Abstract
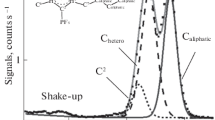
In this study the brominated derivative of pyrimidine, 2-bromopyrimidine, was investigated by photoelectron spectroscopy. Outer valence photoelectron spectra recorded at 21.22, 45 and 100 eV photon energy for this compound are presented. The recorded spectra have a higher resolution than that previously reported in the literature. The bromine 3d and 3p edge photoelectron spectra have also been recorded in a photon impact experiment at 100 and 225 eV. All measurements were performed using Double Toroidal Coincidence Spectrometer showing its potential as a versatile apparatus for spectroscopic studies.
Similar content being viewed by others
References
W.C. Dewey, R.M. Humphrey, Radiat. Res. 26, 538 (1965)
A.W. Potts, D.M.P. Holland, A.B. Trofimov, J. Schirmer, L. Karlsson, K. Siegbahn, J. Phys. B: At. Mol. Opt. Phys. 36, 3129 (2003)
P. O’Keeffe, P. Bolognesi, A.R. Casavola, D. Catone, N. Zema, S. Turchini, L. Avaldi, Molec. Phys. 107, 2025 (2009)
P. Bolognesi, G. Mattioli, P. O’Keeffe, V. Feyer, O. Plekan, Y. Ovcharenko, K.C. Prince, M. Coreno, A. Amore Bonapasta, L. Avaldi, J. Phys. Chem. A 113, 13593 (2009)
L. Storchi, F. Tarantelli, S. Veronesi, P. Bolognesi, E. Fainelli, L. Avaldi, J. Chem. Phys. 129, 154309 (2008)
P. Bolognesi, P. OKeeffe, Y. Ovcharenko, M. Coreno, L. Avaldi, V. Feyer, O. Plekan, K.C. Prince, W. Zhang, V. Carravetta, J. Chem. Phys. 133, 034302 (2010)
J.D. Builth-Williams, S.M. Bellm, D.B. Jones, H. Chaluvadi, D.H. Madison, C.G. Ning, B. Lohmann, M.J. Brunger, J. Chem. Phys. 136, 024304 (2012)
T.J. Reddish, G. Richmond, G.W. Bagley, J.P. Wightman, S. Cvejanovic, Rev. Sci. Instrum. 68, 2685 (1997)
A.E. Slattery, J.P. Wightman, M.A. MacDonald, S. Cvejanovic, T.J. Reddish, J. Phys. B: At. Mol. Opt. Phys. 33, 4833 (2000)
Y.F. Hu, L. Zuin, G. Wright, R. Igarashi, M. McKibben, T. Wilson, S.Y. Chen, T. Johnson, D. Maxwell, B.W. Yates, T.K. Sham, R. Reininger Rev. Sci. Instrum. 78, 083109 (2007)
D.M.P. Holland, A.W. Potts, L. Karlsson, I.L. Zaytseva, A.B. Trofimov, J. Schirmer, Chem. Phys. 352, 205 (2008)
B.H. Boo, N. Saito, J. Electron. Spectrosc. Relat. Phenom. 127, 139 (2002)
B.H. Boo, I. Koyano, J. Korean Phys. Soc. 40, 826 (2002)
T. Ohta, T. Fujikawa, H. Kuroda, Bull. Chem. Soc. Jpn. 48, 2017 (1975)
R. Spohr, T. Bergmark, N. Magnusson, L.O. Werme, C. Nordiing, K. Siegbahn, Phys. Scr. 2, 31 (1970)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Śmiałek, M.A., Szymańska, E., MacDonald, M. et al. Photoelectron spectroscopy of brominated derivative of pyrimidine: 2-bromopyrimidine. Eur. Phys. J. Spec. Top. 222, 2361–2366 (2013). https://doi.org/10.1140/epjst/e2013-02017-8
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1140/epjst/e2013-02017-8