Abstract
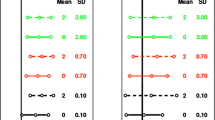
In the drug development process, clinical trials are conducted for different diseases or indications. Generally, the assessment of drug effectiveness is performed by only utilizing data from clinical trials within a particular indication. However, in some cases, different indications are closely related. They may be etiologically or pathophysiologically similar. Even if the indications are less closely related, the general purpose of therapy may be very similar. Evaluating data from all of these related studies together enables researchers to access the information, and give a systematic overview of the treatment effect in a broader scope. In this paper, we use an example in the allergy therapeutic area to illustrate a Bayesian approach to synthesizing evidence from trials aimed at closely related indications. This approach accounts for the heterogeneity of treatment effects across different indications as well as across different studies. It also allows us to quantify the treatment benefit difference between different indications in a more meaningful way. Demonstration of beneficial drug effects on different indications in the context of all related data can cross-substantiate a claim of effectiveness for each indication and hence strengthen the evidence of treatment efficacy.
Similar content being viewed by others
References
Food and Drug Administration. Guidance for Industry-Providing Clinical Evidence of Effectiveness for Human Drugs and Biological Products. Bethesda, MD; US Department of Health and Human Services, Food and Drug Administration; 1998.
Allen IE, Kupelnick B, Kumashiro M, Luo D, Ross S, Wolin M. Efficacy of Interleukin-2 in the treatment of metastatic melanoma: Systematic review and metaanalysis. Cancer Therapeut. 1998;3:168–173.
Dersimonian R, Laird N. Meta-analysis in clinical trials. Controll Clin Trials. 1986;7:177–188.
Tweedie RZ, Mengersen KL. Lung cancer and passive smoking: Reconciling the biochemical and epidemiological approach. Br J Cancer. 1992;66:700–705.
Gelman A, Carlin JB, Stern HS, Rubin DB. Bayesian Data Analysis. New York, NY: Chapman & Hall; 1995.
Chalmers TC. Problems induced by meta-analysis. Stat Med. 1991;10:971–980.
DuMouchel W. Bayesian Meta-analysis. In: Berry D, ed. Statistical Methods for Pharmacology. New York, NY: Marcel Dekker, 1990:509–529.
He Z, Sun D. Hierarchical Bayesian estimation of hunting success rates with spatial correlations. Biometrics. 2000;56:360–367.
Carlin JB. Meta-analysis for 2 x 2 tables: a Bayesian approach. Stat Med. 1992;11:141–158.
Ibrahim JG, Chen M. Prior distributions and Bayesian computation for proportional hazards models. Indian J Stat. 1998;60:48–64.
Spiegelhalter DJ, Thomas A, Best N, Gilks WR. BUGS Examples, Version 0.50. Technical report. Cambridge, England: Institute of Public Health; 1995.
Spiegelhalter DJ, Freedman LS, Darmar MK. Bayesian approaches to randomized trials. J Roy Stat Soc. 1994;357–416.
Begg CB, Pilote L. A model for incorporating historic controls into a meta-analysis. Biometrics. 1991; 47:899–906.
Olkin I. Meta-analysis: methods for combining independent studies. Stat Sci. 1992;7:226.
Light R, Pillemer D. Summing Up: The Science of Reviewing Research. Cambridge, MA: Harvard University Press; 1984.
Dural S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56: 450–463.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, S., Suresh, R. Evaluating Data from Closely Related Clinical Trials. Ther Innov Regul Sci 35, 1317–1326 (2001). https://doi.org/10.1177/009286150103500428
Published:
Issue Date:
DOI: https://doi.org/10.1177/009286150103500428