MJ: Blood donations and risk of coronary heart disease in men. Circulation. 2001, 103: 52-57.
Gillum RF, Sempos CT, Makuc DM, Looker AC, Chien CY, Ingram DD: Serum transferrin saturation, stroke incidence, and mortality in women and men. The NHANES I Epidemiologic Followup Study. National Health and Nutrition Examination Survey. Am J Epidemiol. 1996, 144: 59-68.
Baer DM, Tekawa IS, Hurley LB: Iron stores are not associated with acute myocardial infarction. Circulation. 1994, 89: 2915-2918.
Liao Y, Cooper RS, McGee DL: Iron status and coronary heart disease: negative findings from the NHANES I epidemiologic follow-up study. Am J Epidemiol. 1994, 139: 704-712.
Manttari M, Manninen V, Huttunen JK, Palosuo T, Ehnholm C, Heinonen OP, et al: Serum ferritin and ceruloplasmin as coronary risk factors. Eur Heart J. 1994, 15: 1599-1603. 10.1056/NEJM199404213301604.
Sempos CT, Looker AC, Gillum RF, Makuc DM: Body iron stores and the risk of coronary heart disease. N Engl J Med. 1994, 330: 1119-1124. 10.1016/S1047-2797(00)00068-5.
Sempos CT, Looker AC, Gillum RE, McGee DL, Vuong CV, Johnson CL: Serum ferritin and death from all causes and cardiovascular disease: the NHANES II Mortality Study. National Health and Nutrition Examination Study. Ann Epidemiol. 2000, 10: 441-448. 10.1016/S1047-2797(00)00068-5.
Danesh J, Appleby P: Coronary heart disease and iron status: meta-analyses of prospective studies. Circulation. 1999, 99: 852-854. 10.1001/jama.297.6.603.
Zacharski LR, Chow BK, Howes PS, Shamayeva G, Baron JA, Dalman RL, et al: Reduction of iron stores and cardiovascular outcomes in patients with peripheral arterial disease: a randomized controlled trial. JAMA. 2007, 297: 603-610. 10.1001/jama.297.6.603.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2261/9/34/prepub
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
AM participated in the analysis and interpretation of the data and drafted the manuscript. JMFR and PM participated in interpretation of the data and revised the manuscript. EG conceived the study, participated in the analysis and interpretation of the data, and revised the manuscript. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Menke, A., Fernández-Real, J.M., Muntner, P. et al. The association of biomarkers of iron status with peripheral arterial disease in US adults. BMC Cardiovasc Disord 9, 34 (2009). https://doi.org/10.1186/1471-2261-9-34
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2261-9-34