Abstract
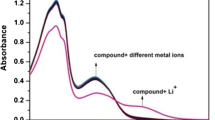
Many photo-physical studies have been reported for the detection of Hg2+ ions. Here we present the effect of Hg 2+2 ions on the absorption and fluorescence of indole-2-carboxylic acid (I2C). Experimental evidence, supported by density functional theory B3LYP/LANL2DZ/PCM, for the formation of a I2C-Hg 2+2 complex, is reported for the first time. It was observed that I2C forms a ground-state complex with Hg 2+2 ions in a ratio of 3:1. The possibility of I2C to be used as a selective novel chemical sensor for the spectrophotometric detection of mercurous ions is described.
Similar content being viewed by others
References
D. Caric, V. Tomisic, M. Kveder, N. Galic, G. Pifat, V. Magnus and M. Soskic, Biophys. Chem. 111, 247 (2004).
J. O. Nriagu and J. M. Pacyna, Nature 333, 134 (1988).
C. Orvig and M. J. Adams, Chem. Rev. 99, 2200 (1999).
M. Tabak, G. Sartor and P. Cavatorta, J. Luminesc. 43, 355 (1989).
J. Pesek, H. Abpikar and J. Becker, Appl. Spectrosc. 42, 473 (1988).
A. Tine and J. J. Aaron, Anal. Chim. Acta 227, 181 (1989).
A. Jonsson, in: Encyclopedia of Plant Physiology, W. Ruhland (Ed.), Vol. 14, p. 959. Springer, Berlin (1961).
J. Westall, in: Aquatic Surface Chemistry: Chemical Processes at the Particle-Water Interface. W. Stumm (Ed.), p. 3. Wiley, New York, NY (1987).
N. S. Bloom, G. A. Gill, S. Cappellino, C. Dobbs, L. McShea, C. Driscoll, R. Mason and J. Rudd, Environ. Sci. Technol. 33, 7 (1999).
K. Z. Braininaa, N. Y. Stozhko and Z. V. Shlygina, Anal. Chem. 57, 945 (2002).
A. B. Descalzo, R. Martinez-Mañez, R. Redeglia, K. Rurack and J. Soto, J. Am. Chem. Soc. 125, 3418 (2003).
J. Yoon, N. E. Ohler, D. H. Vance, W. D. Aumiller and A. W. Czarnic, Tetrahedron Lett. 38, 3845 (1997).
E. M. Nolan and S. J. Lippard, J. Am. Chem. Soc. 125, 14270 (2003).
E. Palomares, R. Vilar and J. Durrant, Chem. Commun. 4, 362 (2004).
A. Ono and H. Togashi, Angew. Chem. Int. Edn. 43, 4300 (2004).
M. Krishnamuthy, A. Mishra and S. K. Dogra, Photochem. Photobiol. 45, 359 (1987).
M. Krishnamuthy, H. K. Sinha and S. K. Dogra, J. Luminesc. 35, 343 (1985).
P. Bangal and S. Chakravorti, J. Phys. Chem. A, 103, 8585 (1999).
J. J. Aaron, A. Tine, M. E. Wojciechowska and C. Parkanyi, J. Luminesc. 33, 33 (1985).
Z. D. Hill and P. MacCarthy, J. Chem. Educ. 63, 162 (1986).
J. Franke and F. Vogtle, Top. Curr. Chem. 132, 13 (1986).
A. D. Becke, J. Chem. Phys. 98, 5648 (1993).
A. D. Becke, J. Chem. Phys. 104, 1040 (1996).
C. Lee, W. Yang and R. G. Parr, Phys. Rev. B37, 785 (1987).
N. Rega, M. Cossi and V. Barone, J. Chem Phys. 105, 11060 (1996).
V. Barone, M. Cossi and J. Tomasi, J. Comput. Chem. 18, 404 (1998).
G. Klopman, J. Am. Chem. Soc. 90, 223 (1968).
L. Salem, J. Am. Chem. Soc. 90, 543 (1968).
L. Salem, J. Am. Chem. Soc. 90, 553 (1968).
I. Fleming, in: Frontier Orbitals and Organic Chemical Reactions, p. 34. Wiley, London (1976).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rele, M., Kapoor, S., Hedge, S. et al. Photophysical characteristics and density functional theory calculations of indole 2-carboxylic acid in the presence of mercurous ions. Res Chem Intermed 32, 637–645 (2006). https://doi.org/10.1163/156856706778400299
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1163/156856706778400299