Abstract
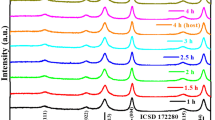
The structure of iron and managanese ions substituted in the framework of nanoporous AlPO-5 is determined by ex situ and in situ X-ray absorption spectroscopy. Fe K-edge XANES and EXAFS studies clearly indicate that iron ions are present as Fe(III) in octahedral coordination in the assynthesised material and tetrahedral coordination in the calcined material in both pure FeAlPO-5 and FeMnalPO-5. XANES and EXAFS results also indicate that reaction with hydrogen peroxide causes the removal of Fe(III) ions from the framework. Mn K-edge XANES and EXAFS of FeMnAlPO-5 samples indicate that Mn(II) ions are present in the framework, tetrahedrally coordinated, in the as-synthesised material but upon calcination it is found that the Mn(II) ions are removed from the framework, suggesting a different synthesis strategy is necessary to stabilise the Mn(II) ions in the framework simultaneously with Fe(III) ions.
Similar content being viewed by others
References
M. E. Davis, Acc. Chem. Res. 26, 111 (1993).
J. M. Thomas, Angew. Chem. Int. Edn Engl. 33, 913 (1994).
A. Corma, Chem. Rev. 97, 2373 (1997).
J. M. Thomas, Angew. Chem. Int. Edn 38, 3589 (1999).
M. Hartmann and L. Kevan, Res. Chem. Intermed. 28, 625 (2002).
A. Corma, J. Catal. 216, 298 (2003).
S. T. Wilson, B. M. Lok, C. A. Messina, T. R. Cannan and E. M. Flaningen, US Patent 4,310,440 (1982).
S. T. Wilson, B. M. Lok, C. A. Messina, T. R. Cannan and E. M. Flanigen, J. Am. Chem. Soc. 104, 1146 (1982).
E. M. Flanigen, B. M. Lok, R. L. Patton and S. T. Wilson, Pure Appl. Chem. 58, 1351 (1986).
S. T. Wilson and E. M. Flanigen, US Patent 4,567,029 (1986).
S. T. Wilson and E. M. Flanigen, ACS Symp. Ser. 398, 329 (1989).
J. M. Thomas, R. Raja, G. Sankar and R. G. Bell, Acc. Chem. Res. 34, 191 (2001).
P. A. Barrett, R. H. Jones, J. M. Thomas, G. Sankar, I. J. Shannon and C. R. A. Catlow, Chem. Commun, 2001 (1996).
I. L. Franklin, A. M. Beale and G. Sankar, Catal. Today 81, 623 (2003).
G. Sankar, R. Raja and J. M. Thomas, Catal. Lett. 55, 15 (1998).
R. Raja, G. Sankar and J. M. Thomas, Chem. Commun., 829 (1999).
J. M. Thomas, R. Raja, G. Sankar and R. G. Bell, Nature 398, 227 (1999).
N. R. Shiju, S. Fiddy, O. Sonntag, M. Stockenhuber and G. Sankar, Chem. Commun., 4955 (2006).
A. Simmen, L. B. McCusker, C. Baerlocher and W. M. Meier, Zeolites 11, 654 (1991).
M. A. Estermann, L. B. McCusker and C. Baerlocher, J. Appl. Crystallogr. 25, 539 (1992).
L. M. Bull, A. K. Cheetham, P. D. Hopkins and B. M. Powell, J. Chem. Soc. Chem. Commun., 1196 (1993).
W. H. Baur, W. Joswig, D. Kassner, J. Kornatowski and G. Finger, Acta Crystallogr. B: Struct. Sci. 50, 290 (1994).
L. M. Bull, A. K. Cheetham, B. M. Powell, J. A. Ripmeester and C. I. Ratcliffe, J. Am. Chem. Soc. 117, 4328 (1995).
L. Smith, A. K. Cheetham, R. E. Morris, L. Marchese, J. M. Thomas, P. A. Wright and J. Chen, Science 271, 799 (1996).
A. Meden, C. Baerlocher and L. B. McCusker, Microporous Mater. 11, 247 (1997).
V. Ramaswamy, L. B. McCusker and C. Baerlocher, Microporous Mesoporous Mater. 31, 1 (1999).
A. Martucci, A. Alberti, G. Cruciani, A. Frache, S. Coluccia and L. Marchese, J. Phys. Chem. B 107, 9655 (2003).
G. Sankar, J. K. Wyles, R. H. Jones, J. M. Thomas, C. R. A. Catlow, D. W. Lewis, W. Clegg, S. J. Coles and S. J. Teat, Chem. Commun., 117 (1998).
G. Sankar and J. M. Thomas, Top. Catal. 8, 1 (1999).
J. M. Thomas and G. Sankar, Acc. Chem. Res. 34, 571 (2001).
J. M. Thomas and G. Sankar, J. Synchrotron Radiat. 8, 55 (2001).
C. Zenonos, G. Sankar, F. Cora, D. W. Lewis, Q. A. Pankhurst, C. R. A. Catlow, J. M. Thomas, Phys. Chem. Chem. Phys. 4, 5421 (2002).
S. Bordiga, F. Boscherini, S. Coluccia, F. Genoni, C. Lamberti, G. Leofanti, L. Marchese, G. Petrini, G. Vlaic and A. Zecchina, Catal. Lett. 26, 195 (1994).
S. Bordiga, S. Coluccia, C. Lamberti, L. Marchese, A. Zecchina, F. Boscherini, F. Buffa, F. Genoni, G. Leofanti, G. Petrini and G. Vlaic, J. Phys. Chem. 98, 4125 (1994).
F. Farges, G. E. Brown and J. J. Rehr, Geochim. Cosmochim. Acta 60, 3023 (1996).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sankar, G., Shiju, N.R., Watts, I.D. et al. Structure of iron and manganese ions substituted in the framework of nanoporous AlPO-5 material. Res. Chem. Intermed. 34, 649–658 (2008). https://doi.org/10.1163/156856708784795644
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1163/156856708784795644