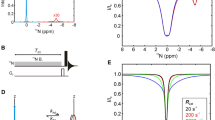
Abstract
Continuous wave time-resolved EPR (CW-TREPR) and pulsed Fourier transform EPR (FT-EPR) experiments with a resolution of ≈10 ns were carried out to investigate the Type I photocleavage. A two-pulse echo detection method was used to eliminate the dead-time problem in the FID measurements. An analysis of the buildup kinetics of the FT-EPR signal intensity due to t-butyl radicals provided the rates of the α-cleavage of t-butylphenyl ketone at various temperatures. We obtained two kinetic parameters at low and high temperatures which correspond to the non-radiative decay rate of the excited triplet state of the ketone and the rate of the Type I photocleavage, respectively.
Similar content being viewed by others
References
F.D. Lewis and J.G. Magyar, J. Org. Chem. 37, 2102 (1972).
M.V. Encina, E.A. Lissi, E. Lemp, A. Zanocco, and J.C. Scaiano, J. Am. Chem. Soc. 105, 1856 (1983).
For Recent Reviews see H. van Willingen, P.R. Levestein, and M.H. Ebersole, Chem. Rev., 93, 173 (1993) and also M.K. Bowman, In: Modern Pulsed and Continuous-Wave Electron Spin Resonance; L. Kevan, and M.K. Bowman, (Eds.), Wiley New York, 1990, pp 1–42.
K. Akiyama, S. Tero-Kubota, and Y. Ikegami, J. Am. Chem. Soc., 106, 8322 (1984).
K. Munger and H. Fischer, Int. J. Chem. Kinetics 17, 809 (1985).
H. Fischer, J. Phys. Chem. 73, 3834 (1969); b) P.W. Atkins, A.J. Dobbs, and K.A. McLauchlan, J. Chem. Soc., Faraday Trans. 2 71, 1269 (1975).
T. Ikoma, K. Akiyama, S. Tero-Kubota, and Y. Ikegami, Chem. Lett. 1491 (1990).
P. Jaegermann, F. Lendzian, G. Rist, and K. Mobius, Chem. Phys. Lett., 140, 615 (1987); b) M. Koyanagi, H. Futami, M. Mukai, and S. Yamauchi, Chem. Phys. Lett., 154, 577 (1989).
J.P. Fouassier, Prog. Org. Coating 18, 229 (1990).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Akiyama, K., Tero-Kubota, S. Time-resolved and fourier transform EPR studies of type I photochemical reaction. Direct determination of kinetic parameters of C−C bond cleavage. Res. Chem. Intermed. 22, 103–113 (1996). https://doi.org/10.1163/156856796X00557
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1163/156856796X00557